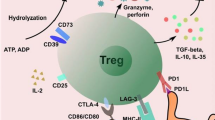
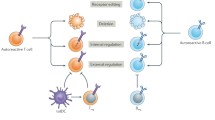
Abstract
It has become increasingly clear that the innate and adaptive arms of the immune response cooperate in generating autoimmune damage in the pathogenesis of rheumatoid arthritis and juvenile idiopathic arthritis. Treatment targets the immunologic pathophysiology of the disease and is based on regaining immune tolerance. Recently introduced biological agents neutralize or simply block cytokines and their proinflammatory pathways, with favorable clinical outcome. However, major downsides are their lack of specificity and the need of continuous administration to be effective. Possibly, more can be gained from a specific approach. Indeed, recent findings suggest that targeting antigen-specific T cells can reinstate regulatory mechanisms and thus induce immune tolerization. This improved understanding has paved the way to novel immunotherapeutic approaches, some of which will be discussed here.
Similar content being viewed by others
References and Recommended Reading
Petty RE, Southwood TR, Baum J, et al.: Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol 1998, 25:1991–1994.
Sakkas LI, Platsoucas CD: Immunopathogenesis of juvenile rheumatoid arthritis: role of T cells and MHC. Immunol Res 1995, 14:218–236.
Salmon M, Gaston JS: The role of T-lymphocytes in rheumatoid arthritis. Br Med Bull 1995, 51:332–345.
Matzinger P: Tolerance, danger, and the extended family. Ann Rev Immunol 1994, 12:991–1045.
Prinz JC: Disease mimicry—a pathogenetic concept for T cellmediated autoimmune disorders triggered by molecular mimicry? Autoimmun Rev 2004, 3:10–15.
Bonnin D, Prakken B, Samodal R, et al.: Ontogeny of synonymous T cell populations with specificity for a self MHC epitope mimicked by a bacterial homologoue: an antigenspecific T cell analysis in a non-transgenic system. Eur J Immunol 1999, 29:3826–3836.
Medzhitov R, Janeway C, Jr.: The toll receptor family and microbial recognition. Trends Microbiol 2000, 8:452–456.
Kimbrell DA, Beutler B: The evolution and genetics of innate immunity. Nat Rev Genet 2001, 2:256–267.
Aderem A, Ulevitch RJ: Toll-like receptors in the induction of the innate immune response. Nature 2000, 406:782–787.
Albani S, Ravelli A, Massa M, et al.: Immune responses to the Escherichia coli dnaJ heat shock protein in juvenile rheumatoid arthritis and their correlation with disease activity. J Pediatr 1994, 124:561–565.
Abulafia-Lapid R, Elias D, Raz I, et al.: T cell proliferative responses of type 1 Diabetes patients and healthy individuals to human hsp60 and its peptides. J Autoimmunity 1999, 12:121–129.
de Graeff-Meeder ER, Zee R, Rijkers GT, et al.: Recognition of human 60 kD heat shock protein by mononuclear cells from patients with juvenile chronic arthritis. Lancet 1991, 337:1368–1372.
de Kleer IM, Kamphuis SM, Rijkers GT, et al.: The spontaneous remission of juvenile idiopathic arthritis is characterized by CD30+ T cells directed to human heat-shock protein 60 capable of producing the regulatory cytokine interleukin-10. Arthritis Rheum 2003, 48:2001–2010.
Prakken ABJ, Van Eden W, Rijkers GT, et al.: Autoreactivity to human heat-shock protein 60 predicts disease remission in oligoarticular juvenile rheumatoid arthritis. Arthritis Rheum 1996, 39:1826–1832.
Abbas AK: The control of T cell activation vs. tolerance. Autoimmun Rev 2003, 2:115–118.
Pasare C, Medzhitov R: Toll-like receptors: balancing host resistance with immune tolerance. Curr Opin Immunol 2003, 15:677–682. This paper describes the role of the receptors of the innate immune system on induction of an adaptive immune response. Also suggests a pathogenic role for malfunction of this pathway.
Nolan KF, Strong V, Soler D, et al.: IL-10-conditioned dendritic cells, decommissioned for recruitment of adaptive immunity, elicit innate inflammatory gene products in response to danger signals. J Immunol 2004, 172:2201–2209. This paper shows regulatory effects of an adaptive response on the innate immune system. A very good example of the crosstalk between the two arms of the immune system.
La Cava A, Matarese G: The weight of leptin in immunity. Nat Rev Immunol 2004, 4:371–379.
Moreno C, Merino J, Vazquez B, et al.: Anti-inflammatory cytokines induce lipopolysaccharide tolerance in human monocytes without modifying toll-like receptor 4 membrane expression. Scand J Immunol 2004, 59:553–558.
Wolkers MC, Brouwenstijn N, Bakker AH, et al.: Antigen bias in T cell cross-priming. Science 2004, 304:1314–1317.
Tze LE, Baness EA, Hippen KL, Behrens TW: Ig light chain receptor editing in anergic B cells. J Immunol 2000, 165:6796–6802.
Berg LJ: Generation of the T cell repertoire. Curr Opin Immunol 1989, 2:87–92.
Hammerling GJ, Schonrich G, Ferber I, Arnold B: Peripheral tolerance as a multi-stepmechanism. Immunol Rev 1993, 133:93–104.
Ohashi PS, Oehen S, Buerki K, et al.: Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 1991, 65:305–317.
Oldstone MB, Nerenberg M, Southern P, et al.: Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell 1991, 65:319–331.
Jonuleit H, Schmitt E, Stassen M, et al.: Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med 2001, 193:1285–1294.
Bluestone JA, Abbas AK: Natural versus adaptive regulatory T cells. Nat Rev Immunol 2003, 3:253–257. A major breakthrough in how Treg is thought of. Proposes the coexistance of two major subsets of Treg and discusses their development.
Giannini EH, Brewer EJ, Kuzmina N, et al.: Methotrexate in resistant juvenile rheumatoid arthritis. Results of the USAUSSR double-blind, placebo-controlled trial. N Engl J Med 1992, 326:1043–1049.
Han J, Ulevitch RJ: Emerging targets for anti-inflammatory therapy. Nat Cell Biol 1999, 1:E39-E40.
Ehrenstein MR, Evans JG, Singh A, et al.: Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med 2004, 200:277–285. Interesting report suggesting that anti-TNFα therapy does not only suppress the inflammatory immune response, but also actively modulates the immune response.
Wendling D, Racadot E, Wijdenes J: Treatment of severe rheumatoid arthritis by anti-interleukin 6 monoclonal antibody. J Rheumatol 1993, 20:259–262.
Nishimoto N, Kishimoto T, Yoshizaki K: Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Ann Rheum Dis 2000, 59(Suppl 1):i21-i27.
Nishimoto N, Yoshizaki K, Maeda K, et al.: Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis. Phase I/II clinical study. J Rheumatol 2003, 30:1426–1435.
Choy EH, Isenberg DA, Garrood T, et al.: Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, doseescalation trial. Arthritis Rheum 2002, 46:3143–3150.
Cutolo M: [IL-1Ra: its role in rheumatoid arthritis]. Reumatismo 2004, 56:41–45.
Silverman GJ, Weisman S: Rituximab therapy and autoimmune disorders: prospects for anti-B cell therapy. Arthritis Rheum 2003, 48:1484–1492.
Edwards JC, Szczepanski L, Szechinski J, et al.: Efficacy of Bcell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004, 350:2572–2581.
De Vita S, Zaja F, Sacco S, et al.: Efficacy of selective B cell blockade in the treatment of rheumatoid arthritis: evidence for a pathogenetic role of B cells. Arthritis Rheum 2002, 46:2029–2033.
Schulze-Koops H, Davis LS, Haverty TP, et al.: Reduction of Th1cell activity in the peripheral circulation of patients with rheumatoid arthritis after treatment with a non-depleting humanized monoclonal antibody to CD4. J Rheumatol 1998, 25:2065–2076.
Chatenoud L: CD3-specific antibody-induced active tolerance: from bench to bedside. Nat Rev Immunol 2003, 3:123–132.
Moreland LW, Alten R, Van den BF, et al.: Costimulatory blockade in patients with rheumatoid arthritis: a pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum 2002, 46:1470–1479.
Eagar TN, Karandikar NJ, Bluestone JA, Miller SD: The role of CTLA-4 in induction and maintenance of peripheral T cell tolerance. Eur J Immunol 2002, 32:972–981.
Weiner HL: Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med 1997, 48:341–351.
Xiao BG, Link H: Mucosal tolerance: a two-edged sword to prevent and treat autoimmune diseases. Clin Immunol Immunopathol 1997, 85:119–128.
Barnett ML, Combitchi D, Trentham DE: A pilot trial of oral type II collagen in the treatment of juvenile rheumatoid arthritis. Arthritis Rheum 1996, 39:623–628.
Myers LK, Higgins GC, Finkel TH, et al.: Juvenile arthritis and autoimmunity to type II collagen. Arthritis Rheum 2001, 44:1775–1781.
Steenbakkers PG, Baeten D, Rovers E, et al.: Localization of MHC class II/human cartilage glycoprotein-39 complexes in synovia of rheumatoid arthritis patients using complex-specific monoclonal antibodies. J Immunol 2003, 170:5719–5727.
Baeten D, Steenbakkers PG, Rijnders AM, et al.: Detection of major histocompatibility complex/human cartilage gp-39 complexes in rheumatoid arthritis synovitis as a specific and independent histologic marker. Arthritis Rheum 2004, 50:444–451.
Steinman RM, Mellman I: Immunotherapy: bewitched, bothered, and bewildered no more. Science 2004, 305:197–200. This article provides an update on state-of-the-art immunotherapy in basic research and clinic. It also describes difficulties to overcome and where future research should be focused on.
Prakken B, Kuis W, Van Eden W, Albani S: Heat shock proteins in juvenile idiopathic arthritis: keys for understanding remitting arthritis and candidate antigens for immune therapy. Curr Rheumatol Rep 2002, 4:466–473.
Welch WJ, Kang HS, Beckmann RP, Mizzen LA: Response of mammalian cells to metabolic stress; changes in cell physiology and structure/function of stress proteins. Curr Top Microbiol Immunol 1991, 167:31–55.
Jones DB, Coulson AFW, Duff GW: Sequence homologies between hsp60 and autoantigens. Immunol Today 1993, 14:115–118.
Albani S, Keystone EC, Nelson JL, et al.: Positive selection in autoimmunity: abnormal immune responses to a bacterial dnaJ antigenic determinant in patients with early rheumatoid arthritis. Nature Med 1995, 1:448–452.
Windhagen A, Nicholson LB, Weiner HL, et al.: Role of Th1 and Th2 cells in neurologic disorders. Chem Immunol 1996, 63:171–186.
Van Eden W, Thole JER, van der Zee R, et al.: Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 1988, 331:171–173.
Van Eden W: Heat-shock proteins as immunogenic bacterial antigens with the potential to induce and regulate autoimmune arthritis. Immunol Rev 1991, 121:5–28.
Albani S, Keystone EC, Nelson JL, et al.: Positive selection in autoimmunity: abnormal immune responses to a bacterial dnaJ antigenic determinant in patients with early rheumatoid arthritis. Nature Med 1995, 1:448–452.
La Cava A, Nelson JL, Ollier WER, et al.: Genetic bias in immune responses to a cassette shared by different microorganisms in patients with rheumatoid arthritis. J Clin Invest 1997, 100:658–663.
Prakken BJ, Samodal R, Le TD, et al.: Epitope-specific immunotherapy induces immune deviation of proinflammatory T cells in rheumatoid arthritis. Proc Natl Acad Sci U S A 2004, 101:4228–4233. Well designed phase I clinical trial that shows the ability of a mucosal route to induce tolerance to a specific antigen systemically. Most likely achieved by the induction of Treg.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Teklenburg, G., Albani, S. The role of immune tolerance in preventing and treating arthritis. Curr Rheumatol Rep 6, 434–441 (2004). https://doi.org/10.1007/s11926-004-0022-3
Issue Date:
DOI: https://doi.org/10.1007/s11926-004-0022-3