Abstract
Purpose of Review
Researchers suggests that patients with COVID-19 develop neuropathic pain within weeks or months following infection and that patients with neuropathic pain and COVID-19 sometimes present with deterioration of neurologic complications and pain exacerbation. The objective of this systematic review is to discuss the case-reports having neuropathic pain during and after COVID-19 infection.
Recent Findings
Case reports that has described about patients getting neuropathy or neuropathic pain around the disease either immediately or late post COVID were included. The data was extracted and qualitatively synthesised. Literature was searched and 939 articles were found. 12 articles were screened as per the eligibility criteria and finally, 6 case reports on neuropathic pain in Covid-19 were selected from the database and manual search and finalised for analysis. 2 cases of herpes zoster and post herpetic neuralgia, 2 cases of intense burning pain, 1 case of trigeminal neuralgia and 1 of brachial plexopathy included for the review.
Summary
Covid 19 viral neurogenic invasion is something very newly discovered topic of discussion in the field of research. With the passage of time, more cases will emerge and more data will be available for research. The review is registered in Prospero with no. CRD42021257060.
Similar content being viewed by others
Introduction
Neuropathic pain is the pain that results due to any lesion or disease of the somatosensory nervous system, as per the International Association for the Study of Pain (IASP) [1, 2]. There are different causative neurobiological mechanisms, clinical features, and aetiology of the present lesion for this broad category of pain. Non-nociceptive pain is the most broadly known and accepted term for neuropathic pain because there is no nociceptive stimulus present. Neuropathic pain can be very disturbing for the patient and may have chronic effects if not treated timely and properly. Neuropathic pain is described as throbbing, piercing, or burning pain by the patients. Patients also complain of hyperalgesia, which is a feeling of more pain than it should be felt when a normally causing pain stimulus is given. Patients complain of allodynia which is the pain caused by painless stimulus. Neuropathy and neuropathic pain can be triggered by many viral infections. Peripheral neuropathy is caused by immune-mediated mechanisms and not by direct nerve disease. This is supported by viral genome studies on peripheral neuropathy [3, 6]. Inflammation of the peripheral nerves and ganglia as well as tissue damage causes the development of pain. Some viral infections, such as herpes zoster virus, human immunodeficiency virus (HIV), Epstein-Barr virus, cytomegalovirus, enteroviruses, and tropical viruses, have been shown to cause neuropathic pain [4, 5]. Neuropathic pain has also been documented in patients recovering from the SARS virus, a coronavirus that caused a worldwide outbreak in 2003 [5]. Viral infections can injure the peripheral nervous system, by directly effecting the microbe and secondary immune overactivation. Infection through SARS-CoV-2 and any neurogenic symptoms points towards post-infectious response resulting from compromised immunity [1]. SARS‐CoV‐2 infection-associated Guillain–Barre syndrome (GBS)-related case reports have been documented in China and Europe [7,8,9].
This review will specifically throw light on the case reports of patients with neuropathic pain, developed around COVID-19 infection.
Methods
Data Source and Searches
Literature for this systematic review (PROSPERO Registration: CRD42021257060) has been searched electronically from January 2020 to July 2021 in Web of Science, Medline (PUBMED), SCOPUS, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL). Search was done using highly sensitive strategies in combination with the following terms: “Covid-19”, “Covid”, “Corona”, “Pain”, “Neuropathy”, “Neurological”, “Neuropathic pain”, “Neuralgia”, “Case study”, and “Case-report”. Publications in English language only will be included. Additional studies were searched by analysing the references from relevant articles.
Study Selection and Data Extraction
Two reviewers (NRD and DJ) independently evaluated and scrutinised the full text articles for eligibility as per criteria for eligibility. Any kind of conflict between reviewers was sorted by involving third reviewer (VG) and mutual consent. Both individually extracted data in accordance with the year of publication, type of study, and each and every information related to participants like the disease onset, epidemiology, and signs/symptoms, investigations, pain severity, and conclusions. We included case reports with pain related to neurological manifestations and any kind of neuropathic pain associated with SARS‐CoV‐2 infection. Interventional, comparative, and prospective studies were excluded.
Integration of Results
The data was extracted and qualitatively synthesised. Because the number of reports and patients is restricted, and also the study design, underlying pathology, condition, intervention, investigations procedure, and outcome measures were of heterogenous nature, quantitative analysis could not be conducted.
Ethical Permit
Published case reports have been included in this systematic review. This work did not include any details about the personal lives of the patients. This study did not include any human participants. We did not seek ethical approval from a committee.
Evaluation of the Risk of Biases
Moola et al. [10] devised the critical evaluation checklist for case reports. We incorporated it for the evaluation of risk of bias in the included studies. If a case report met 5 of the 8 appraisal criteria, it was considered to be of acceptable quality and hence included in the systematic review. Assessment was done by two independent reviewers (NRD and DJ).
Results
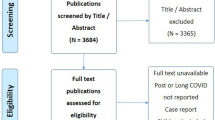
Literature was searched and 939 articles were found. Fourteen articles were screened as per the eligibility criteria, and finally, 8 case reports on neuropathic pain in COVID-19 were selected from the database and manual search and finalised for analysis. The process for search criteria, selection, and exclusion of studies is represented in Fig. 1.
Evaluating Bias Risk
Table 1 displays the bias risk that has been evaluated with the help of the Critical Appraisal Checklist for Case Reports [10]. The first assessment criterion was patient demographics; five of six studies included information on gender, age, and, in some cases, employment (Yes: 5). The second factor was patient histories and timelines; just one study out of six did not include these (Yes: 5, No: 1). The third criteria were an accurate description of the patients’ present clinical status; all six studies met this requirement (Yes: 6). The fourth criterion was a detailed explanation of the clinical state, evaluation, and outcomes; all studies, with the exception of one, addressed these in depth (Yes: 6). The fifth assessment criterion is a clear explanation of intervention and therapy processes; in all six investigations, broad remarks were given about them (Yes: 6). The sixth requirement required a detailed account of post-intervention clinical circumstances, which were not provided. Pain and symptom reduction were clearly documented in most of the six investigations, although not in as much detail as pre-intervention settings (Yes: 6). The seventh criteria were information on unforeseen or unfavourable occurrences; just one research did not explain this (Yes: 5). Finally, the eighth criteria were if the case report gave takeaway lessons; four studies were deemed beneficial since they covered numerous testing and thorough explanations for unusual occurrences.
The majority of case reports adequately recorded the patient’s demographic features, clinical history according to chronology, the patient’s current clinical condition at presentation, diagnostic tests or evaluation procedures, and the outcomes. Following the pre-specified criteria, all 8 case reports were assessed to be of acceptable quality and so included in this systematic review. Four of the eight investigations (Andrew R. Shors [14]; Timo Siepmann [17]; Javier Molina-Gil MD et al. [15]; Mario Cacciavillani et al. [18]) did not describe the important takeaways. Table 1 summarises the findings.
Evidence Synthesis
Cases were from the USA (n = 3), the UK (n = 1), Spain (n = 1), China (n = 1), Germany (n = 1), and Italy (n = 1). Age of patients ranged from 40 to 70 years overall, and there were 5 men and 3 women (Table 2). In all patients, COVID-19 was diagnosed with RT-PCR.
Neuropathic pain and neuropathic symptoms started between 4 to 45 days after the symptoms onset and diagnosis of COVID-19 in 4 patients, but conversely, in four case reports, onset of neuropathic symptoms developed 3 to 15 days before COVID-19 diagnosed (Table 2).
Two case reports had patients with herpes zoster [13, 14], one with intense burning pain [12], one with trigeminal neuralgia [15], one with brachial plexopathy [11], one with small fibre polyneuropathy [16], and two with neuralgic amyotrophy [17, 18]. Three patients, one with burning pain, and two with herpes zoster infection, did not have any investigations done.
Gabapentin, oxycodone, acetaminophen, amitriptyline, nortriptyline, pregabalin, and diazepam were the choice of drugs to control neuropathic symptoms in patients with brachial plexopathy, intense burning pain, neuropathic pain, small fibre polyneuropathy, sensory neuralgic amyotrophy, and trigeminal neuralgia. Tramadol, analgesia, ibuprofen, infrared therapy, and topical lidocaine were given to relieve pain. Acyclovir was given in both patients of herpes zoster and post-herpetic neuralgia.
Patient with brachial plexopathy observes severe weakness in the left upper extremity along with neuropathic pain in the left hand and forearm. One patient had intense burning pain in neck and back along C1 to L5 region, including the paraspinal area and trapezius muscle area. Patients with herpes zoster and post-herpes neuralgia had rash on waist and papules on face with burning sensation and severe pain. Trigeminal neuralgia patient felt sudden severe pain in the right side of face that was triggered by light touch. A severe burning sensation throughout the body followed by tingling, numbness, and burning pain in both feet and hands was observed in patient who had neuropathic pain due to small fibre polyneuropathy. Severe constant pain in the right upper limb followed by paraesthesia and progressive weakness and left upper limb followed by hypoesthesia and dysesthesia along with motor weakness was seen in both cases of neuralgic amyotrophy respectively.
All patients showed gradual improvement in symptoms.
Discussion
Neuropathic pain is the pain observed directly due to peripheral or central nervous system dysfunction without stimulation of nociceptors [19]. The problem in neuropathic pain is due to the abnormal processing of perception and is usually seen in people with neurological diseases. Nociceptive pain is a physiological response as a result of tissue damage that will initiate the pain. Unlike neurogenic pain, neuropathic pain does not involve transient disturbances of the central or peripheral nervous system and therefore produces prolonged pain [20].
Coronaviruses can cause neuropathic pain either directly or indirectly. Recent evidence suggests that angiotensin-converting enzyme 2 (ACE2) serves as a “cytokine storm” doorway for SARS-CoV-2 entrance into cells and the release of tumour necrosis factor alpha (TNF-a) [21]. COVID-19 patients suffer from neurological symptoms, including peripheral neuropathies. Loss of olfactory and gustatory senses, visual damage, and neuropathic pain are the most commonly reported peripheral nerve symptoms. Myalgia or joint pain (arthralgia), sore throat, and headache are all pain-related symptoms. Many individuals with COVID-19 have experienced peripheral nervous system involvement, including painful neuropathies. Mao et al. reported dysgeusia (5.6%), dyssomnia (5.1%), visual disturbances (1.4%), and neuralgia (2.3%) as peripheral nervous system effects. This might be due to invasion of virus in the peripheral nerves, extended immobilisation during a severe illness, or both [22].
During the COVID-19 outbreak, patients exhibiting neurological signs and experiencing various sorts of discomfort must not be neglected. This is the very first systematic review performed to analyse cases/patients (case reports) of neuropathic pain developed due to underlying neurological disorder during or post-COVID-19 infection. Though many case reports are published on neurological involvement post-COVID-19 viral infection, we took only those patients who had pain as the most dominant symptom. Of the 939 studies searched in the database, only eight studies were selected and reviewed. Eight case reports of patients belonged to different regions of the world are reviewed. They all got neuropathic pain, went through the necessary tests and treatments, and eventually recovered.
Reviews on neurological symptoms, manifestations, complications, and comorbidities post-COVID-19 have been conducted in the last 2 years [22,23,24,25,26,27,28,29,30]. One literature commentary described the long-term pain syndromes like testicular pain, headache, chronic pain, and chest pain related to COVID-19 infection [31]. The first study to report neurological involvement in SARS-CoV-2 patients indicated that neurological symptoms were substantially more prevalent in individuals with “severe” illness, as characterised by respiratory symptoms [23]. Headache, insomnia, myalgia, ageusia, and dizziness were common neurological manifestations of COVID; less common were cases of seizure, stroke, Miller fisher syndrome, encephalitis, and GBS [25,26,27,28]. One systematic review has been published in 2020, reviewing the case reports on post−COVID−19 Guillain–Barre Syndrome [32]. We, that is why, did not consider GBS patient’s case reports for this review. We specifically concentrated on patients with neuropathic pain or neurological conditions causing pain as one of the most prominent symptom. Neuropathic pain is very less reported worldwide.
Mechanism for viral invasion in the nervous system in the brachial plexopathy case would be the formation of microthrombi resulted due to COVID−19−induced hypercoagulable state [33]. These microthrombi caused nerves infarction supplying the brachial plexus, thence resulting in brachial plexopathy. The cell−mediated immune response, particularly T cells, is declined, mostly CD4+ and CD8+, caused by immunosuppressive illnesses like COVID−19 [34,35,36]. This decline in immunity and dysregulation of T cells causes the reactivation of latent varicella zoster which lies dormant in the body, resulting in herpes zoster infection.
COVID-19 virus infects epithelial, dendritic, and mononuclear cells early in the infection, generating enhanced production of cytokines and chemokines. The virus transmission is facilitated by the late and inadequate production of interferons, which play a role in building innate immunity against viral infections [37]. Nerve injury due to pro-inflammatory cytokines and other mediators of the systemic immune dysregulation might be the reason for intense burning pain in the patient.
There is a direct mechanism associated with COVID-19 headache and facial pain generation due to the invasion and dissemination of the virus in the CNS which occurs when the virus binds to the superficial receptors of the angiotensin converting enzyme type 2 found in the trigeminal nerve terminals of the nasal cavity [38]. An indirect stimulation of the trigeminal-vascular system in response to a cytokine storm, with a rise in systemic inflammatory indicators such as calcitonin gene-related peptide, is also hypothesised.
Post-infectious small fibre neuropathy due to involvement of small somatic or autonomic fibres and their functions might be the possible mechanism behind neuropathic pain in Small fibre Polyneuropathy patient. Neuralgic amyotrophy in two patients would be caused by a direct viral infection of the brachial plexus or the autoimmune response to preceding SARS-COV infection [39].
Conclusion
Synthesis and analysis case reports on neuropathic pain post-developed post-COVID-19 infection are performed in this review. Although not many cases are reported worldwide, there are many reviews discussing the neurological expression of COVID-19 and other coronavirus infections. Practitioners and investigators should keep neuropathic symptoms in mind as they see COVID-19 patients and conduct the diagnostic as well as treatment procedure accordingly. One limitation of the present review is not recruiting studies having cases of GBS and such neurological disorders that did not have pain as one of the significant symptom.
References
International Association for the Study of Pain. IASP Taxonomy. Pain terms. Neuropathic pain. Updated 2017 Dec 14.
Murnion BP. Neuropathic pain: current definition and review of drug treatment. Aust Prescr 2018;41:60–3.
Attal N, Martinez V, Bouhassira D. Potential for increased prev- alence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021;6(1): e884.
Stainsby B, Howitt S, Porr J. Neuromusculoskeletal disorders following SARS: a case series. J Can Chiropr Assoc. 2011;55(1):32–9.
Magdy R, et al. Characteristics and risk factors of persistent neuropathic pain in recovered COVID-19 patients. Pain Med. 2021;00:1–8.
Brizzi KT, Lyons JL. Peripheral nervous system manifestations of infectious diseases. Neurohospitalist. 2014;4:230–40.
Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–4.
Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–5.
Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–6.
Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, Currie M, Qureshi R, Mattis P, Lisy K, Mu P-F. chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z. Joanna Briggs Institute Reviewer's Manual.The Joanna Briggs Institute, 2017.
Han CY, Tarr AM, et al. Brachial plexopathy as a complication of COVID-19. BMJ Case Rep. 2021;2021(14): e237459.
Aksan F, Nelson EA, Swedish KA. A COVID-19 patient with intense burning pain. J Neurovirol 2020 Oct;26(5):800–801
Cao X, Zhang X, Meng W, Zheng H. (2020) Herpes zoster and post herpetic neuralgia in an elderly patient with critical COVID-19: a case report. J Pain Res. 2020;22(13):2361–5.
Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection JAAD Case Rep. 2020 Jul;6(7):656–657.
Molina‐Gil J, González‐Fernández L, García‐Cabo C. Trigeminal neuralgia as the sole neurological manifestation of COVID-19: a case report Headache. 2021 Mar;61(3):560–562.
McWilliam M, Samuel M, Alkufri FH. Neuropathic pain post-COVID-19: a case report. J Case Rep. 2021 Jul 22;14(7).
Siepmann T, Kitzler HH, Lueck C, Platzek I, Reichmann H, Barlinn K. Neuralgic amyotrophy following infection with SARS-CoV-2. Muscle Nerve. 2020; https://doi.org/10.1002/mus.27035. Online ahead of print.
Cacciavillani M, Salvalaggio A, Briani C. Pure sensory neuralgic amyotrophy in COVID-19 infection. Muscle Nerve. 2021;63(1):E7–8.
Ziegler D. Treatment of neuropathic pain. In: Gries FA, Cameron NE, Low PA, Ziegler D, editors. Textbook of diabetic neuropathy. New York: Georg Thieme Verlag; 2003. p. 21–4.
Hanson P. Neuropathic pain: clinical characteristics and diagnostic work up. Eur J Pain. 2002; 6 (SupplA) :47–50.
Su S, Cui H, Wang T, Shen X, Ma C. Pain: a potential new label of COVID-19. Brain Behav Immun. 2020;87:159–60.
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with corona virus disease 2019 in Wuhan. China JAMA Neurol. 2020;77(6):863–90.
van Montal V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other corona virus infections: a systematic review. Clin Neurol Neurosurg. 2020;194: 105921.
Pleasure SJ, Green AJ, Josephson SA. The spectrum of neurologic disease in the severe acute respiratory syndrome corona virus 2 pandemic infection: neurologists move to the front lines. JAMA Neurol. 2020;77:679–80.
Whittake A, Anson M, Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020;2020(00):1–9.
Correia AO, Feitosa PW, de Sousa Moreira JL, Nogueira SÁ, Fonseca RB, Nobre ME. Neurological manifestations of COVID-19 and other coronaviruses: a systematic review. Neurology, Psychiatry and Brain Research. 2020 Sep 1;37:27-32.
Vitalakumar D et al. Neurological manifestations in COVID-19 patients: a meta-analysis. ACS Chemical Neuroscience 2021 12(15); 2776–2797.
Tsai ST, Lu MK, San S, Tsai CH. The neurologic manifestations of coronavirus disease 2019 pandemic: a systemic review. Frontiers in neurology. 2020 May 19;11:498.
Reza-Zaldívar EE, Hernández-Sapiéns MA, Minjarez B, Gómez-Pinedo U, Márquez-Aguirre AL, Mateos-Díaz JC, Matias-Guiu J, Canales-Aguirre AA. Infection mechanism of SARS-COV-2 and its implication on the nervous system. Frontiers in immunology. 2021:3738.
Mc Farland AJ, Yousuf MS, Shiers S, Price TJ. Neurobiology of SARS-CoV-2 Yousuf, Stephanie Shiers, Theodore J. Neurobiology of SARS-CoV-2 interactions with the peripheral nervous system: implications for COVID-19 and pain. Pain Rep. 2020 Jan 7;6(1):e885.
Fiala K, Martens J, Abd-Elsayed A (2022) Post-COVID pain syndromes. Curr Pain Headache Rep Mar 10;1–5.
Carrillo-Larco RM, et al. (2020) COVID-19 and Guillain-Barre syndrome: a systematic review of case reports. Wellcome Open Res. 2020;21(5):107.
Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13.
Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–8.
Arvin AM. Humoral and cellular immunity to varicella-zoster virus: an overview. J Infect Dis. 2008;197(Suppl 2):S58–60.
Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster N Engl J Med. 2002;347(5):340–6.
Ye Q, et al. The pathogenesis and treatment of the ‘cytokine storm’’ in COVID-19’. J Infect: Published online June; 2020. p. 1.
Bobker SM, Robbins MS. COVID-19 and headache: a primer for trainees. Headache. 2020;60:1806–11.
Van Rosmalen M, Lieba-Samal D, Pillen S, van Alfen N. Ultrasound of peripheral nerves in neuralgic amyotrophy. Muscle Nerve. 2019;59(1):55–9.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: DJ; Investigation: DJ, NRD, VG, AK; Visualising: RNC, AP; Supervision: VNM, VKS; Writing: NRD, VG.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Informed Consent
Not applicable.
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuropathic Pain
Rights and permissions
About this article
Cite this article
Joshi, D., Gyanpuri, V., Pathak, A. et al. Neuropathic Pain Associated with COVID-19: a Systematic Review of Case Reports. Curr Pain Headache Rep 26, 595–603 (2022). https://doi.org/10.1007/s11916-022-01065-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11916-022-01065-3