Abstract
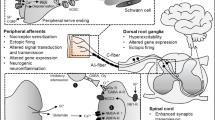
Numerous mechanisms are implicated in the perception of pain. Although many anatomical, molecular, and functional components have been identified, a comprehensive and integrated theory of pain perception has yet to be firmly established that fits the diverse clinical experience. Acute pain involves the activation of several varieties of small primary sensory neurons, collectively termed nociceptors, which have small-caliber unmyelinated or myelinated axons (C and Aδ fibers, respectively) that innervate all body tissues. They are stimulated by noxious stimuli that activate ion channels on the endings either directly or through the release of cytokines from damaged or stressed tissues. A variety of drugs successfully treats acute pain by targeting these ion channels or cytokine interactions. Paradoxically, several chronic neuropathic pain conditions are associated with a loss of small-caliber axons and have an unpredictable and poor response to current drugs, especially at doses that do not cause severe side effects. In an attempt to further an integrated theory of pain perception, this review focuses upon the presumed role of small-caliber innervation, particularly to the epidermis and cutaneous vasculature, and the clinical manifestations, diagnosis, and treatment of pathologies of this innervation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Rice F, Albrecht P: Cutaneous mechanisms of tactile perception: morphological and chemical organization of the innervation to the skin. In The Senses: A Comprehensive Reference. Edited by Kaas J, Gardner E. New York: Elsevier; 2008:1–32. This comprehensive review offers a solid basis for interpreting the roles of cutaneous innervation under normal and pathologic conditions, particularly highlighting the array of targets innervated by small-caliber afferents.
Devor M: Neurobiology of normal and pathophysiological pain. In Evaluation and Treatment of Chronic Pain, edn 3. Edited by Aronoff G. Baltimore: Williams & Wilkins; 1999:11–26.
Smith ES, Lewin GR: Nociceptors: a phylogenetic view. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 2009, 195:1089–1106.
Andratsch M, Mair N, Constantin CE, et al.: A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci 2009, 29:13473–13483.
Jankowski MP, Lawson JJ, McIlwrath SL, et al.: Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci 2009, 29:1636–1647.
Reichling DB, Levine JD: Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 2009, 32:611–618.
•• Dray A: Neuropathic pain: emerging treatments. Br J Anaesth 2008,101:48–58. This review nicely covers all the current and novel ideas of pain and treatment strategies. Importantly, this article points to the dramatic number of chronic pain conditions with peripheral origins.
• O’Connor AB, Dworkin RH: Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 2009, 122:S22–S32. This paper reviews current methodologic parameters and treatment guidelines for chronic pain patients.
• Argoff CE, Albrecht P, Irving G, Rice F: Multimodal analgesia for chronic pain: rationale and future directions. Pain Med 2009, 10(Suppl 2):S53–S66. This review focuses on the potential use of multiple pharmacologic agents for additive or synergistic analgesic efficacy, as well as the use of nontraditional therapies to promote a comprehensive management of chronic pain patients.
Dworkin RH, O’Connor AB, Backonja M, et al.: Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain 2007, 132:237–251.
Wallace JM: Update on pharmacotherapy guidelines for treatment of neuropathic pain. Curr Pain Headache Rep 2007, 11:208–214.
Lee MC, Mouraux A, Iannetti GD: Characterizing the cortical activity through which pain emerges from nociception. J Neurosci 2009, 29:7909–7916.
Loeser JD, Treede RD: The Kyoto protocol of IASP Basic Pain Terminology. Pain 2008, 137:473–477.
Price DD: Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv 2002, 2:392–403, 339.
Campero M, Baumann TK, Bostock H, Ochoa JL: Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol 2009, 587:5633–5652.
• Roosterman D, Goerge T, Schneider SW, et al.: Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev 2006, 86:1309–1379. This extensive review discusses the numerous aspects and signaling systems involved in skin/neural interactions.
Devor M: Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009, 196:115–128.
•• Dussor G, Koerber HR, Oaklander AL, et al.: Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain Res Rev 2009, 60:24–35. This paper offers a succinct and thorough review of cutaneous mechanisms involved in pain transmission.
Green BG: Temperature perception on the hand during static versus dynamic contact with a surface. Atten Percept Psychophys 2009, 71:1185–1196.
Jimenez-Andrade JM, Mantyh WG, Bloom AP, et al.: A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone 2010, 46:306–313.
•• Bowsher D, Geoffrey Woods C, Nicholas AK, et al.: Absence of pain with hyperhidrosis: a new syndrome where vascular afferents may mediate cutaneous sensation. Pain 2009, 147:287–298. This paper describes vascular innervation alterations in rare individuals that revealed a previously unappreciated function of vascular afferents, which may underlie several forms of chronic pain and/or disease states.
Nolano M, Crisci C, Santoro L, et al.: Absent innervation of skin and sweat glands in congenital insensitivity to pain with anhidrosis. Clin Neurophysiol 2000, 111:1596–1601.
• Craig AD: A new view of pain as a homeostatic emotion. Trends Neurosci 2003, 26:303–307. This seminal paper outlines the basic premise that pain is generated as a result of homeostatic functioning of small-fiber afferents.
Steinhoff M, Ständer S, Seeliger S, et al.: Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol 2003, 139:1479–1488.
Zylka MJ, Rice FL, Anderson DJ: Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 2005, 45:17–25.
Binshtok AM, Wang H, Zimmermann K, et al.: Nociceptors are interleukin-1beta sensors. J Neurosci 2008, 28:14062–14073.
Kwan KY, Glazer JM, Corey DP, et al.: TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 2009, 29:4808–4819.
Namer B, Hilliges M, Orstavik K, et al.: Endothelin 1 activates and sensitizes human C-nociceptors. Pain 2008, 137:41–49.
•• Zylka MJ, Sowa NA, Taylor-Blake B, et al.: Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron 2008, 60:111–122. This eloquent work describes a potential analgesic functioning of nonpeptidergic axons, thereby offering a counter to the algesic function proposed for peptidergic afferents.
Rau KK, McIlwrath SL, Wang H, et al.: Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci 2009, 29:8612–8619.
Hardie RC: TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol 2007, 578:9–24.
Wong GY, Gavva NR: Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: recent advances and setbacks. Brain Res Rev 2009, 60:267–277.
Ro JY, Lee JS, Zhang Y: Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain 2009, 144:270–277.
Lingueglia E: Acid-sensing ion channels in sensory perception. J Biol Chem 2007, 282:17325–17329.
Cannon KE, Chazot PL, Hann V, et al.: Immunohistochemical localization of histamine H3 receptors in rodent skin, dorsal root ganglia, superior cervical ganglia, and spinal cord: potential antinociceptive targets. Pain 2007, 129:76–92.
Anand U, Otto WR, Sanchez-Herrera D, et al.: Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain 2008, 138:667–680.
Tracey I, Johns E: The pain matrix: reloaded or reborn as we image tonic pain using arterial spin labelling. Pain 2010, 148:359–360.
Lawson SN: Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp Physiol 2002, 87:239–244.
Denda M, Nakatani M, Ikeyama K, et al.: Epidermal keratinocytes as the forefront of the sensory system. Exp Dermatol 2007, 16:157–161.
Koizumi S, Fujishita K, Inoue K, et al.: Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J 2004, 380:329–338.
•• Mandadi S, Sokabe T, Shibasaki K, et al.: TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch 2009, 458:1093–1102. This paper offers great evidence for the involvement of terminal tissue cells in the transduction and integration of sensory stimuli and the generation of normal perceptions.
Ulmann L, Rodeau JL, Danoux L, et al.: Trophic effects of keratinocytes on the axonal development of sensory neurons in a coculture model. Eur J Neurosci 2007, 26:113–125.
Zhao P, Barr TP, Hou Q, et al.: Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: evidence for a role in pain. Pain 2008, 139:90–105.
Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, Bigliardi-Qi M: Opioids and the skin–where do we stand? Exp Dermatol 2009, 18:424–430.
Fink E, Oaklander AL: Small-fiber neuropathy: answering the burning questions. Sci Aging Knowledge Environ 2006, 2006:pe7.
Tavee J, Zhou L: Small fiber neuropathy: a burning problem. Cleve Clin J Med 2009, 76:297–305.
Backonja MM, Walk D, Edwards RR, et al.: Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain 2009, 25:641–647.
Walk D: Role of skin biopsy in the diagnosis of peripheral neuropathic pain. Curr Pain Headache Rep 2009, 13:191–196.
Oaklander AL, Rissmiller JG, Gelman LB, et al.: Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy). Pain 2006, 120:235–243.
Orstavik K, Jorum E: Microneurographic findings of relevance to pain in patients with erythromelalgia and patients with diabetic neuropathy. Neurosci Lett 2010, 470:180–184.
Bednarik J, Vlckova-Moravcova E, Bursova S, et al.: Etiology of small-fiber neuropathy. J Peripher Nerv Syst 2009, 14:177–183.
Krishnan ST, Quattrini C, Jeziorska M, et al.: Abnormal LDIflare but normal quantitative sensory testing and dermal nerve fiber density in patients with painful diabetic neuropathy. Diabetes Care 2009, 32:451–455.
Souayah N, Ajroud-Driss S, Sander HW, et al.: Small fiber neuropathy following vaccination for rabies, varicella or Lyme disease. Vaccine 2009, 27:7322–7325.
Gemignani F, Brindani F, Vitetta F, et al.: Restless legs syndrome in diabetic neuropathy: a frequent manifestation of small fiber neuropathy. J Peripher Nerv Syst 2007, 12:50–53.
Yilmaz Z, Renton T, Yiangou Y, et al.: Burning mouth syndrome as a trigeminal small fibre neuropathy: increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci 2007, 14:864–871.
Treede RD, Jensen TS, Campbell JN, et al.: Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008, 70:1630–1635.
Visser N, McGhee CN, Patel DV: Laser-scanning in vivo confocal microscopy reveals two morphologically distinct populations of stromal nerves in normal human corneas. Br J Ophthalmol 2009, 93:506–509.
• Nebuchennykh M, Loseth S, Lindal S, Mellgren SI: The value of skin biopsy with recording of intraepidermal nerve fiber density and quantitative sensory testing in the assessment of small fiber involvement in patients with different causes of polyneuropathy. J Neurol 2009, 256:1067–1075. (Erratum in J Neurol 2009, 256:1034.) This paper explores the utility of combining tools available for the assessment of small-fiber neuropathy to advance diagnosis and improve treatments.
Devigili G, Tugnoli V, Penza P, et al.: The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008,131:1912–1925.
Hlubocky A, Wellik K, Ross MA, et al.: Skin biopsy for diagnosis of small fiber neuropathy: a critically appraised topic. Neurologist,16:61–63.
Albrecht PJ, Hines S, Eisenberg E, et al.: Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain 2006,120:244–266.
Oaklander AL, Fields HL: Is reflex sympathetic dystrophy/complex regional pain syndrome type I a small-fiber neuropathy? Ann Neurol 2009,65:629–638.
Paré M, Albrecht PJ, Noto CJ, et al.: Differential hypertrophy and atrophy among all types of cutaneous innervation in the glabrous skin of the monkey hand during aging and naturally occurring type 2 diabetes. J Comp Neurol 2007,501:543–567.
Smith AG, Howard JR, Kroll R, et al.: The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci 2005,228:65–69.
Sorensen L, Molyneaux L, Yue DK: The level of small nerve fiber dysfunction does not predict pain in diabetic Neuropathy: a study using quantitative sensory testing. Clin J Pain 2006,22:261–265.
Petersen KL, Rice FL, Suess F, et al.: Relief of post-herpetic neuralgia by surgical removal of painful skin. Pain 2002,98:119–126.
• Ho TW, Backonja M, Ma J, et al.: Efficient assessment of neuropathic pain drugs in patients with small fiber sensory neuropathies. Pain 2009,141:19–24. This paper describes the use of skin biopsy to focus patient inclusion for clinical drug trials and demonstrates improved endpoint significance due to decreased rates of placebo response.
Disclosure
Dr. Phillip J. Albrecht and Dr. Frank L. Rice have received fair market compensation for performing basic research contracts and/or consultation on behalf of Johnson & Johnson, AstraZeneca, Endo Pharmaceuticals, Vertex Pharmaceuticals, Novartis, Forest Laboratories, and Eli Lilly. Neither author has any conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albrecht, P.J., Rice, F.L. Role of Small-Fiber Afferents in Pain Mechanisms With Implications on Diagnosis and Treatment. Curr Pain Headache Rep 14, 179–188 (2010). https://doi.org/10.1007/s11916-010-0105-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11916-010-0105-y