Abstract
Purpose
To present a review of insights gained from investigating the question as to whether red haired individuals have altered sensitivity to pain.
Methods
A narrative review of the literature.
Results
Anecdotal observations from anaesthesiologists have suggested that individuals with red hair require more analgesia on average than members of the general population. This observation has been confirmed and the redheaded phenotype is associated with an altered sensitivity to pain across a wide range of different pain types. Through the use of mouse models, a central mechanism for this altered pain sensitivity has been proposed involving both the melanocortin and opioid receptor systems, despite the causative mutation for this phenotype occurring in melanocortin 1 receptors (MC1Rs) on peripheral melanocytes.
Conclusions
Understanding the endocrine imbalance caused by this loss of function mutation helps us to further explore the mechanisms behind pain sensitivity. It also facilitates a discussion about how pharmacogenomics can be exploited to personalise and subsequently optimise treatment.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anecdotally it has long been noted that individuals with natural red hair often required more anaesthesia than other patients, and this has subsequently been confirmed for inhalational anaesthesia [1]. Since the determination of minimum alveolar concentration (MAC) involves the response to a noxious stimulus [2] this also implies that redheads are more sensitive to pain. This has now been confirmed in several clinical studies, with the proviso that the type of pain stimulus may matter [3,4,5,6]. Redheads may be more sensitive to thermal induced pain [3] but have equal sensitivity to ischaemic pain [4]. It is important to note that not all studies agree: Gradwohl et al. were unable to find any differences between anaesthetic or pain sensitivity in a study of patients undergoing surgery [5]. While an advantage of their study was that it was in a clinical rather than experimental setting, one disadvantage was that they used only phenotype and not genotype to identify red-haired patients. Other studies have in fact reported reduced sensitivity to (capsaicin-induced) pain, and the interpretation of these contradictory results will be discussed later [6]. In dental surgery, no difference in response to local anaesthetic has been found [7], but intriguingly the rate of anxiety in the red-haired is higher than others [7, 8]. Thus, clinical studies offer the stimulus to investigate this hypothesis in more detail, but do not offer sufficiently clear information from which to draw robust conclusions. Not including studies that assess potential general anaesthetic sensitivities to red hair (something quite different from, and not necessarily related to, pain sensitivity [9]), there are relatively few human trials examining this specific question. The literature in the animal, genomic and molecular science of red hair and pain appears wider and is the predominant focus of this review. We seek though to link results back to the human condition.
2 Genomics of red hair
The redheaded phenotype arises through a mutation in the melanocortin 1 receptor (MC1R) [10,11,12] found on epidermal melanocytes, with loss of receptor function causing disrupted pigment production. Instead of the darker eumelanin, a stable molecule that resists photodamage by ultra-violet (UV) radiation [13, 14], sulphated pheomelanin, a red/yellow pigment, is instead produced. The phenotype is a combination of red hair and fair skin. The red-haired phenotype has long been associated with Celtic populations, and is mostly concentrated around North and Northwest Europe, particularly in Iceland, Ireland and Great Britain [15]. The exact number of redheads worldwide is debated, with inconsistent data, but is frequently quoted to be around 2% of the global population.
There are several alleles coding for MC1R, and it is debated whether the existence of these confers any survival advantage. One theory is that these alleles are present in European populations to maximise production of vitamin D from UV light; the disadvantage being an increased risk for developing UV damage-associated skin cancers such as melanoma [16, 17]. However, distribution of these alleles do not conform to the Hardy‐Weinberg equilibrium suggesting that there is a neutral survival advantage [18, 19]. The alleles are virtually absent in African populations, indicating large survival disadvantage to red hair in that environment.
The natural, main ligand of MC1R, α-melanocyte stimulating hormone (α-MSH), is a cleavage product of adrenocorticotrophin hormone (ACTH), which is in turn a product of the prohormone proopiomelanocortin (POMC) within the hypothalamic-pituitary system (Fig. 1) [20]. Additionally, α-MSH is produced in keratinocytes and melanocytes following UV exposure [21], which is clearly an advantage in terms of UV protection. α-MSH therefore can arise from melanotrophs within the pituitary gland or POMC cleavage in the periphery, and has been shown to have a high affinity with the MC1R receptor in human melanocytes [22, 23].
Metabolic pathways involving ɑ-MSH (alpha-melanocyte stimulating hormone). ɑ-MSH is a major agonist of the melanocortin system including MC1R and MC4R (melanocortin receptors 1 and 4), is a cleavage product of the prohormone POMC (proopiomelanocortin) via another product, ACTH (adrenocorticotrophin hormone). PC (proconvertase) 1 and 2 are the enzymes responsible for peptide cleavage to produce the active products. POMC is the precursor for a range of different products, with a large range of different effects extending from metabolic to pigmentation—some products other than ɑ-MSH have been shown on the diagram below, including N-pro-opiomelanocortin (N-POC (pro- γ-MSH)), γ-MSH (gamma-melanocyte stimulating hormone), CLIP (corticotrophin-like intermediate lobe peptide), β-LPH (beta-lipotropin), β-END (beta-endorphin), γ-LPH (gamma-lipotropin) and β-MSH (beta-melanocyte stimulating hormone)
3 Lessons from animal models
One of the main issues within the literature surrounding the study of pain is the significant discrepancy in tests used between human and animal models. Often, little regard is put into the type of pain that is being elicited, and the differences in signalling that will occur as a result — this is a likely cause of the contradictory responses that exist within the literature [3]. It is important to note the differences in assays available between human and animal studies, as the tests available for the latter have more definitive end-points at which results are collated, and are also available to investigate a wider range of different pain types.
Clear types of pain were demonstrated by Mogil et al., who used 11 random mouse strains to investigate differences in response across 12 standardised pain tests [24, 25]. Through genetic analysis, this group was able to organise different pain types into clusters which shared similar modalities [25]. These concepts were built upon to characterise five fundamental types of nociception and hypersensitivity; baseline thermal nociception, spontaneous responses to noxious chemical stimuli, thermal hypersensitivity, mechanical hypersensitivity and afferent input-independent hypersensitivity [26], which can all be investigated using different pain assays. For example, baseline thermal nociception in rodents can be studied using tail withdrawal tests [4, 27], where the latency to remove the tail from a water bath or hot plate at a set ‘hot’ temperature is measured. For chemical types of pain, 0.9% acetic acid can be injected into the abdomen of mice, and the subsequent number of reflexive abdominal constrictions within a certain timeframe is measured to estimate the spontaneous response to pain [27].
In humans, however, endpoints are more subjective: thermal nociception can be determined by finding a ‘nociceptive threshold’ using temperature probes in a specific sequence to prevent sensitisation or habituation of cutaneous receptors [3]. This reinforces a key difference in how pain is measured between human and animal models, as the conscious determination of a nociceptive threshold can be influenced by a large number of different factors, including psychological [28] and even placebo [29]. This makes it difficult to compare between individuals or draw objective conclusions from human data.
Nevertheless, animal models have been largely consistent with many human studies, including the variability of response with type of pain stimulus and some inconsistencies in results. Thus, MC1R variants in labrador dogs result in increased sensitivity to thermal (but not mechanical) pain [30]. In both animal and human studies, two findings have emerged that require further exploration. One is that the potential influence of red hair is further modulated by sex; a second is the differential influence of red hair on response to opioids.
Delaney et al. studied mutant mice lacking MC1R (termed Mc1re/e) and found an influence as expected, but surprisingly this was an increased tolerance, not sensitivity, to thermal and inflammatory (formalin-induced) pain (but not neuropathic pain following peripheral nerve injury) which was confined to females. Male mutants showed similar responses in all conditions [31]. Note that sex differences in pain sensitivity are well established [32] with females generally displaying greater sensitivity [33, 34], and this is in turn influenced by species strains. Thus, male Sprague Dawley rats are more sensitive than females to thermal pain, while it is the reverse in Long Evans rats [35].
Mogil et al. reported an increased sensitivity to opioid analgesia in red hair in a mouse model. Thus even if pain sensitivity is increased as many studies report, this seems advantageously balanced in therapeutic terms by the increased sensitivity to analgesia [4]. They used quantitative locus mapping to indicate that Mc1r allele was associated with the site of action of the broad κ-opioid agonist, U50,488. This association was identified in female mice only. They then elegantly translated this finding to study women with two variant Mc1r alleles and confirmed a greater response to κ-opioids, such as pentazocine, than dark-haired controls. This study weaves in the MC1R pathway with opioid response and sex [36, 37]. One limitation of their study is that they did not employ positional cloning to reduce the confidence interval surrounding the quantitative trait locus, eliminating all other genes on distal chromosome 8. Quantitative trait locus (QTL) analysis is a statistical method that links phenotypic (trait measurement) and genotypic data (molecular markers) to explain the genetic basis of variation in complex traits, and Mogil et al. argued that their success in linking their mouse study to human responses superceded the need to undertake more exact positional cloning. A second, perhaps more practical limitation is the κ-opioid system has currently limited therapeutic potential in terms of treating pain, in contrast to the μ-opioid system. Drugs targeting these receptors tend to cause significant dysphoric, rather than euphoric, psychotomimetic side effects, indicating that another major function of this system in mood regulation [38]. There are few specific indications for pentazocine, a κ agonist, and buprenorphine, another commonly used opioid, is in fact a κ antagonist.
However, focus on the endogenous ligand for the κ-opioid receptor, dynorphin, may offer more scientific insight as it also binds with high specificity and antagonises MC1R [39]. Moreover, in addition to their peripheral localization, MC1Rs are expressed in brain glial cells [40] and the ventral periaqueductal gray [41], which are regions putatively relevant to nociception [42].
Mogil et al. later turned attention to the μ-opioid system. They studied spontaneous Mc1re/e mice versus wild-type controls, and in parallel humans with red and dark hair, using a variety of both acute and tonic pain assays to confirm that mice mutants mimicking redhaired humans were less sensitive to pain [27]. They also investigated the response to the morphine metabolite morphine-8-glucuronide, M6G, as the μ-opioid receptor agonist. Mutants and redheads showed an increased analgesic response, which the authors confirmed was likely a true pharmacodynamic, rather than metabolic/pharmacokinetic effect, as circulating levels of the drug were similar in both groups [27]. Interestingly, Mogil et al. did not find any sex-dependent effects in mice or humans, in contrast to their previous report for the κ-opioid system. However, extending the results of MC1R interaction with both κ- and μ-opioid systems raises the possibility that in dark-haired humans there is an endogenous pain inhibition system resulting from MC1R activation. Reduced or absent in redheads, this results in anti-analgesia.
Mc1r is not the only member of the melanocortin receptor family to be investigated in the context of pain, as there have been explorations into rat models and the involvement of allele for melanocortin 4 receptor (Mc4r) in centralised pain phenomena, such as allodynia [43, 44]. Allodynia is the experience of a painful response to a non-nociceptive stimulus and can involve both peripheral sensitisation and central maladaptation, depending on the mode [45]. The co-localisation of μ-opioid and melanocortin receptors in the spinal cord, and their supposed antagonistic relationship, has led to the consideration of melanocortin 4 receptor (MC4R) action in this central sensitisation response. Starowicz et al. compared the anti-allodynic effects of melanocortin receptor antagonist SHU-9119 and μ-opioid receptor agonist morphine, finding that targeting the melanocortin system had a more significant response [43]. This further implicates other members of the melanocortin in the control of pain responses and suggests an important interplay with our endogenous opioid system.
4 Confounders and more complex genomics
When reporting that red-haired women were significantly more sensitive to thermal nociception, Liem et al. also found an increased resistance to subcutaneous lidocaine [3]. This is unexpected as this drug should act primarily on the voltage gated sodium channels of peripheral C fibres. One possibility is that anti-nociceptive actions of lidocaine are recognised via other putative systems [46], although this is at different concentrations from those achieved by subcutaneous injection [46, 47]. It is conceivable that the MC1R system acts in a more complex way in respect of the analgesia conferred by lidocaine block of peripheral nerves (e.g., a modulation of sodium channel sensitivity).
The study of pain has been recognised to be complex [48]. Pain being a subjective sensation is difficult to measure, and as studies cited above show, responses can depend on the stimulus type being used. The stimulus intensity that can be administered in humans is understandably limited, as also it is in animal studies. Pain is ultimately a bio-psycho-social condition where immeasurable factors ideally need to be considered. Acute pain can evolve into chronic pain, which underlines these issues and therefore, pain can exist or continue in the absence of any apparent noxious stimulus. Moreover, the necessary treatment of pain can lead to complexities created by dependence or hyperalgesia [49]. Research has indicated that genotype can at least somewhat determine an individual’s response to different types of pain assay [24,25,26]. Animal studies offer the advantage of creating genomic variants and more invasive techniques, but animals cannot report pain, so all measures are surrogates [50].
Another confounding factor within less genotypically controlled human groups could be the exact mutation in the MC1R gene: within the ‘red-haired’ human phenotype, there is a wide degree of variation which is understandably reflected by a high degree of polymorphism in this gene [51, 52] and variable expression. This concept was further explored through a retrospective study by Zorina-Lichtenwalter et al. who used the data from an orofacial pain study with full genotyping to investigate the correlation between 17 specific polymorphisms in MC1R and the prevalence of chronic pain conditions. While red hair per se was associated with a greater sensitivity to pain, the association between the polymorphisms and experience of pain was more complex. Individuals with the most common missense variants in alleles for MC1R statistically had fewer chronic pain conditions than those with no such mutation [53]. Thus, mutation in the MC1R allele is associated with an alteration in pain sensitivity, irrespective of hair colour.
5 Redheads and pain: an imbalance in opioid signalling?
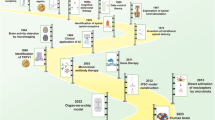
Robinson et al. [54] recently conducted an extensive series of experiments presented within a single paper, and it is worth presenting their results in the logical sequential order they undertook them to unravel the relationships between red hair, MC1R and opioid signalling. For a summary of the different mouse models used by Robinson et al., see Table 1.
They first excluded skin pigment per se as an influence on pain sensitivity in normal and Mc1re/e mice, by crossing these with albino mice (Tyrc/c), in whom melanocyte numbers were unaltered but lacked pigment. Mutant Mc1re/e always exhibited higher pain tolerance than other types, regardless of the actual skin and coat colour, indicating that melanocytes were important rather than the eumelanin pigment. Next, they assessed whether melanocyte numbers were influential by studying genetically matched (C57BL/6J) mouse models that differed in melanocyte numbers. Mice with increased epidermal melanocytes (K14-SCF) were more sensitive to pain than mice lacking melanocytes (Mitf mi−wh/mi−wh), who in turn displayed higher pain tolerance than wild type (WT), WT mice (i.e., the pain sensitivity was K14-SCF (most sensitive) > WT > Mitf mi−wh/mi−wh). This indicates that the number of epidermal melanocytes—independent of MC1R function—modulates pain sensitivity.
Then, they crossed red-haired (Mc1re/e) mice with the Mitf mi−wh/mi−wh mice lacking melanocytes, to test the potential role of MC1R function in nonmelanocytic cells. Genetic absence of melanocytes now inhibited the ability of MC1R to influence pain sensitivity, suggesting that this is dependent on MC1R function in melanocytes rather than in other cell types.
Robinson et al. then considered β-endorphin as a potential modulator of pain sensitivity. β-endorphin is a posttranslational cleavage product of proopiomelanocortin (POMC), expressed in melanocytes; POMC is induced by adenosine 3′,5′-monophosphate (cAMP) in other cell types and low cAMP levels in red-haired Mc1r mutant melanocytes may affect POMC expression and in turn, β-endorphin levels. They confirmed that red-haired mice did indeed exhibit lower plasma levels of β-endorphin. However, this appeared contrary to the higher pain tolerance found in these mice as β-endorphin promotes rather than diminishes analgesia. Moreover, they discovered that β-endorphin levels were also inversely associated with pain thresholds in the other mouse genotypes: K14-SCF mice (more melanocytes; more sensitive to pain) and Mitfmi−wh/mi−wh mice (absent melanocytes; more tolerant to pain). Thus, overall, melanocyte numbers and MC1R function were inversely correlated with pain sensitivity, despite themselves being directly related to plasma β-endorphin levels.
Next, to determine whether the reduced pain sensitivity to pain was a result of compensation for—or adaptation to—the lower β-endorphin levels, Robinson et al. employed mice carrying homozygous POMC mutation that expresses all melanocortin peptides except β-endorphin (β-end−/−), and a strain lacking μ-opioid receptor for β-endorphin (Oprm1−/−). There was no effect of the knockout on the pain threshold differences in black versus red-haired mice. Lack of μ-receptor had no effect on the pain thresholds in black mice, consistent with previous studies [55]. However, the absence of μ-receptor abolished the elevated nociceptive thresholds in red-haired mice. Separately, opioid receptor antagonists (naloxone and cyprodime) both altered pain sensitivity of red-haired mice to those of black mice.
All these results suggested that pain thresholds in red-haired mice are not dependent on a β-endorphin-opioid receptor signalling pathway. The increased μ-opioid receptor signalling in the context of low plasma β-endorphin levels could be due to (a) increased expression of some other endogenous opioid, (b) μ-receptor adaptation, or (c) reduction of a pathway that is antagonistic to opioid signalling. The first (a) was excluded by a finding of similar dynorphin, enkephalin, and endomorphin levels between red-haired and black mice.
Robinson et al. noted that plasma levels of α-MSH (a melanocortin agonist, like β-endorphin, also encoded within POMC) varied across different-pigmented mice, paralleling β-endorphin levels (i.e., higher α-MSH levels in mice with more melanocytes; lower in red-haired mice). This variation is therefore consistent with the respective changes in pain thresholds, unlike β-endorphin which is inversely related. To test whether the relationship could be causative, Robinson et al. ‘rescued’ low endogenous α-MSH levels in red-haired mice pharmacologically: melanotan II, (that mimics α-MSH action) increased (i.e., reversed) pain sensitivity in red-haired but not black mice, in a sex-independent manner. Thus, loss of function in MC1R signalling results in increased tolerance to pain, but an MSH receptor other than MC1R is likely involved.
Robinson et al. noted that the different melanocortin receptor, MC4R has been implicated in pain control [56]. The peptide SHU‐9119 antagonizes MC4R, despite being an MC1R agonist, and was found to be analgesic in black mice, mimicking the red-haired (Mc1re/e) genotype. This was also the case in the cross of K14-SCF with Mc1re/e mice. Since these mice have wild-type pain thresholds, and levels of all POMC products, but lack MC1R function, these results strongly suggest that the analgesic effects of SHU‐9119 are MC1R independent and likely due to ligand effects on MC4R (or MC3R, another related receptor). Thus, MC4R signalling may balance opioid signalling in modulating responses to pain.
Robinson et al. undertook a series of experiments to explore the putative MC4R system: (i) Mc4r null mice exhibited reduced pain sensitivity; (ii) the absence of MC4R on a genetically black background resulted response to opioid antagonists similar to red-haired mice, suggesting that pain threshold may be a balance between Mu opioid receptor 1 (OPRM1) and MC4R signalling; (iii) naloxonazine, a specific μ-receptor antagonist restores nociceptive thresholds in both Mc4r null and red-haired mice; (iv) melanocortin agonist treatment reduced the elevated pain thresholds of red-haired, but not Mc4r null mice, suggesting that MC4R is the key melanocortin target for MSH; (v) intraperitoneal administration of naltrexone, an opioid receptor antagonist that crosses the blood–brain barrier (BBB) reduces differences between black and red-haired mice, but administration of methylated naltrexone that does not cross the BBB does not, suggesting the influence on opioid signalling in red-haired mice is a central, not peripheral phenomenon – this implicates α-MSH balanced opioid receptor–mediated regulation of central nociception; (vi) in rat primary hypothalamic neurons the melanocortin agonist [Nle4,D-Phe7]-α-MSH increased cAMP content, whereas morphine significantly inhibited this melanocortin-induced cAMP elevation suggesting a mutual antagonistic interaction in these cells.
The detailed work of Robinson et al. suggests that the reduced pain sensitivity in the red-haired arises from reduced α-MSH levels caused by a reduced POMC production in melanocytes, in turn leading to diminished MC4R signalling. The antagonism of opioid signalling within the central nervous system (CNS) is thereby reduced which occurs despite a reduced β-endorphin production consequent on the reduced POMC production. The theory is a ‘net melanocortin deficiency’, relative to opioid signalling, altering the balance to favour μ-opioid receptor–induced analgesia in the red-haired (Fig. 2). Note that these conclusions are entirely compatible with the suggestions of Zorina-Lichtenwalter et al. that it is the underlying genotype, not the phenotype of red hair, that is the relevant influence and therefore, this work may be revealing of a more generalised mechanism in responses to pain.
Diagrammatic summary of the proposed mechanism for altered pain sensitivity in redheads. The details and adaptation is from Robinson et al.’s paper [54], with proposed conclusions considered in the above discussion
6 So, what can we learn from the study of redheads in context of pain?
Understanding how polymorphisms interact with various pharmacological agents is the core concept of pharmacogenomics, with the aim of personalising therapy as mainstream practice. This will make therapy more effective and reduce side-effects. That said, Mogil (whose work has been cited above), writing more than 10 years before the Robinson et al. study, bemoaned the state of genomics in pain [57]. He cited Max and Stewart as writing that ‘pain has come late to the genetics party’ [58] and observed that transgenic, linkage mapping and microarray studies in rodents might predict the existence of many hundreds of pain-relevant genes, yet focus has been on just a handful: COMT (encoding catechol-O-methyltransferase), GCH1 (encoding guanosine triphosphate (GTP) cyclohydrolase 1), and OPRM1 (discussed above). His summary of the general results applies to the discussion above: poor replicability of genetic association studies, conclusions have become less straightforward than initially imagined—there is no binary yes/no association or influence on pain—and subsequent studies after the initial have reported no association, or sex-dependent association, or significant associations but of different single nucleotide polymorphisms or haplotypes, and significant associations, but of different phenotypes than those initially suspected. All this is attributed variously to data set heterogeneity, stratification of populations, and genomic statistical underpowering. Both selective publication and at times scientific fraud have had their part to play in pain therapy research [59, 60]. If pointing to one gene as ‘responsible for pain’ is oversimplistic, then widening to net to include many more—whilst acknowledging the reality that pain is indeed a complex bio-psycho-social phenomenon—then runs the risk that all genes are ‘pain genes’. Mogil cites as Goldstein: ‘‘in pointing at everything, genetics would point at nothing” [61].
That said, the elegant work of Robinson et al., and others cited, represents an incremental advance and does indeed push forward the genomic understanding of pain, albeit from this limited perspective of the influence of red hair. Examples of the success of genomics include the use of abacavir in HIV treatment, where the presence of a specific human leukocyte antigen (HLA) allele can cause severe side effects [62], preventable by prior genotyping [63, 64]. Particular low-function mutations in the cytochrome P450 2D6 metabolic enzyme (CYP2D6) are thought to limit the metabolic pathway that forms codeine’s physiologically active metabolite, morphine [65], resulting in lack of analgesic effect. Because the phenotype associated with MC1R mutation is self-evident, identification of these individuals with potentially altered nociceptive sensitivity, and perhaps anaesthetic efficacy, is easy (at the expense of making blinded trials difficult).
7 Conclusions
Although the model proposed in Fig. 2 represents progress, there remain fundamental questions that require resolution before results can be translated to clinical practice. One is that the direction of influence of red hair/MC1R remains unclear, with some studies reporting increased pain sensitivity and others the reverse (or none). The influence of sex remains unresolved. And of the opioid receptors the δ- or ζ-receptors, or their specific subtypes have not been investigated. Whilst the model presented by Robinson et al. nicely demonstrates the influence of red hair/MC1R on the balance between opioid tone and melatonin signalling, it is possible that other unknown factors may affect input from the opioid system. These other factors may have nothing to do with red hair or the melanocortin system but may lead to confounding results in redhaired individuals. We are a long way off from specific therapies, but the work shows that a simple question about redheads can lead to unravelling of complex pain-relevant pathways within the body.
Availability of data and materials
Not applicable.
Abbreviations
- ACTH:
-
Adrenocorticotrophin hormone
- α-MSH:
-
α-Melanocyte stimulating hormone
- BBB:
-
Blood-brain barrier
- β-END:
-
Beta-endorphin
- β-end − / − :
-
Genetic notation for mutation that expresses all melanocortin peptides except β-endorphin
- β-LPH:
-
Beta-lipotropin
- β-MSH:
-
Beta-melanocyte stimulating hormone
- cAMP:
-
Adenosine 3′,5′-monophosphate
- C57BL/6J:
-
Species of mouse (wild type)
- CLIP:
-
Corticotrophin-like intermediate lobe peptide
- CNS:
-
Central nervous system
- COMT :
-
Gene encoding catechol-O-methyltransferase)
- CYP2D6:
-
Cytochrome P450 2D6 metabolic enzyme
- γ-LPH:
-
gamma-lipotropin
- GCH1 :
-
Gene encoding GTP (guanosine triphosphate) cyclohydrolase 1
- GTP:
-
Guanosine triphosphate
- HLA:
-
Human leukocyte antigen
- K14-SCF:
-
Notation for mice with increased epidermal melanocytes
- MAC:
-
Minimum alveolar concentration
- MC1R:
-
Melanocortin 1 receptor
- Mc1r :
-
Alleles for MC1R
- Mc1r e/e :
-
Mutant mice lacking MC1R
- MC3R:
-
Melanocortin 3 receptor
- MC4R:
-
Melanocortin 4 receptors
- Mc4r :
-
Allele for MC4R
- Mitf mi − wh/mi − wh :
-
Allele notation for mice lacking melanocytes
- [Nle4,D-Phe7]-α-MSH:
-
Melanocortin agonist
- N-POC:
-
N-pro-opiomelanocortin
- OPRM1:
-
μ-Opioid receptor for β-endorphin
- Oprm1 −/− :
-
Genetic notation for a strain lacking μ-opioid receptor for β-endorphin
- PC:
-
Proconvertase
- POMC:
-
Proopiomelanocortin
- QTL:
-
Quantitative trait locus
- SHU-9119:
-
Melanocortin receptor antagonist
- Tyr c/c :
-
Genetic notation for albino mice
- U50,488:
-
Broad κ-opioid agonist
- UV:
-
Ultra-violet
- WT:
-
Wild type
References
Liem EB, Lin C, Suleman M, Doufas AG, Gregg RG, Veauthier JM, et al. Anesthetic requirement is increased in redheads. Anesthesiology. 2004;101(2):279–83. https://doi.org/10.1097/00000542-200408000-00006.
Aranake A, Mashour GA, Avidan MS. Minimum alveolar concentration: ongoing relevance and clinical utility. Anaesthesia. 2013;68(5):512–22. https://doi.org/10.1111/anae.12168.
Liem EB, Joiner TV, Tsueda K, Sessler DI. Increased sensitivity to thermal pain and reduced subcutaneous lidocaine efficacy in redheads. Anesthesiology. 2005;102(3):509–14. https://doi.org/10.1097/00000542-200503000-00006.
Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, Lariviere WR, et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci USA. 2003;100(8):4867–72. https://doi.org/10.1073/pnas.0730053100.
Gradwohl SC, Aranake A, Abdallah AB, McNair P, Lin N, Fritz BA, et al. Intraoperative awareness risk, anesthetic sensitivity, and anesthetic management for patients with natural red hair: a matched cohort study. Can J Anesth. 2015;62:345–55. https://doi.org/10.1007/s12630-014-0305-8.
Andresen T, Lunden D, Drewes AM, Arendt-Nielsen L. Pain sensitivity and experimentally induced sensitisation in red haired females. Scand J Pain. 2011;2(1):3–6. https://doi.org/10.1016/j.sjpain.2010.08.005.
Droll B, Drum M, Nusstein J, Reader AI, Beck M. Anesthetic efficacy of the inferior alveolar nerve block in red-haired women. J Endod. 2012;38(12):1564–9. https://doi.org/10.1016/j.joen.2012.08.014.
Binkley CJ, Beacham A, Neace W, Gregg RG, Liem EB, Sessler DI. Genetic variations associated with red hair color and fear of dental pain, anxiety regarding dental care and avoidance of dental care. J Am Dent Assoc. 2009;140(7):896–905. https://doi.org/10.14219/jada.archive.2009.0283.
Myles PS, Buchanan FF, Bain CR. The effect of hair colour on anaesthetic requirements and recovery time after surgery. Anaesth Intensive Care. 2012;40(4):683–9. https://doi.org/10.1177/0310057X1204000415.
Beaumont KA, Wong SS, Ainger SA, Liu YY, Patel MP, Millhauser L, et al. Melanocortin MC1 receptor in human genetics and model systems. Eur J Pharmacol. 2011;660(1):103–10. https://doi.org/10.1016/j.ejphar.2010.11.040.
Box NF, Wyeth JR, O’Gorman LE, Martin NG, Sturm RA. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet. 1997;6(11):1891–7. https://doi.org/10.1093/hmg/6.11.1891.
Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte–stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet. 1995;11(3):328–30. https://doi.org/10.1038/ng1195-328.
Krol ES, Liebler DC. Photoprotective actions of natural and synthetic melanins. Chem Res Toxicol. 1998;11(12):1434–40. https://doi.org/10.1021/tx980114c.
Scherer D. Kumar R Genetics of pigmentation in skin cancer — a review. Mut Res/Rev Mut Res. 2010;705(2):141–53. https://doi.org/10.1016/j.mrrev.2010.06.002.
Katsara MA, Nothnagel M. True colors: a literature review on the spatial distribution of eye and hair pigmentation. Forensic Sci Int Genet. 2019;39:109–18. https://doi.org/10.1016/j.fsigen.2019.01.001.
Haddadeen C, Lai C, Cho S, Healy E. Variants of the melanocortin-1 receptor: do they matter clinically? Exp Dermatol. 2015;24(1):5–9. https://doi.org/10.1111/exd.12540.
Valverde P, Healy E, Sikkink S, Haldane F, Thody AJ, Carothers A, et al. The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum Mol Genet. 1996;5(10):1663–6. https://doi.org/10.1093/hmg/5.10.1663.
Harding RM, Healy E, Ray AJ, Ellis NS, Flanagan N, Todd C, et al. Evidence for variable selective pressures at MC1R. Am J Hum Genet. 2000;66(4):1351–61.
Pandit JJ. ‘Hardy’s Law’ and genomics in Anaesthesia. Anaesthesia. 2008;63(12):1284–7. https://doi.org/10.1111/j.1365-2044.2008.05736.x.
Cawley NX, Li Z, Loh YP. 60 years of POMC: biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J Mol Endocrinol. 2016;56(4):T77–97. https://doi.org/10.1530/JME-15-0323.
Wolf Horrell EM, Boulanger MC, D’Orazio JA. Melanocortin 1 receptor: structure, function, and regulation. Front Genetics. 2016;7:95. https://doi.org/10.3389/fgene.2016.00095.
De Luca M, Siegrist W, Bondanza S, Mathor M, Cancedda R, Eberle AN. Alpha melanocyte stimulating hormone (alpha MSH) stimulates normal human melanocyte growth by binding to high-affinity receptors. J Cell Sci. 1993;105(4):1079–84. https://doi.org/10.1242/jcs.105.4.1079.
Donatien PD, Hunt G, Pieron C, Lunec J, Taïeb A, Thody AJ. The expression of functional MSH receptors on cultured human melanocytes. Arch Dermatol Res. 1992;284(7):424–6. https://doi.org/10.1007/BF00372074.
Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, et al. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80(1–2):67–82. https://doi.org/10.1016/s0304-3959(98)00197-3.
Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, et al. Heritability of nociception II. “Types” of nociception revealed by genetic correlation analysis. Pain. 1999;80(1–2):83–93. https://doi.org/10.1016/s0304-3959(98)00196-1.
Lariviere WR, Wilson SG, Laughlin TM, Kokayeff A, E West EE, Adhikari SM, et al. Heritability of nociception. III. Genetic relationships among commonly used assays of nociception and hypersensitivity. Pain. 2002;97:75–86. https://doi.org/10.1016/s0304-3959(01)00492-4.
Mogil JS, Ritchie J, Smith SB, Strasburg K, Kaplan L, Wallace MR, et al. Melanocortin-1 receptor gene variants affect pain and mu-opioid analgesia in mice and humans. J Med Genet. 2005;42(7):583–7. https://doi.org/10.1136/jmg.2004.027698.
Linton SJ, Shaw WS. Impact of psychological factors in the experience of pain. Phys Ther. 2011;91(5):700–11. https://doi.org/10.2522/ptj.20100330.
Forsberg JT, Martinussen M, Flaten MA. The placebo analgesic effect in healthy individuals and patients: a meta-analysis. Psychosom Med. 2017;79(4):388–94. https://doi.org/10.1097/PSY.0000000000000432.
Perez TE, Mealey KL, Burke NS, Grubb TL, Court MH, Greene SA. Relationship between the melanocortin-1 receptor (MC1R) variant R306ter and physiological responses to mechanical or thermal stimuli in Labrador Retriever dogs. Vet Anaesth Analg. 2017;44(2):370–4.
Delaney A, Keighren M, Fleetwood-Walker S, Jackson IJ. Involvement of the melanocortin-1 receptor in acute pain and pain of inflammatory but not neuropathic origin. PloS One. 2010;5(9):e12498.
Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2(3):137–45.
Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20(3):371–513. https://doi.org/10.1017/s0140525x97221485.
Riley JLI II, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74(2–3):181–7. https://doi.org/10.1016/s0304-3959(97)00199-1.
Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev. 2000;24(3):375–89. https://doi.org/10.1016/S0149-7634(00)00015-4.
Tershner SA, Mitchell JM, Fields HL. Brainstem pain modulating circuitry is sexually dimorphic with respect to mu and kappa opioid receptor function. Pain. 2000;85(1–2):153–9. https://doi.org/10.1016/S0304-3959(99)00257-2.
Liu N, Gintzler AR. Prolonged ovarian sex steroid treatment of male rats produces antinociception: identification of sex-based divergent analgesic mechanisms. Pain. 2000;85(1–2):273–81. https://doi.org/10.1016/S0304-3959(99)00278-X.
Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa opioid receptor: from addiction to depression, and back. Front Psych. 2014;5:170. https://doi.org/10.3389/fpsyt.2014.00170.
Quillan JM, Sadée W. Dynorphin peptides: antagonists of melanocortin receptors. Pharm Res. 1997;14(6):713–9. https://doi.org/10.1023/A:1012185919153.
Wikberg JES. Melanocortin receptors: perspectives for novel drugs. Eur J Pharmacol. 1999;375(1–3):295–310. https://doi.org/10.1016/S0014-2999(99)00298-8.
Xia Y, Wikberg JE, Chhajlani V. Expression of melanocortin 1 receptor in periaqueductal gray matter. NeuroReport. 1995;6(16):2193–6. https://doi.org/10.1097/00001756-199511000-00022.
Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–38. https://doi.org/10.1146/annurev.ne.07.030184.001521.
Starowicz K, Przewlocki R, Gispen WH, Przewlocka B. Modulation of melanocortin-induced changes in spinal nociception by μ-opioid receptor agonist and antagonist in neuropathic rats. NeuroReport. 2002;13(18):2447–52. https://doi.org/10.1097/00001756-200212200-00015.
Vrinten DH, Gispen WH, Groen GJ, Adan RAH. Antagonism of the melanocortin system reduces cold and mechanical allodynia in mononeuropathic rats. J Neurosci. 2000;20(21):8131–7. https://doi.org/10.1523/jneurosci.20-21-08131.2000.
Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924–35. https://doi.org/10.1016/S1474-4422(14)70102-4.
Hermanns H, Hollmann MW, Stevens MF, Lirk P, Brandenburger T, Piegeler T, et al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 2019;123(3):335–49. https://doi.org/10.1016/j.bja.2019.06.014.
Pandit JJ, McGuire N. Unlicensed intravenous lidocaine for postoperative pain: always a safer ‘licence to stop’ than to start. Anaesthesia. 2021;76(2):156–60. https://doi.org/10.1111/anae.15286.
Marchand S. Mechanisms challenges of the pain phenomenon. Front Pain Res. 2021;1:574370.
Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia—when is enough too much? a review of opioid-induced tolerance and hyperalgesia. The Lancet. 2019;393(10180):1558–68. https://doi.org/10.1016/S0140-6736(19)30430-1.
Mogil JS. Animal models of pain: progress and challenges. Nature Rev Neurosci. 2009;10(4):283–94. https://doi.org/10.1038/nrn2606.
Ringholm A, Klovins J, Rudzish R, Phillips S, Rees JL, Schiöth HB. Pharmacological characterization of loss of function mutations of the human melanocortin 1 receptor that are associated with red hair. J Invest Dermatol. 2004;123(5):917–23. https://doi.org/10.1111/j.0022-202X.2004.23444.x.
Smith R, Healy E, Siddiqui S, Flanagan N, Steijlen PM, Rosdahl I, et al. Melanocortin 1 Receptor Variants in an Irish Population. J Invest Dermatol. 1998;111(1):119–22. https://doi.org/10.1046/j.1523-1747.1998.00252.x.
Zorina-Lichtenwalter K, Maixner W, Diatchenko L. Detangling red hair from pain: phenotype-specific contributions from different genetic variants in melanocortin-1 receptor. Pain. 2020;161(5):938–48. https://doi.org/10.1097/j.pain.0000000000001780.
Robinson KC, Kemény LV, Fell GL, Hermann AL, Allouche J, Ding W, et al. Reduced MC4R signaling alters nociceptive thresholds associated with red hair. Sci Adv. 2022;7(14):eabd1310. https://doi.org/10.1126/sciadv.abd1310.
Matthes HWD, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the µ-opioid-receptor gene. Nature. 1996;383(6603):819–23. https://doi.org/10.1038/383819a0.
Tanabe K, Gamo K, Aoki S, Wada K, Kiyama H. Melanocortin receptor 4 is induced in nerve-injured motor and sensory neurons of mouse. J Neurochem. 2007;101(4):1145–52. https://doi.org/10.1111/j.1471-4159.2006.04432.x.
Mogil JS. Are we getting anywhere in human pain genetics? Pain. 2009;146(3):231–2. https://doi.org/10.1016/j.pain.2009.07.023.
Max MB, Stewart WF. The molecular epidemiology of pain: a new discipline for drug discovery. Nature Rev Drug Disc. 2008;7(8):647–58. https://doi.org/10.1038/nrd2595.
Bornemann-Cimenti H, Szilagyi IS, Sandner-Kiesling A. Perpetuation of retracted publications using the example of the Scott S. Reuben case: incidences, reasons and possible improvements. Sci Eng Ethics. 2016;22(4):1063–72.
Pandit JJ. On statistical methods to test if sampling in trials is genuinely random. Anaesthesia. 2012;67(5):456–62. https://doi.org/10.1111/j.1365-2044.2012.07114.x.
Goldstein DB. Common genetic variation and human traits. N Engl J Med. 2009;360(17):1696–8. https://doi.org/10.1056/NEJMp0806284.
Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359(9308):727–32. https://doi.org/10.1016/S0140-6736(02)07873-X.
Mallal S, Phillips E, Carosi G, Molina JM, Workman C, Tomazic J, et al. HLA-B*5701 Screening for hypersensitivity to abacavir. N Engl J Med. 2008;358(6):568–79. https://doi.org/10.1056/NEJMoa0706135.
Hughes DA, Vilar FJ, Ward CC, Alfirevic A, Park BK, Pirmohamed M. Cost-effectiveness analysis of HLA B*5701 genotyping in preventing abacavir hypersensitivity. Pharmacogen Genomics. 2004;14(6):335–42. https://doi.org/10.1097/00008571-200406000-00002.
Crews KR, Monte AA, Huddart R, Caudle KE, Kharasch ED, Gaedigk A, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2D6, OPRM1, and COMT genotypes and select opioid therapy. Clin Pharmacol Ther. 2021;110(4):888–96. https://doi.org/10.1002/cpt.2149.
Acknowledgements
Not applicable.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
The authors jointly conceived of the project; JJP defined the scope and title; HRW conducted the preliminary literature search and prepared the first draft. Subsequent drafts were prepared by JJP; both authors approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Williams, H.R., Pandit, J.J. Red hair and pain sensitivity: insights into genomics of pain?. APS 1, 15 (2023). https://doi.org/10.1007/s44254-023-00017-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44254-023-00017-3