Abstract
Purpose of the Review
The purpose of this review is to summarize the role of the osteocyte in muscle atrophy in cancer patients, sarcopenia, spinal cord injury, Duchenne’s muscular dystrophy, and other conditions associated with muscle deterioration.
Recent Findings
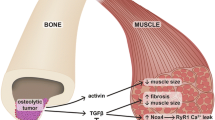
One type of bone cell, the osteocyte, appears to play a major role in muscle and bone crosstalk, whether physiological or pathological. Osteocytes are cells living within the bone-mineralized matrix. These cells are connected to each other by means of dendrites to create an intricately connected network. The osteocyte network has been shown to respond to different types of stimuli such as mechanical unloading, immobilization, aging, and cancer by producing osteocytes-derived factors. It is now becoming clear that some of these factors including sclerostin, RANKL, TGF-β, and TNF-α have detrimental effects on skeletal muscle.
Summary
Bone and muscle not only communicate mechanically but also biochemically. Osteocyte-derived factors appear to contribute to the pathogenesis of muscle disease and could be used as a cellular target for new therapeutic approaches.

Similar content being viewed by others
References
Bonewald L. Use it or lose it to age: a review of bone and muscle communication. Bone. 2019;120:212–8. https://doi.org/10.1016/j.bone.2018.11.002.
Rosenberg N, Rosenberg O, Soudry M. Osteoblasts in bone physiology-mini review. Rambam Maimonides Med J. 2012;3(2):e0013. https://doi.org/10.5041/RMMJ.10080.
Feng X, Teitelbaum SL. Osteoclasts: new Insights. Bone Res. 2013;1(1):11–26. https://doi.org/10.4248/BR201301003.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. https://doi.org/10.1002/jbmr.320.
Robling AG, Bonewald LF. The osteocyte: new insights. Annu Rev Physiol. 2020;82:485–506. https://doi.org/10.1146/annurev-physiol-021119-034332.
Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, et al. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283(39):26374–82. https://doi.org/10.1074/jbc.M803136200.
Yoshimoto T, Kittaka M, Doan AAP, Urata R, Prideaux M, Rojas RE, et al. Osteocytes directly regulate osteolysis via MYD88 signaling in bacterial bone infection. Nat Commun. 2022;13(1):6648. https://doi.org/10.1038/s41467-022-34352-z.
Xiong J, Piemontese M, Onal M, Campbell J, Goellner JJ, Dusevich V, et al. Osteocytes, not osteoblasts or lining cells, are the main source of the rankl required for osteoclast formation in remodeling bone. PLoS One. 2015;10(9):e0138189. https://doi.org/10.1371/journal.pone.0138189.
Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020;40:2. https://doi.org/10.1186/s41232-019-0111-3.
Xiong J, O’Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. J Bone Miner Res. 2012;27(3):499–505. https://doi.org/10.1002/jbmr.1547.
O’Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, et al. RANK-independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheumatol. 2016;68(12):2889–900. https://doi.org/10.1002/art.39837.
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19(13):1842–4. https://doi.org/10.1096/fj.05-4221fje.
Wang JS, Mazur CM, Wein MN. Sclerostin and osteocalcin: candidate bone-produced hormones. Front Endocrinol (Lausanne). 2021;12:584147. https://doi.org/10.3389/fendo.2021.584147.
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–75. https://doi.org/10.1074/jbc.M705092200.
Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/beta-catenin pathway. JBMR Plus. 2017;1(2):86–100. https://doi.org/10.1002/jbm4.10015.
Tsourdi E, Jahn K, Rauner M, Busse B, Bonewald LF. Physiological and pathological osteocytic osteolysis. J Musculoskelet Neuronal Interact. 2018;18(3):292–303.
Parfitt AM. The cellular basis of bone turnover and bone loss: a rebuttal of the osteocytic resorption–bone flow theory. Clin Orthop Relat Res. 1977;127:236–47.
van der Plas A, Aarden EM, Feijen JH, de Boer AH, Wiltink A, Alblas MJ, et al. Characteristics and properties of osteocytes in culture. J Bone Miner Res. 1994;9(11):1697–704. https://doi.org/10.1002/jbmr.5650091105.
Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jahn K, Kato S, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27(5):1018–29. https://doi.org/10.1002/jbmr.1567.
Jahn K, Kelkar S, Zhao H, Xie Y, Tiede-Lewis LM, Dusevich V, et al. Osteocytes acidify their microenvironment in response to PTHrP in vitro and in lactating mice in vivo. J Bone Miner Res. 2017;32(8):1761–72. https://doi.org/10.1002/jbmr.3167.
Yamazaki M, Michigami T. Osteocytes and the pathogenesis of hypophosphatemic rickets. Front Endocrinol (Lausanne). 2022;13:1005189. https://doi.org/10.3389/fendo.2022.1005189.
Qing H, Bonewald LF. Osteocyte remodeling of the perilacunar and pericanalicular matrix. Int J Oral Sci. 2009;1(2):59–65. https://doi.org/10.4248/ijos.09019.
Youlten SE, Kemp JP, Logan JG, Ghirardello EJ, Sergio CM, Dack MRG, et al. Osteocyte transcriptome mapping identifies a molecular landscape controlling skeletal homeostasis and susceptibility to skeletal disease. Nat Commun. 2021;12(1):2444. https://doi.org/10.1038/s41467-021-22517-1.
Mo C, Zhao R, Vallejo J, Igwe O, Bonewald L, Wetmore L, et al. Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation. Cell Cycle. 2015;14(10):1507–16. https://doi.org/10.1080/15384101.2015.1026520.
Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang AV, Kraft P, et al. Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science. 2021;371(6528). https://doi.org/10.1126/science.abc8059.
Jurimae J, Karvelyte V, Remmel L, Tamm AL, Purge P, Gruodyte-Raciene R, et al. Serum sclerostin concentration is associated with specific adipose, muscle and bone tissue markers in lean adolescent females with increased physical activity. J Pediatr Endocrinol Metab. 2021;34(6):755–61. https://doi.org/10.1515/jpem-2020-0662.
Kim JA, Roh E, Hong SH, Lee YB, Kim NH, Yoo HJ, et al. Association of serum sclerostin levels with low skeletal muscle mass: The Korean Sarcopenic Obesity Study (KSOS). Bone. 2019;128:115053. https://doi.org/10.1016/j.bone.2019.115053.
Wood CL, Pajevic PD, Gooi JH. Osteocyte secreted factors inhibit skeletal muscle differentiation. Bone Rep. 2017;6:74–80. https://doi.org/10.1016/j.bonr.2017.02.007.
Traub J, Bergheim I, Eibisberger M, Stadlbauer V. Sarcopenia and liver cirrhosis-comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019. Nutrients. 2020;12(2). https://doi.org/10.3390/nu12020547.
Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–37. https://doi.org/10.1038/s41574-018-0062-9.
Keramidaki K, Tsagari A, Hiona M, Risvas G. Osteosarcopenic obesity, the coexistence of osteoporosis, sarcopenia and obesity and consequences in the quality of life in older adults >/=65 years-old in Greece. J Frailty Sarcopenia Falls. 2019;4(4):91–101. https://doi.org/10.22540/JFSF-04-091.
Busse B, Djonic D, Milovanovic P, Hahn M, Puschel K, Ritchie RO, et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9(6):1065–75. https://doi.org/10.1111/j.1474-9726.2010.00633.x.
D’Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136(2):284–95. https://doi.org/10.1016/j.cell.2008.11.037.
Modder UI, Hoey KA, Amin S, McCready LK, Achenbach SJ, Riggs BL, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26(2):373–9. https://doi.org/10.1002/jbmr.217.
Medeiros MC, Rocha N, Bandeira E, Dantas I, Chaves C, Oliveira M, et al. Serum sclerostin, body composition, and sarcopenia in hemodialysis patients with diabetes. Int J Nephrol. 2020;2020:4596920. https://doi.org/10.1155/2020/4596920.
Ahn SH, Jung HW, Lee E, Baek JY, Jang IY, Park SJ, et al. Decreased serum level of sclerostin in older adults with sarcopenia. Endocrinol Metab (Seoul). 2022. https://doi.org/10.3803/EnM.2022.1428.
Kim SP, Frey JL, Li Z, Kushwaha P, Zoch ML, Tomlinson RE, et al. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A. 2017;114(52):E11238–47. https://doi.org/10.1073/pnas.1707876115.
Dufresne SS, Dumont NA, Boulanger-Piette A, Fajardo VA, Gamu D, Kake-Guena SA, et al. Muscle RANK is a key regulator of Ca2+ storage, SERCA activity, and function of fast-twitch skeletal muscles. Am J Physiol Cell Physiol. 2016;310(8):C663–72. https://doi.org/10.1152/ajpcell.00285.2015.
Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111(8):1221–30. https://doi.org/10.1172/JCI17215.
Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest. 2019;129(8):3214–23. https://doi.org/10.1172/JCI125915.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. https://doi.org/10.1016/S1470-2045(10)70218-7.
von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers-update 2014. J Cachexia Sarcopenia Muscle. 2014;5(4):261–3. https://doi.org/10.1007/s13539-014-0164-8.
Pourquie O. Vertebrate somitogenesis. Annu Rev Cell Dev Biol. 2001;17:311–50. https://doi.org/10.1146/annurev.cellbio.17.1.311.
Waning DL, Mohammad KS, Reiken S, Xie W, Andersson DC, John S, et al. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat Med. 2015;21(11):1262–71. https://doi.org/10.1038/nm.3961.
Hesse E, Schröder S, Brandt D, Pamperin J, Saito H, Taipaleenmäki H. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight. 2019;5(9):e125543. https://doi.org/10.1172/jci.insight.125543.
Narasimhan A, Shahda S, Kays JK, Perkins SM, Cheng L, Schloss KNH, et al. Identification of potential serum protein biomarkers and pathways for pancreatic cancer cachexia using an aptamer-based discovery platform. Cancers (Basel). 2020;12(12):3787. https://doi.org/10.3390/cancers12123787.
Bonetto A, Kays JK, Parker VA, Matthews RR, Barreto R, Puppa MJ, et al. Differential bone loss in mouse models of colon cancer cachexia. Front Physiol. 2016;7:679. https://doi.org/10.3389/fphys.2016.00679.
Huot JR, Novinger LJ, Pin F, Narasimhan A, Zimmers TA, O’Connell TM, et al. Formation of colorectal liver metastases induces musculoskeletal and metabolic abnormalities consistent with exacerbated cachexia. JCI Insight. 2020;5(9):e136687. https://doi.org/10.1172/jci.insight.136687.
Huot JR, Pin F, Essex AL, Bonetto A. MC38 tumors induce musculoskeletal defects in colorectal cancer. Int J Mol Sci. 2021;22(3):1486. https://doi.org/10.3390/ijms22031486.
Berent TE, Dorschner JM, Craig TA, Drake MT, Westendorf JJ, Kumar R. Lung tumor cells inhibit bone mineralization and osteoblast activity. Biochem Biophys Res Commun. 2019;519(3):566–71. https://doi.org/10.1016/j.bbrc.2019.09.045.
Pin F, Barreto R, Kitase Y, Mitra S, Erne CE, Novinger LJ, et al. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: a new model for the study of cancer cachexia. J Cachexia Sarcopenia Muscle. 2018;9(4):685–700. https://doi.org/10.1002/jcsm.12311.
Pin F, Prideaux M, Huot JR, Essex AL, Plotkin LI, Bonetto A, et al. Non-bone metastatic cancers promote osteocyte-induced bone destruction. Cancer Lett. 2021;520:80–90. https://doi.org/10.1016/j.canlet.2021.06.030.
Pin F, Jones AJ, Huot JR, Narasimhan A, Zimmers TA, Bonewald LF, et al. RANKL blockade reduces cachexia and bone loss induced by non-metastatic ovarian cancer in mice. J Bone Miner Res. 2022;37(3):381–96. https://doi.org/10.1002/jbmr.4480.
Wang H, Xia Y, Li B, Li Y, Fu C. Reverse adverse immune microenvironments by biomaterials enhance the repair of spinal cord injury. Front Bioeng Biotechnol. 2022;10:812340. https://doi.org/10.3389/fbioe.2022.812340.
Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29(5):489–500. https://doi.org/10.1080/10790268.2006.11753898.
Invernizzi M, Carda S, Rizzi M, Grana E, Squarzanti DF, Cisari C, et al. Evaluation of serum myostatin and sclerostin levels in chronic spinal cord injured patients. Spinal Cord. 2015;53(8):615–20. https://doi.org/10.1038/sc.2015.61.
Maimoun L, Ben Bouallegue F, Gelis A, Aouinti S, Mura T, Philibert P, et al. Periostin and sclerostin levels in individuals with spinal cord injury and their relationship with bone mass, bone turnover, fracture and osteoporosis status. Bone. 2019;127:612–9. https://doi.org/10.1016/j.bone.2019.07.019.
Qin W, Li X, Peng Y, Harlow LM, Ren Y, Wu Y, et al. Sclerostin antibody preserves the morphology and structure of osteocytes and blocks the severe skeletal deterioration after motor-complete spinal cord injury in rats. J Bone Miner Res. 2015;30(11):1994–2004. https://doi.org/10.1002/jbmr.2549.
Zhao W, Li X, Peng Y, Qin Y, Pan J, Li J, et al. Sclerostin antibody reverses the severe sublesional bone loss in rats after chronic spinal cord injury. Calcif Tissue Int. 2018;103(4):443–54. https://doi.org/10.1007/s00223-018-0439-8.
Phillips EG, Beggs LA, Ye F, Conover CF, Beck DT, Otzel DM, et al. Effects of pharmacologic sclerostin inhibition or testosterone administration on soleus muscle atrophy in rodents after spinal cord injury. PLoS ONE. 2018;13(3):e0194440. https://doi.org/10.1371/journal.pone.0194440.
Yiu EM, Kornberg AJ. Duchenne muscular dystrophy. J Paediatr Child Health. 2015;51(8):759–64. https://doi.org/10.1111/jpc.12868.
Buckner JL, Bowden SA, Mahan JD. Optimizing bone health in duchenne muscular dystrophy. Int J Endocrinol. 2015;2015:928385. https://doi.org/10.1155/2015/928385.
Gao X, Tang Y, Amra S, Sun X, Cui Y, Cheng H, et al. Systemic investigation of bone and muscle abnormalities in dystrophin/utrophin double knockout mice during postnatal development and the mechanisms. Hum Mol Genet. 2019;28(10):1738–51. https://doi.org/10.1093/hmg/ddz012.
Dufresne SS, Boulanger-Piette A, Bosse S, Argaw A, Hamoudi D, Marcadet L, et al. Genetic deletion of muscle RANK or selective inhibition of RANKL is not as effective as full-length OPG-fc in mitigating muscular dystrophy. Acta Neuropathol Commun. 2018;6(1):31. https://doi.org/10.1186/s40478-018-0533-1.
Hamoudi D, Marcadet L, Piette Boulanger A, Yagita H, Bouredji Z, Argaw A, et al. An anti-RANKL treatment reduces muscle inflammation and dysfunction and strengthens bone in dystrophic mice. Hum Mol Genet. 2019;28(18):3101–12. https://doi.org/10.1093/hmg/ddz124.
Akhtar Ali S, Kang H, Olney R, Ramos-Platt L, Ryabets-Lienhard A, Cheung C, et al. Evaluating RANKL and OPG levels in patients with Duchenne muscular dystrophy. Osteoporos Int. 2019;30(11):2283–8. https://doi.org/10.1007/s00198-019-05077-5.
Belavy DL, Baecker N, Armbrecht G, Beller G, Buehlmeier J, Frings-Meuthen P, et al. Serum sclerostin and DKK1 in relation to exercise against bone loss in experimental bed rest. J Bone Miner Metab. 2016;34(3):354–65. https://doi.org/10.1007/s00774-015-0681-3.
Frings-Meuthen P, Boehme G, Liphardt AM, Baecker N, Heer M, Rittweger J. Sclerostin and DKK1 levels during 14 and 21 days of bed rest in healthy young men. J Musculoskelet Neuronal Interact. 2013;13(1):45–52.
Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, et al. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 2012;97(9):E1736–40. https://doi.org/10.1210/jc.2012-1579.
Brent MB, Bruel A, Thomsen JS. Anti-sclerostin antibodies and abaloparatide have additive effects when used as a countermeasure against disuse osteopenia in female rats. Bone. 2022;160:116417. https://doi.org/10.1016/j.bone.2022.116417.
Speacht TL, Lang CH, Donahue HJ. Soluble RANKL exaggerates hindlimb suspension-induced osteopenia but not muscle protein balance. J Orthop Res. 2021;39(9):1860–9. https://doi.org/10.1002/jor.24917.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimonty, A., Bonewald, L.F. & Pin, F. Role of the Osteocyte in Musculoskeletal Disease. Curr Osteoporos Rep 21, 303–310 (2023). https://doi.org/10.1007/s11914-023-00788-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-023-00788-5