Abstract
Purpose of Review
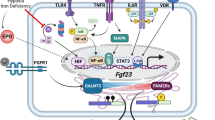
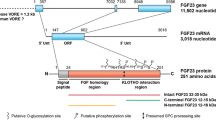
Fibroblast growth factor 23 (FGF23) is a bone- and bone marrow-derived hormone that is critical to maintain phosphate homeostasis. The principal actions of FGF23 are to reduce serum phosphate levels by decreasing kidney phosphate reabsorption and 1,25-dihydroxyvitamin D synthesis. FGF23 deficiency causes hyperphosphatemia and ectopic calcifications, while FGF23 excess causes hypophosphatemia and skeletal defects. Excess FGF23 also correlates with kidney disease, where it is associated with increased morbidity and mortality. Accordingly, FGF23 levels are tightly regulated, but the mechanisms remain incompletely understood.
Recent Findings
In addition to bone mineral factors, additional factors including iron, erythropoietin, inflammation, energy, and metabolism regulate FGF23. All these factors affect Fgf23 expression, while some also regulate FGF23 protein cleavage. Conversely, FGF23 may have a functional role in regulating these biologic processes.
Summary
Understanding the bi-directional relationship between FGF23 and non-bone mineral factors is providing new insights into FGF23 regulation and function.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G, Cholesterol, et al. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112(17):2627–33.
Forbes RM, Cooper AR, Mitchell HH. The composition of the adult human body as determined by chemical analysis. J Biol Chem. 1953;203(1):359–66.
Farrow EG, White KE. Recent advances in renal phosphate handling. Nat Rev Nephrol. 2010;6(4):207–17.
Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92(1):131–55.
Bergwitz C, Juppner H. FGF23 and syndromes of abnormal renal phosphate handling. Adv Exp Med Biol. 2012;728:41–64.
Consortium A. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–8.
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001;98(11):6500–5.
Moe OW. Familial tumoral calcinosis: a valuable vehicle for discovery. Nephrol Dial Transplant. 2014;29(12):2155–7.
Leaf DE, Jacob KA, Srivastava A, Chen ME, Christov M, Juppner H, et al. Fibroblast growth factor 23 levels associate with AKI and death in critical illness. J Am Soc Nephrol. 2017;28(6):1877–85.
Christov M, Waikar SS, Pereira RC, Havasi A, Leaf DE, Goltzman D, Pajevic PD, Wolf M, Jüppner H. Plasma FGF23 levels increase rapidly after acute kidney injury. Kidney Int. 2013;84(4):776–85.
Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–92.
Clinkenbeard EL, White KE. Systemic control of bone homeostasis by FGF23 signaling. Curr Mol Biol Rep. 2016;2(1):62–71.
Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–9.
Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–52.
Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M, the Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25(2):349–60.
Ishigami J, Taliercio JT, Feldman HI, Srivastava A, Townsend RR, Cohen DL, Horwitz EJ, Rao P, Charleston J, Fink JC, Ricardo AC, Sondheimer J, Chen TK, Wolf M, Isakova T, Appel LJ, Matsushita K, for the CRIC study Investigators. Fibroblast growth factor 23 and risk of hospitalization with infection in chronic kidney disease: the Chronic Renal Insufficiency Cohort (CRIC) study. J Am Soc Nephrol. 2020;31(8):1836–46.
Edmonston D, Wojdyla D, Mehta R, Cai X, Lora C, Cohen D, Townsend RR, He J, Go AS, Kusek J, Weir MR, Isakova T, Pencina M, Wolf M, Appel LJ, Feldman HI, Lash JP, Rao PS, Rahman M, et al. Single measurements of carboxy-terminal fibroblast growth factor 23 and clinical risk prediction of adverse outcomes in CKD. Am J Kidney Dis. 2019;74(6):771–81. In patients with CKD, single measurements of cFGF-23 improve prediction of risks for all-cause mortality and heart failure admission, but not cardiovascular mortality, ESRD, or atherosclerotic events.
Leaf DE, Christov M, Juppner H, Siew E, Ikizler TA, Bian A, et al. Fibroblast growth factor 23 levels are elevated and associated with severe acute kidney injury and death following cardiac surgery. Kidney Int. 2016;89(4):939–48.
Leaf DE, Siew ED, Eisenga MF, Singh K, Mc Causland FR, Srivastava A, Alp Ikizler T, Ware LB, Ginde AA, Kellum JA, Palevsky PM, Wolf M, Waikar SS. Fibroblast growth factor 23 associates with death in critically ill patients. Clin J Am Soc Nephrol. 2018;13(4):531–41.
Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104.
Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–8.
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–408.
Bockmann I, Lischka J, Richter B, Deppe J, Rahn A, Fischer DC, et al. FGF23-mediated activation of local RAAS promotes cardiac hypertrophy and fibrosis. Int J Mol Sci. 2019;20(18):4634.
Lindner M, Mehel H, David A, Leroy C, Burtin M, Friedlander G, Terzi F, Mika D, Fischmeister R, Prié D. Fibroblast growth factor 23 decreases PDE4 expression in heart increasing the risk of cardiac arrhythmia; Klotho opposes these effects. Basic Res Cardiol. 2020;115(5):51.
Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstadt HJ, Meersch M, et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016;126(3):962–74.
Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, Czaja MJ, Bartz R, Abraham R, di Marco GS, Brand M, Wolf M, Faul C. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016;90(5):985–96.
Pastor-Arroyo EM, Gehring N, Krudewig C, Costantino S, Bettoni C, Knopfel T, et al. The elevation of circulating fibroblast growth factor 23 without kidney disease does not increase cardiovascular disease risk. Kidney Int. 2018;94(1):49–59. Mice with X-linked hypophosphatemia have high FGF23 levels, but have no left ventricular hypertrophy and no alteration of cardiac function.
Dussold C, Gerber C, White S, Wang X, Qi L, Francis C, Capella M, Courbon G, Wang J, Li C, Feng JQ, Isakova T, Wolf M, David V, Martin A. DMP1 prevents osteocyte alterations, FGF23 elevation and left ventricular hypertrophy in mice with chronic kidney disease. Bone Res. 2019;7:12.
Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai MM, Cattley RC, Wronski TJ, Xia X, Li X, Henley C, Eschenberg M, Richards WG. FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest. 2012;122(7):2543–53.
Clinkenbeard EL, Noonan ML, Thomas JC, Ni P, Hum JM, Aref M, et al. Increased FGF23 protects against detrimental cardio-renal consequences during elevated blood phosphate in CKD. JCI Insight. 2019;4(4):e123817. Mice with bone specific Fgf23 knock out have more profound aortic calcification and cardiac hypertrophy versus wild type controls in the mouse model of CKD.
Wheeler JA, Clinkenbeard EL. Regulation of fibroblast growth factor 23 by iron, EPO, and HIF. Curr Mol Biol Rep. 2019;5(1):8–17.
Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;96(11):3541–9.
Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ, Vidal R, Chan RJ, Goodwin CB, Hui SL, Peacock M, White KE. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108(46):E1146–55.
Babitt JL, Eisenga MF, Haase VH, Kshirsagar AV, Levin A, Locatelli F, Małyszko J, Swinkels DW, Tarng DC, Cheung M, Jadoul M, Winkelmayer WC, Drüeke TB, Abu-Alfa AK, Afsar B, Pai AB, Besarab A, Moore GB, Casadevall N, et al. Controversies in optimal anemia management: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021;99:1280–95.
Wang CY, Babitt JL. Hepcidin regulation in the anemia of inflammation. Curr Opin Hematol. 2016;23(3):189–97.
David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, Zumbrennen-Bullough KB, Sun CC, Lin HY, Babitt JL, Wolf M. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89(1):135–46.
Mehta R, Cai X, Hodakowski A, Lee J, Leonard M, Ricardo A, Chen J, Hamm L, Sondheimer J, Dobre M, David V, Yang W, Go A, Kusek JW, Feldman H, Wolf M, Isakova T, CRIC Study Investigators. Fibroblast growth factor 23 and anemia in the chronic renal insufficiency cohort study. Clin J Am Soc Nephrol. 2017;12(11):1795–803.
Nam KH, Kim H, An SY, Lee M, Cha MU, Park JT, Yoo TH, Lee KB, Kim YH, Sung SA, Lee J, Kang SW, Choi KH, Ahn C, Han SH. Circulating fibroblast growth factor-23 levels are associated with an increased risk of anemia development in patients with nondialysis chronic kidney disease. Sci Rep. 2018;8(1):7294.
Yamashita K, Mizuiri S, Nishizawa Y, Kenichiro S, Doi S, Masaki T. Oral iron supplementation with sodium ferrous citrate reduces the serum intact and c-terminal fibroblast growth factor 23 levels of maintenance haemodialysis patients. Nephrology (Carlton). 2017;22(12):947–53.
Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28(8):1793–803.
Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight. 2018;3(23):e124486.
Wolf M, Rubin J, Achebe M, Econs MJ, Peacock M, Imel EA, Thomsen LL, Carpenter TO, Weber T, Brandenburg V, Zoller H. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323(5):432–43. In 2 randomized trials of patients with iron-deficiency anemia, iron isomaltoside compared with ferric carboxymaltose, resulted in lower incidence of hypophosphatemia over 35 days.
Gilmartin CE, Hoang T, Cutts BA, Leung L. Retrospective cohort study comparing the adverse reactions and efficacy of intravenous iron polymaltose with ferric carboxymaltose for iron deficiency anemia. Int J Gynaecol Obstet. 2018;141(3):315–20.
Urbina T, Belkhir R, Rossi G, Carbonnel F, Pavy S, Collins M, Mariette X, Seror R. Iron supplementation-induced phosphaturic osteomalacia: FGF23 is the culprit. J Bone Miner Res. 2018;33(3):540–2.
Klein K, Asaad S, Econs M, Rubin JE. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018;2018:bcr2017222851.
Block GA, Block MS, Smits G, Mehta R, Isakova T, Wolf M, Chertow GM. A pilot randomized trial of ferric citrate coordination complex for the treatment of advanced CKD. J Am Soc Nephrol. 2019;30(8):1495–504.
Block GA, Pergola PE, Fishbane S, Martins JG, LeWinter RD, Uhlig K, et al. Effect of ferric citrate on serum phosphate and fibroblast growth factor 23 among patients with nondialysis-dependent chronic kidney disease: path analyses. Nephrol Dial Transplant. 2019;34(7):1115–24.
Francis C, Courbon G, Gerber C, Neuburg S, Wang X, Dussold C, Capella M, Qi L, Isakova T, Mehta R, Martin A, Wolf M, David V. Ferric citrate reduces fibroblast growth factor 23 levels and improves renal and cardiac function in a mouse model of chronic kidney disease. Kidney Int. 2019;96(6):1346–58.
Sasaki A, Yasukawa H, Shouda T, Kitamura T, Dikic I, Yoshimura A. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J Biol Chem. 2000;275(38):29338–47.
Flamme I, Ellinghaus P, Urrego D, Kruger T. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS One. 2017;12(10):e0186979.
Hanudel MR, Eisenga MF, Rappaport M, Chua K, Qiao B, Jung G, Gabayan V, Gales B, Ramos G, de Jong MA, van Zanden JJ, de Borst MH, Bakker SJL, Nemeth E, Salusky IB, Gaillard CAJM, Ganz T. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol Dial Transplant. 2019;34(12):2057–65. Serum EPO levels are associated with total FGF23, but not intact FGF23 in mice and patients with CKD after the kidney transplant.
Clinkenbeard EL, Hanudel MR, Stayrook KR, Appaiah HN, Farrow EG, Cass TA, Summers LJ, Ip CS, Hum JM, Thomas JC, Ivan M, Richine BM, Chan RJ, Clemens TL, Schipani E, Sabbagh Y, Xu L, Srour EF, Alvarez MB, et al. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica. 2017;102(11):e427–e30.
Toro L, Barrientos V, Leon P, Rojas M, Gonzalez M, Gonzalez-Ibanez A, et al. Erythropoietin induces bone marrow and plasma fibroblast growth factor 23 during acute kidney injury. Kidney Int. 2018;93(5):1131–41. In AKI in mice and humans there is an increase in erythropoietin and circulating FGF23, while in mice erythropoietin receptor blockade prevented the induction of FGF23.
Roszko KL, Brown S, Pang Y, Huynh T, Zhuang Z, Pacak K, Collins MT. C-terminal, but not intact, FGF23 and EPO are strongly correlatively elevated in patients with gain-of-function mutations in HIF2A: Clinical Evidence for EPO Regulating FGF23. J Bone Miner Res. 2021;36(2):315–21.
Ishii S, Suzuki T, Wakahashi K, Asada N, Kawano Y, Kawano H, Sada A, Minagawa K, Nakamura Y, Mizuno S, Takahashi S, Matsui T, Katayama Y. FGF-23 from erythroblasts promotes hematopoietic progenitor mobilization. Blood. 2021;137(11):1457–67. Bone marrow erythroblasts induce G-CSF-induced hematopoietic progenitor cell mobilization via FGF-23 production as an intrinsic suppressor of chemoattraction.
Noonan ML, Clinkenbeard EL, Ni P, Swallow EA, Tippen SP, Agoro R, Allen MR, White KE. Erythropoietin and a hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHDi) lowers FGF23 in a model of chronic kidney disease (CKD). Phys Rep. 2020;8(11):e14434.
Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993;90(9):4304–8.
Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest. 2007;117(7):1926–32.
Zhang Q, Doucet M, Tomlinson RE, Han X, Quarles LD, Collins MT, Clemens TL. The hypoxia-inducible factor-1alpha activates ectopic production of fibroblast growth factor 23 in tumor-induced osteomalacia. Bone Res. 2016;4:16011.
Fernandez-Sanchez R, Berzal S, Sanchez-Nino MD, Neria F, Goncalves S, Calabia O, Tejedor A, J. Calzada M, Caramelo C, J.P. Deudero J, Ortiz A. AG490 promotes HIF-1alpha accumulation by inhibiting its hydroxylation. Curr Med Chem. 2012;19(23):4014–23.
Hum JM, Clinkenbeard EL, Ip C, Cass TA, Allen M, White KE. The metabolic bone disease associated with the Hyp mutation is independent of osteoblastic HIF1alpha expression. Bone Rep. 2017;6:38–43.
. Hanudel MR, Wong S, Jung G, Qiao B, Gabayan V, Zuk A, et al. Amelioration of chronic kidney disease-associated anemia by vadadustat in mice is not dependent on erythroferrone. Kidney Int. 2021;100:79–89.
Agoro R, Montagna A, Goetz R, Aligbe O, Singh G, Coe LM, Mohammadi M, Rivella S, Sitara D. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J. 2018;32(7):3752–64. FGF23 is a causative factor in the development of renal anemia and iron deficiency, and blocking FGF23 signaling stimulates erythropoiesis in CKD mouse model.
Coe LM, Madathil SV, Casu C, Lanske B, Rivella S, Sitara D. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J Biol Chem. 2014;289(14):9795–810.
Agoro R, Park MY, Le Henaff C, Jankauskas S, Gaias A, Chen G, et al. C-FGF23 peptide alleviates hypoferremia during acute inflammation. Haematologica. 2021;106(2):391–403.
Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M, for the Chronic Renal Insufficiency Cohort. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol. 2012;7(7):1155–62.
Hanks LJ, Casazza K, Judd SE, Jenny NS, Gutierrez OM. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS One. 2015;10(3):e0122885.
Dounousi E, Torino C, Pizzini P, Cutrupi S, Panuccio V, D'Arrigo G, Abd ElHafeez S, Tripepi G, Mallamaci F, Zoccali C. Intact FGF23 and alpha-Klotho during acute inflammation/sepsis in CKD patients. Eur J Clin Investig. 2016;46(3):234–41.
Sato H, Kazama JJ, Murasawa A, Otani H, Abe A, Ito S, et al. Serum fibroblast growth factor 23 (FGF23) in patients with rheumatoid arthritis. Intern Med. 2016;55(2):121–6.
Resende AL, Elias RM, Wolf M, Dos Reis LM, Graciolli FG, Santos GD, et al. Serum levels of fibroblast growth factor 23 are elevated in patients with active lupus nephritis. Cytokine. 2017;91:124–7.
Yamazaki M, Kawai M, Miyagawa K, Ohata Y, Tachikawa K, Kinoshita S, Nishino J, Ozono K, Michigami T. Interleukin-1-induced acute bone resorption facilitates the secretion of fibroblast growth factor 23 into the circulation. J Bone Miner Metab. 2015;33(3):342–54.
Durlacher-Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh-Many T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94(2):315–25. IL-6 increases FGF23 transcription via STAT3 phosphorylation in AKI and CKD models in mice.
Glosse P, Fajol A, Hirche F, Feger M, Voelkl J, Lang F, Stangl GI, Föller M. A high-fat diet stimulates fibroblast growth factor 23 formation in mice through TNFalpha upregulation. Nutr Diabetes. 2018;8(1):36.
Pathak JL, Bakker AD, Luyten FP, Verschueren P, Lems WF, Klein-Nulend J, Bravenboer N. Systemic inflammation affects human osteocyte-specific protein and cytokine expression. Calcif Tissue Int. 2016;98(6):596–608.
Ito N, Wijenayaka AR, Prideaux M, Kogawa M, Ormsby RT, Evdokiou A, Bonewald LF, Findlay DM, Atkins GJ. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol. 2015;399:208–18.
Han X, Li L, Yang J, King G, Xiao Z, Quarles LD. Counter-regulatory paracrine actions of FGF-23 and 1,25(OH)2 D in macrophages. FEBS Lett. 2016;590(1):53–67.
Zhang B, Yan J, Umbach AT, Fakhri H, Fajol A, Schmidt S, Salker MS, Chen H, Alexander D, Spichtig D, Daryadel A, Wagner CA, Föller M, Lang F. NFkappaB-sensitive Orai1 expression in the regulation of FGF23 release. J Mol Med (Berl). 2016;94(5):557–66.
Feger M, Hase P, Zhang B, Hirche F, Glosse P, Lang F, Föller M. The production of fibroblast growth factor 23 is controlled by TGF-beta2. Sci Rep. 2017;7(1):4982.
Guillaume Courbon CF, Gerber C, Neuburg S, Wang X, Lynch E, Isakova T, Babitt JL, Wolf M, Martin A, David V. Lipocalin 2 stimulates bone fibroblast growth factor 23 production in chronic kidney disease. Bone Res. 2021;9:35. LCN2 is an important mediator of FGF23 induction by inflammation, and not by iron deficiency or high phosphorus in the context of CKD.
Radhakrishnan K, Kim YH, Jung YS, Kim DK, Na SY, Lim D, et al. Orphan nuclear receptor ERR-gamma regulates hepatic FGF23 production in acute kidney injury. Proc Natl Acad Sci U S A. 2021;118(16):e2022841118. IL-6 via ERR-g regulates FGF23 in the liver in mouse models of AKI. There is a correlation between IL6 and FGF23 in patients undergoing cardiac surgery who will develop AKI.
McKnight Q, Jenkins S, Li X, Nelson T, Marlier A, Cantley LG, Finberg KE, Fretz JA. IL-1beta drives production of FGF-23 at the onset of chronic kidney disease in mice. J Bone Miner Res. 2020;35(7):1352–62.
Egli-Spichtig D, Imenez Silva PH, Glaudemans B, Gehring N, Bettoni C, Zhang MYH, Pastor-Arroyo EM, Schönenberger D, Rajski M, Hoogewijs D, Knauf F, Misselwitz B, Frey-Wagner I, Rogler G, Ackermann D, Ponte B, Pruijm M, Leichtle A, Fiedler GM, et al. Tumor necrosis factor stimulates fibroblast growth factor 23 levels in chronic kidney disease and non-renal inflammation. Kidney Int. 2019;96(4):890–905.
Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, et al. The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab. 2010;95(4):1741–8.
Portale AA, Wolf M, Juppner H, Messinger S, Kumar J, Wesseling-Perry K, et al. Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol. 2014;9(2):344–53.
Masuda Y, Ohta H, Morita Y, Nakayama Y, Miyake A, Itoh N, Konishi M. Expression of Fgf23 in activated dendritic cells and macrophages in response to immunological stimuli in mice. Biol Pharm Bull. 2015;38(5):687–93.
Zhang X, Guo K, Xia F, Zhao X, Huang Z, Niu J. FGF23(C-tail) improves diabetic nephropathy by attenuating renal fibrosis and inflammation. BMC Biotechnol. 2018;18(1):33.
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–8.
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51.
Kuro-o M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat Rev Nephrol. 2013;9(11):650–60.
Stubbs JR, Liu S, Tang W, Zhou J, Wang Y, Yao X, Quarles LD. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18(7):2116–24.
Marsell R, Mirza MA, Mallmin H, Karlsson M, Mellstrom D, Orwoll E, et al. Relation between fibroblast growth factor-23, body weight and bone mineral density in elderly men. Osteoporos Int. 2009;20(7):1167–73.
di Giuseppe R, Kuhn T, Hirche F, Buijsse B, Dierkes J, Fritsche A, et al. Potential predictors of plasma fibroblast growth factor 23 concentrations: cross-sectional analysis in the EPIC-Germany study. PLoS One. 2015;10(7):e0133580.
Garland JS, Holden RM, Ross R, Adams MA, Nolan RL, Hopman WM, Morton AR. Insulin resistance is associated with fibroblast growth factor-23 in stage 3-5 chronic kidney disease patients. J Diabetes Complicat. 2014;28(1):61–5.
Vervloet MG, van Zuilen AD, Heijboer AC, ter Wee PM, Bots ML, Blankestijn PJ, et al. Fibroblast growth factor 23 is associated with proteinuria and smoking in chronic kidney disease: an analysis of the MASTERPLAN cohort. BMC Nephrol. 2012;13:20.
Vidal A, Rios R, Pineda C, Lopez I, Munoz-Castaneda JR, Rodriguez M, et al. Direct regulation of fibroblast growth factor 23 by energy intake through mTOR. Sci Rep. 2020;10(1):1795.
Glosse P, Feger M, Mutig K, Chen H, Hirche F, Hasan AA, Gaballa MMS, Hocher B, Lang F, Föller M. AMP-activated kinase is a regulator of fibroblast growth factor 23 production. Kidney Int. 2018;94(3):491–501.
Bar L, Feger M, Fajol A, Klotz LO, Zeng S, Lang F, et al. Insulin suppresses the production of fibroblast growth factor 23 (FGF23). Proc Natl Acad Sci U S A. 2018;115(22):5804–9. Insulin/IGF1-dependent PI3K/PKB/Akt/FOXO1 signaling is suppressor of FGF23 production in vitro and in vivo in mice and in humans.
Riley MS, Schade DS, Eaton RP. Effects of insulin infusion on plasma phosphate in diabetic patients. Metabolism. 1979;28(3):191–4.
Yeung SMH, Bakker SJL, Laverman GD, De Borst MH. Fibroblast growth factor 23 and adverse clinical outcomes in type 2 diabetes: a bitter-sweet symphony. Curr Diab Rep. 2020;20(10):50.
Simic P, Kim W, Zhou W, Pierce KA, Chang W, Sykes DB, Aziz NB, Elmariah S, Ngo D, Pajevic PD, Govea N, Kestenbaum BR, de Boer IH, Cheng Z, Christov M, Chun J, Leaf DE, Waikar SS, Tager AM, et al. Glycerol-3-phosphate is an FGF23 regulator derived from the injured kidney. J Clin Invest. 2020;130(3):1513–26. Glycerol-3-phosphate, by-product of glycolysis, is secreted from the kidney in response to AKI and regulates FGF23 in the bone and bone marrow.
He Q, Shumate LT, Matthias J, Aydin C, Wein MN, Spatz JM, et al. A G protein-coupled, IP3/protein kinase C pathway controlling the synthesis of phosphaturic hormone FGF23. JCI Insight. 2019;4(17):e125007. A G protein-coupled, IP3/protein kinase C pathway is required for the synthesis of FGF23 in mice.
Matthias J, Cui Q, Shumate LT, Plagge A, He Q, Bastepe M. Extra-large galpha protein (XLalphas) deficiency causes severe adenine-induced renal injury with massive FGF23 elevation. Endocrinology. 2020;161(1):bqz025.
Funding
PS is supported by the National Institutes of Health grant K08DK124568. JLB is supported by the National Institutes of Health grants RO1-DK087727 and R01-DK128068 and the Patricia and Scott Eston Massachusetts General Hospital Research Scholar Award.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
This review article does not present any previously unpublished original research, and ethical approval is therefore not applicable.
Conflict of Interest
JLB has been a consultant for Incyte Corporation and Alnylam Pharmaceuticals and owns equity in Ferrumax Pharmaceuticals, a company focused on targeting RGM proteins (including hemojuvelin) and bone morphogenetic protein (BMP/TGF-beta) superfamily signaling as hepcidin modulating agents for the treatment of anemia and other iron disorders. Dr. Babitt’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict of interest policies. PS declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Kidney and Bone
Rights and permissions
About this article
Cite this article
Simic, P., Babitt, J.L. Regulation of FGF23: Beyond Bone. Curr Osteoporos Rep 19, 563–573 (2021). https://doi.org/10.1007/s11914-021-00703-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-021-00703-w