Abstract
Purpose of Review
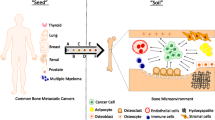
Skeletal metastasis involves the uncoupling of physiologic bone remodeling resulting in abnormal bone turnover and radical changes in bony architecture, density, and quality. Bone strength assessment and fracture risk prediction are critical in clinical treatment decision-making. This review focuses on bone tissue and structural mechanisms altered by osteolytic metastasis and the resulting changes to its material and mechanical behavior.
Recent Findings
Both organic and mineral phases of bone tissue are altered by osteolytic metastatic disease, with diminished bone quality evident at multiple length-scales. The mechanical performance of bone with osteolytic lesions is influenced by a combination of tissue-level and structural changes.
Summary
This review considers the effects of osteolytic metastasis on bone biomechanics demonstrating its negative impact at tissue and structural levels. Future studies need to assess the cumulative impact of cancer treatments on metastatically involved bone quality, and its utility in directing multimodal treatment planning.
Similar content being viewed by others
References
Janjan N. Bone metastases: approaches to management. Semin Oncol. 2001;28:28–34.
Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–76.
Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321.
Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64.
Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–90.
Hoefeler H, Duran I, Hechmati G, Rodriguez CG, Lüftner D, Ashcroft J, et al. Health resource utilization associated with skeletal-related events in patients with bone metastases: results from a multinational retrospective - prospective observational study - a cohort from 4 european countries. J Bone Oncol. 2014;3:40–8.
Hussain A, Lee RJ, Graff JN, Halabi S. The evolution and understanding of skeletal complication endpoints in clinical trials of tumors with metastasis to the bone. Crit Rev Oncol Hematol. 2019;139:108–16.
Vassiliou V, Kalogeropoulou C, Petsas T, Leotsinidis M, Kardamakis D. Clinical and radiological evaluation of patients with lytic, mixed and sclerotic bone metastases from solid tumors: is there a correlation between clinical status of patients and type of bone metastases? Clin Exp Metastasis. 2007;24:49–56.
O’Sullivan GJ. Imaging of bone metastasis: an update. World J Radiol. 2015;7:202.
Singh VA, Haseeb A, Alkubaisi AAHA. Incidence and outcome of bone metastatic disease at University Malaya Medical Centre. Singapore Med J. 2014;55:539–46.
Burke MV, Atkins A, Akens M, Willett TL, Whyne CM. Osteolytic and mixed cancer metastasis modulates collagen and mineral parameters within rat vertebral bone matrix. J Orthop Res. 2016;34:2126–36.
Fohr B, Dunstan CR, Seibel MJ. Markers of bone remodeling in metastatic bone disease. J Clin Endocrinol Metab. 2003;88:5059–75.
Gilkes DM, Chaturvedi P, Bajpai S, Wong CC, Wei H, Pitcairn S, et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 2013;73:3285–96.
Pathi SP, Lin DDW, Dorvee JR, Estroff LA, Fischbach C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials. 2011;32:5112–22.
Won E, Wise-Milestone L, Akens MK, Burch S, Yee AJM, Wilson BC, et al. Beyond bisphosphonates: photodynamic therapy structurally augments metastatically involved vertebrae and destroys tumor tissue. Breast Cancer Res Treat. 2010;124:111–9.
Burke M, Atkins A, Kiss A, Akens M, Yee A, Whyne C. The impact of metastasis on the mineral phase of vertebral bone tissue. J Mech Behav Biomed Mater. 2017;69:75–84.
Choudhari C, Chan K, Akens MK, Whyne CM. μFE models can represent microdamaged regions of healthy and metastatically involved whole vertebrae identified through histology and contrast enhanced μCT imaging. J Biomech. 2016;49:1103–10.
Burke M, Akens M, Kiss A, Willett T, Whyne C. Mechanical behavior of metastatic vertebrae are influenced by tissue architecture, mineral content, and organic feature alterations. J Orthop Res. 2018;36:3013–22.
Nazarian A, Von Stechow D, Zurakowski D, Müller R, Snyder BD. Bone volume fraction explains the variation in strength and stiffness of cancellous bone affected by metastatic cancer and osteoporosis. Calcif Tissue Int. 2008;83:368–79.
Hardisty MR, Akens MK, Hojjat SP, Yee A, Whyne CM. Quantification of the effect of osteolytic metastases on bone strain within whole vertebrae using image registration. J Orthop Res. 2012;30:1032–9.
Hojjat SP, Foltz W, Wise-Milestone L, Whyne CM. Multimodal μcT/μMR based semiautomated segmentation of rat vertebrae affected by mixed osteolytic/osteoblastic metastases. Med Phys. 2012;39:2848–53.
Alexander GE, Gutierrez S, Nayak A, Palumbo BT, Cheong D, Letson GD, et al. Biomechanical model of a high risk impending pathologic fracture of the femur: lesion creation based on clinically implemented scoring systems. Clin Biomech. 2013;28:408–14.
Morton JJ, Bennison M, Lievers WB, Waldman SD, Pilkey AK. Failure behaviour of rat vertebrae determined through simultaneous compression testing and micro-CT imaging. J Mech Behav Biomed Mater. 2018;79:73–82.
Kaneko TS, Bell JS, Pejcic MR, Tehranzadeh J, Keyak JH. Mechanical properties, density and quantitative CT scan data of trabecular bone with and without metastases. J Biomech. 2004;37:523–30.
Lee T. Predicting failure load of the femur with simulated osteolytic defects using noninvasive imaging technique in a simplified load case. Ann Biomed Eng. 2007;35:642–50.
Mann KA, Lee J, Arrington SA, Damron TA, Allen MJ. Predicting distal femur bone strength in a murine model of tumor osteolysis. Clin Orthop Relat Res. 2008;466:1271–8.
Benca E, Reisinger A, Patsch JM, Hirtler L, Synek A, Stenicka S, et al. Effect of simulated metastatic lesions on the biomechanical behavior of the proximal femur. J Orthop Res. 2017;35:2407–14.
Tschirhart CE, Finkelstein JA, Whyne CM. Biomechanics of vertebral level, geometry, and transcortical tumors in the metastatic spine. J Biomech. 2007;40:46–54.
Entezari V, Basto PA, Vartanians V, Zurakowski D, Snyder BD, Nazarian A. Non-invasive assessment of failure torque in rat bones with simulated lytic lesions using computed tomography based structural rigidity analysis. J Biomech. 2011;44:552–6.
Arrington SA, Schoonmaker JE, Damron TA, Mann KA, Allen MJ. Temporal changes in bone mass and mechanical properties in a murine model of tumor osteolysis. Bone. 2006;38:359–67.
Hong J, Cabe GD, Tedrow JR, Hipp JA, Snyder BD. Failure of trabecular bone with simulated lytic defects can be predicted non-invasively by structural analysis. J Orthop Res. 2004;22:479–86.
Alkalay RN, Harrigan TP. Mechanical assessment of the effects of metastatic lytic defect on the structural response of human thoracolumbar spine. J Orthop Res. 2016;34:1808–19.
Oftadeh R, Karimi Z, Villa-Camacho J, Tanck E, Verdonschot N, Goebel R, et al. Curved beam computed tomography based structural rigidity analysis of bones with simulated lytic defect: a comparative study with finite element analysis. Sci Rep. 2016;6:1–12.
Derikx LC, van Aken JB, Janssen D, Snyers A, van der Linden YM, Verdonschot N, et al. The assessment of the risk of fracture in femora with metastatic lesions. J Bone Joint Surg Br. 2012;94-B:1135–42.
Anez-Bustillos L, Derikx LC, Verdonschot N, Calderon N, Zurakowski D, Snyder BD, et al. Finite element analysis and CT-based structural rigidity analysis to assess failure load in bones with simulated lytic defects. Bone. 2014;58:160–7.
Tanck E, van Aken JB, van der Linden YM, Schreuder HWWB, Binkowski M, Huizenga H, et al. Pathological fracture prediction in patients with metastatic lesions can be improved with quantitative computed tomography based computer models. Bone. 2009;45:777–83.
Mirzaei M, Zeinali A, Razmjoo A, Nazemi M. On prediction of the strength levels and failure patterns of human vertebrae using quantitative computed tomography (QCT)-based finite element method. J Biomech. 2009;42:1584–91.
Burke M, Golaraei A, Atkins A, Akens M, Barzda V, Whyne C. Collagen fibril organization within rat vertebral bone modified with metastatic involvement. J Struct Biol. 2017;199:153–64.
Sekita A, Matsugaki A, Ishimoto T, Nakano T. Synchronous disruption of anisotropic arrangement of the osteocyte network and collagen/apatite in melanoma bone metastasis. J Struct Biol. 2017;197:260–70.
Molla MS, Katti DR, Iswara J, Venkatesan R, Paulmurugan R, Katti KS. Prostate cancer phenotype influences bone mineralization at metastasis: a study using an in vitro prostate cancer metastasis testbed. JBMR Plus. 2020;4:e10256.
Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006;17:1514–23.
Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, Van Der Ham F, et al. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005;37:825–32.
Oxlund H, Barckman M, Ørtoft G, Andreassen TT. Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone. 1995;17:365–71.
Boskey AL, Spevak L, Weinstein RS. Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos Int. 2009;20:793–800.
Renders GAP, Mulder L, van Ruijven LJ, Langenbach GEJ, van Eijden TMGJ. Mineral heterogeneity affects predictions of intratrabecular stress and strain. J Biomech. 2011;44:402–7.
Bailey S, Vashishth D. Mechanical characterization of bone: state of the art in experimental approaches - what types of experiments do people do and how does one interpret the results? Curr Osteoporos Rep. 2018;16:423–33.
Hernandez CJ, Keaveny TM. A biomechanical perspective on bone quality. Bone. 2006;39:1173–81.
Martin RB, Boardman DL. The effects of collagen fiber orientation, porosity, density, and mineralization on bovine cortical bone bending properties. J Biomech. 1993;26:1047–54.
Wang X, Shen X, Li X, Mauli AC. Age-related changes in the collagen network and toughness of bone. Bone. 2002;31:1–7.
Zimmermann EA, Busse B, Ritchie RO. The fracture mechanics of human bone: influence of disease and treatment. Bonekey Rep. 2015;4:743.
Launey ME, Buehler MJ, Ritchie RO. On the mechanistic origins of toughness in bone. Annu Rev Mater Res. 2010;40:25–53.
Zysset PK, Edward Guo X, Edward Hoffler C, Moore KE, Goldstein SA. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J Biomech. 1999;32:1005–12.
Lau ML, Lau KT, Yao Yeo YD, Au Yeung CT, Lee JH. Measurement of bovine bone properties through surface indentation technique. Mater Manuf Process. 2010;25:324–8.
Kawamura M, Masaki C, Shibata Y, Kondo Y, Mukaibo T, Miyazaki T, et al. Pentosidine correlates with nanomechanical properties of human jaw bone. J Mech Behav Biomed Mater. 2019;98:20–5.
Burton B, Gaspar A, Josey D, Tupy J, Grynpas MD, Willett TL. Bone embrittlement and collagen modifications due to high-dose gamma-irradiation sterilization. Bone. 2014;61:71–81.
Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17:319–36.
Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, et al. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39:1073–9.
Nass N, Ignatov A, Andreas L, Weißenborn C, Kalinski T, Sel S. Accumulation of the advanced glycation end product carboxymethyl lysine in breast cancer is positively associated with estrogen receptor expression and unfavorable prognosis in estrogen receptor-negative cases. Histochem Cell Biol. 2017;147:625–34.
Chen Y, Terajima M, Yang Y, Sun L, Ahn YH, Pankova D, et al. Lysyl hydroxylase 2 induces a collagen cross-link switch in tumor stroma. J Clin Invest. 2015;125:1147–62.
Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–96.
Wise-Milestone L, Akens MK, Rosol TJ, Hojjat SP, Grynpas MD, Whyne CM. Evaluating the effects of mixed osteolytic/osteoblastic metastasis on vertebral bone quality in a new rat model. J Orthop Res. 2012;30:817–23.
He F, Chiou AE, Loh HC, Lynch M, Seo BR, Song YH, et al. Multiscale characterization of the mineral phase at skeletal sites of breast cancer metastasis. Proc Natl Acad Sci USA. 2017;114(40):10542–7.
Zhu W, Wang M, Fu Y, Castro NJ, Fu SW, Zhang LG. Engineering a biomimetic three-dimensional nanostructured bone model for breast cancer bone metastasis study. Acta Biomater. 2015;14:164–74.
Bi X, Sterling JA, Merkel AR, Perrien DS, Nyman JS, Mahadevan-Jansen A. Prostate cancer metastases alter bone mineral and matrix composition independent of effects on bone architecture in mice - a quantitative study using microCT and raman spectroscopy. Bone. 2013;56:454–60.
Ibrahim T, Leong I, Sanchez-Sweatman O, Khokha R, Sodek J, Tenenbaum HC, et al. Expression of bone sialoprotein and osteopontin in breast cancer bone metastases. Clin Exp Metastasis. 2000;18:253–60.
Nyman JS, Granke M, Singleton RC, Pharr GM. Tissue-level mechanical properties of bone contributing to fracture risk. Curr Osteoporos Rep. 2016;14:138–50.
Richert L, Keller L, Wagner Q, Bornert F, Gros C, Bahi S, et al. Nanoscale stiffness distribution in bone metastasis. World J Nano Sci Eng. 2015;05:219–28.
Mullins LP, Bruzzi MS, McHugh PE. Measurement of the microstructural fracture toughness of cortical bone using indentation fracture. J Biomech. 2007;40:3285–8.
Kruzic JJ, Kim DK, Koester KJ, Ritchie RO. Indentation techniques for evaluating the fracture toughness of biomaterials and hard tissues. J Mech Behav Biomed Mater. 2009;2:384–95.
Ural A, Vashishth D. Cohesive finite element modeling of age-related toughness loss in human cortical bone. J Biomech. 2006;39:2974–82.
Morais JJL, de Moura MFSF, Pereira FAM, Xavier J, Dourado N, Dias MIR, et al. The double cantilever beam test applied to mode I fracture characterization of cortical bone tissue. J Mech Behav Biomed Mater. 2010;3:446–53.
Tertuliano OA, Greer JR. The nanocomposite nature of bone drives its strength and damage resistance. Nat Mater. 2016;15:1195–202.
Schwiedrzik J, Raghavan R, Bürki A, Lenader V, Wolfram U, Michler J, et al. In situ micropillar compression reveals superior strength and ductility but an absence of damage in lamellar bone. Nat Mater. 2014;13:740–7.
Kataruka A, Mendu K, Okeoghene O, Puthuvelil J, Akono AT. Microscopic assessment of bone toughness using scratch tests. Bone Reports. 2017;6:17–25.
Mendu K, Kataruka A, Puthuvelil J, Akono AT. Fragility assessment of bovine cortical bone using scratch tests. J Vis Exp. 2017;2017(129):56488.
Islam A, Neil Dong X, Wang X. Mechanistic modeling of a nanoscratch test for determination of in situ toughness of bone. J Mech Behav Biomed Mater. 2012;5:156–64.
Chapurlat RD, Delmas PD. Bone microdamage: a clinical perspective. Osteoporos Int. 2009;20:1299–308.
Diab T, Vashishth D. Effects of damage morphology on cortical bone fragility. Bone. 2005;37:96–102.
Atkins A, Burke M, Samiezadeh S, Akens MK, Hardisty M, Whyne CM. Elevated microdamage spatially correlates with stress in metastatic vertebrae. Ann Biomed Eng. 2019;47:980–9.
Shah LM, Salzman KL. Imaging of spinal metastatic disease. Int J Surg Oncol. 2011;2011:1–12.
Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–53.
Heindel W, Gübitz R, Vieth V, Weckesser M, Schober O, Schäfers M. The diagnostic imaging of bone metastases. Dtsch Arztebl Int. 2014;111:741–7.
Whealan K, Kwak D, Tedrow JR, Inoue K, Snyder BD. Noninvasive imaging predicts failure load of the spine with simulated osteolytic defects. J Bone Jt Surgery. 2000;326–328:811–4.
Hojjat S-PP, Whyne CM. Automated quantitative microstructural analysis of metastatically involved vertebrae: effects of stereologic model and spatial resolution. Med Eng Phys. 2011;33:188–94.
Alkalay RN. Effect of the metastatic defect on the structural response and failure process of human vertebrae: an experimental study. Clin Biomech. 2015;30:121–8.
Skrinskas T, Clemons M, Freedman O, Weller I, Whyne CM. Automated CT-based analysis to detect changes in the prevalence of lytic bone metastases from breast cancer. Clin Exp Metastasis. 2009;26:97–103.
Hill ME, Richards MA, Gregory WM, Smith P, Rubens RD. Spinal cord compression in breast cancer: a review of 70 cases. Br J Cancer. 1993;68:969–73.
Cunha MVR, Al-Omair A, Atenafu EG, Masucci GL, Letourneau D, Korol R, et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int J Radiat Oncol Biol Phys. 2012;84:343–9.
Whyne CM, Hu SS, Lotz JC. Burst fracture in the metastatically involved spine: development, validation, and parametric analysis of a three- dimensional poroelastic finite-element model. Spine (Phila Pa 1976). 2003;28:652–60.
Tschirhart CE, Nagpurkar A, Whyne CM. Effects of tumor location, shape and surface serration on burst fracture risk in the metastatic spine. J Biomech. 2004;37:653–60.
Campbell JQ, Petrella AJ. Automated finite element modeling of the lumbar spine: using a statistical shape model to generate a virtual population of models. J Biomech. 2016;49:2593–9.
Hardisty M, Gordon L, Agarwal P, Skrinskas T, Whyne C. Quantitative characterization of metastatic disease in the spine. Part I. Semiautomated segmentation using atlas-based deformable registration and the level set method. Med Phys. 2007;34:3127–34.
Whyne C, Hardisty M, Wu F, Skrinskas T, Clemons M, Gordon L, et al. Quantitative characterization of metastatic disease in the spine. Part II. Histogram-based analyses. Med Phys. 2007;34:3279–85.
Klein G, Martel A, Sahgal A, Whyne C. Hardisty M. In: Cai Y, Wang L, Audette M, Zheng G, Li S, editors. Computational methods and clinical applications for spine imaging: metastatic vertebrae segmentation for use in a clinical pipeline. Shenzhen, China: Springer; 2019. p. 15–28.
Clement A. Image based and biomechanical characterization of osteoblastic vertebral metastases [master’s dissertation]. Toronto: [Toronto, ON]: University of Toronto; 2019.
Samiezadeh S, Ebrahimi H, Tong F, Whyne C. A computational model of the post-yield response of vertebral bone using zero-thickness cohesive elements. Poster presented at: Orthopaedic Research Society Annual Meeting. United States: San Diego; 2017 Mar 20.
Boehling NS, Grosshans DR, Allen PK, McAleer MF, Burton AW, Azeem S, et al. Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases: clinical article. J Neurosurg Spine. 2012;16:379–86.
Sahgal A, Atenafu EG, Chao S, Al-Omair A, Boehling N, Balagamwala EH, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the spinal instability Neoplastic score. J Clin Oncol. 2013;31:3426–31.
Bilsky MH, Laufer I, Fourney DR, Groff M, Schmidt MH, Varga PP, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine. 2010;13:324–8.
Fisher CG, Dipaola CP, Ryken TC, Bilsky MH, Shaffrey CI, Berven SH, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the spine oncology study group. Spine (Phila Pa 1976). 2010;35:1221–9.
Joaquim AF, Fernandes YB, Cavalcante RAC, Fragoso RM, Honorato DC, Patel AA. Evaluation of the thoracolumbar injury classification system in thoracic and lumbar spinal trauma. Spine (Phila Pa 1976). 2011;36:33–6.
Fourney DR, Frangou EM, Ryken TC, DiPaola CP, Shaffrey CI, Berven SH, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the Spine Oncology Study Group. J Clin Oncol. 2011;29:3072–7.
Versteeg AL, Verlaan JJ, Sahgal A, Mendel E, Quraishi NA, Fourney DR, et al. The spinal instability neoplastic score: impact on oncologic decision-making. Spine (Phila Pa 1976). 2016;41:S231–7.
Damron TA, Nazarian A, Entezari V, Brown C, Grant W, Calderon N, et al. CT-based structural rigidity analysis is more accurate than Mirels scoring for fracture prediction in metastatic femoral lesions. Clin Orthop Relat Res. 2016;474:643–51.
Snyder BD, Cordio MA, Nazarian A, Kwak SD, Chang DJ, Entezari V, et al. Noninvasive prediction of fracture risk in patients with metastatic cancer to the spine. Clin Cancer Res. 2009;15:7676–83.
Thibault I, Whyne CM, Zhou S, Campbell M, Atenafu EG, Myrehaug S, et al. Volume of lytic vertebral body metastatic disease quantified using computed tomography - based image segmentation predicts fracture risk after spine stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2017;97:75–81.
Hoff B, Toole M, Yablon C, Ross B, Luker G, VanPoznak C, et al. Potential for early fracture risk assessment in patients with metastatic bone disease using parametric response mapping of CT images. Tomography. 2015;1:98–104.
Hardisty M, Wright T, Campbell M, Burke M, Atenafu E, Cawricz M, et al. CT based quantitative measures of the stability of fractured metastatically involved vertebrae treated with spine stereotactic body radiotherapy. Clin Exp Metastasis. 2020;37(5):575–84.
Sternheim A, Giladi O, Gortzak Y, Drexler M, Salai M, Trabelsi N, et al. Pathological fracture risk assessment in patients with femoral metastases using CT-based finite element methods. A retrospective clinical study. Bone. 2018;110:215–20.
Janssen S, Paulino Pereira N, Meijs T, Bredella M, Ferrone M, van Dijk C, et al. Predicting pathological fracture in femoral metastases using a clinical CT scan based algorithm: a case-control study. J Orthop Sci. 2018;23:394–402.
Eggermont F, van der Wal G, Westhoff P, Laar A, de Jong M, Rozema T, et al. Patient-specific finite element computer models improve fracture risk assessments in cancer patients with femoral bone metastases compared to clinical guidelines. Bone. 2020;130:115101.
Roth SE, Mousavi P, Finkelstein J, Chow E, Kreder H, Whyne CM. Metastatic burst fracture risk prediction using biomechanically based equations. Clin Orthop Relat Res. 2004;419:83–90.
Galbusera F, Casaroli G, Bassani T. Artificial intelligence and machine learning in spine research. JOR SPINE. 2019;2:e1044.
Ramos JS, Nogueira-Barbosa MH, Watanabe CYV, Traina AJM. B Growth: an efficient approach for the segmentation of vertebral compression fractures in magnetic resonance imaging. Proc ACM Symp Appl Comput. 2019;(Part F1477):220–7.
Jakubicek R, Chmelik J, Jan J, Ourednicek P, Lambert L, Gavelli G. Learning–based vertebra localization and labeling in 3D CT data of possibly incomplete and pathological spines. Comput Methods Programs Biomed. 2020;183:105081.
Rose PS, Laufer I, Boland PJ, Hanover A, Bilsky MH, Yamada J, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075–9.
Loi M, Nuyttens JJ, Desideri I, Greto D, Livi L. Single-fraction radiotherapy (SFRT) for bone metastases: patient selection and perspectives. Cancer Manag Res. 2019;11:9397–408.
Faruqi S, Tseng C-LL, Whyne C, Alghamdi M, Wilson J, Myrehaug S, et al. Vertebral compression fracture after spine stereotactic body radiation therapy: a review of the pathophysiology and risk factors. Clin Neurosurg. 2018;83:314–22.
Sprave T, Verma V, Förster R, Schlampp I, Hees K, Bruckner T, et al. Local response and pathologic fractures following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy for spinal metastases - a randomized controlled trial. BMC Cancer. 2018;18:4777–8.
Wernle JD, Damron TA, Allen MJ, Mann KA. Local irradiation alters bone morphology and increases bone fragility in a mouse model. J Biomech. 2010;43:2738–46.
Soares PBF, Soares CJ, Limirio PHJO, de Jesus RNR, Dechichi P, Spin-Neto R, et al. Effect of ionizing radiation after-therapy interval on bone: histomorphometric and biomechanical characteristics. Clin Oral Investig. 2019;23:2785–93.
Ehresman J, Schilling A, Pennington Z, Gui C, Chen X, Lubelski D, et al. A novel MRI-based score assessing trabecular bone quality to predict vertebral compression fractures in patients with spinal metastasis. J Neurosurg Spine. 2020;32:499–506.
Savaridas T, Wallace RJ, Dawson S, Simpson AHRW. Effect of ibandronate on bending strength and toughness of rodent cortical bone: possible implications for fracture prevention. Bone Jt Res. 2015;4:99–104.
Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20:887–94.
Acevedo C, Bale H, Gludovatz B, Wat A, Tang SY, Wang M, et al. Alendronate treatment alters bone tissues at multiple structural levels in healthy canine cortical bone. Bone. 2015;81:352–63.
Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008;19:329–37.
Jin A, Cobb J, Hansen U, Bhattacharya R, Reinhard C, Vo N, et al. The effect of long-term bisphosphonate therapy on trabecular bone strength and microcrack density. Bone Jt Res. 2017;6:602–9.
Pazianas M, van der Geest S, Miller P. Bisphosphonates and bone quality. Bonekey Rep. 2014;3:529.
Pelker RR, Friedlaender GE, Panjabi MM, Markham T, Hausman M, Doganis AC, et al. Chemotherapy-induced alterations in the biomechanics of rat bone. J Orthop Res. 1985;3:91–5.
Quach JM, Askmyr M, Jovic T, Baker EK, Walsh NC, Harrison SJ, et al. Myelosuppressive therapies significantly increase pro-inflammatory cytokines and directly cause bone loss. J Bone Miner Res. 2015;30:886–97.
Hall TJ, Jeker H, Schaueblin M. Taxol inhibits osteoclastic bone resorption. Calcif Tissue Int. 1995;57:463–5.
Chung YS, Kang HC, Lee T. Comparative effects of ibandronate and paclitaxel on immunocompetent bone metastasis model. Yonsei Med J. 2015;56:1643–50.
Won E, Akens MK, Hardisty MR, Burch S, Bisland SK, Yee AJM, et al. Effects of photodynamic therapy on the structural integrity of vertebral bone. Spine (Phila Pa 1976). 2010;35:272–7.
Iolascon G, Napolano R, Gioia M, Moretti A, Riccio I, Gimigliano F. The contribution of cortical and trabecular tissues to bone strength: Insights from denosumab studies. Clin Cases Miner Bone Metab. 2013;10:47–51.
Castellano D, Sepulveda JM, García-Escobar I, Rodriguez-Antolín A, Sundlöv A, Cortes-Funes H. The role of RANK-ligand inhibition in cancer: the story of denosumab. Oncologist. 2011;16:136–45.
Dougall WC, Holen I, González SE. Targeting RANKL in metastasis. Bonekey Rep. 2014;3:519.
Dempster DW, Lambing CL, Kostenuik PJ, Grauer A. Role of RANK ligand and denosumab, a targeted RANK ligand inhibitor, in bone health and osteoporosis: a review of preclinical and clinical data. Clin Ther. 2012;34:521–36.
Lo VCK, Akens MK, Wise-Milestone L, Yee AJM, Wilson BC, Whyne CM. The benefits of photodynamic therapy on vertebral bone are maintained and enhanced by combination treatment with bisphosphonates and radiation therapy. J Orthop Res. 2013;31:1398–405.
Arrington SA, Damron TA, Mann KA, Allen MJ. Concurrent administration of zoledronic acid and irradiation leads to improved bone density, biomechanical strength, and microarchitecture in a mouse model of tumor-induced osteolysis. J Surg Oncol. 2007;97:284–90.
O’Carrigan B, Wong MHF, Willson ML, Stockler MR, Pavlakis N, Goodwin A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2017;10:CD003474.
Lockwood M, Banderudrappagari R, Suva LJ, Makhoul I. Atypical femoral fractures from bisphosphonate in cancer patients – review. J Bone Oncol. 2019;18:100259.
Ota S, Inoue R, Shiozaki T, Yamamoto Y, Hashimoto N, Takeda O, et al. Atypical femoral fracture after receiving antiresorptive drugs in breast cancer patients with bone metastasis. Breast Cancer. 2017;24:601–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
I, Cari Whyne, on behalf of all authors, declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomechanics
Rights and permissions
About this article
Cite this article
Whyne, C.M., Ferguson, D., Clement, A. et al. Biomechanical Properties of Metastatically Involved Osteolytic Bone. Curr Osteoporos Rep 18, 705–715 (2020). https://doi.org/10.1007/s11914-020-00633-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00633-z