Abstract
Purpose of Review
To provide insights into the role of peptide receptor radionuclide therapy (PRRT) in patients with advanced neuroendocrine tumors (NET) and an overview of possible strategies to combine PRRT with locoregional and systemic anticancer treatments.
Recent Findings
Research on combining PRRT with other treatments encompasses a wide variety or treatments, both local (transarterial radioembolization) and systemic therapies, chemotherapy (i.e., capecitabine and temozolomide), targeted therapies (i.e., olaparib, everolimus, and sunitinib), and immunotherapies (e.g., nivolumab and pembrolizumab). Furthermore, PRRT shows promising first results as a treatment prior to surgery.
Summary
There is great demand to enhance the efficacy of PRRT through combination with other anticancer treatments. While research in this area is currently limited, the field is rapidly evolving with numerous ongoing clinical trials aiming to address this need and explore novel therapeutic combinations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroendocrine tumors are a broad class of malignancies that can be omnipresent throughout the body but are most commonly found in the gastro-intestinal tract (GEP-NET) and lung. Although regularly classified as rare malignancy, their incidence is increasing worldwide due to an increased awareness and improved diagnostic capabilities to detect NET lesions. Surgical resection of the primary tumor and regional lymph nodes is still the only curative treatment; however, more than 40% of patients initially present with metastatic and advanced disease. At this stage, the disease is considered non-curative, so treatment focusses on controlling tumor volume, reduce tumor related side-effects, improve quality of live (QoL), and prolong survival [1, 2]. In patients with advanced well-differentiated somatostatin-receptor (SSTR) positive (functional) NETs, somatostatin analogs (SSA) are generally recommended as first-line treatment. In patients who progress on SSA, there are a number of systemic therapies that can be considered, including cytotoxic chemotherapy, multityrosine kinase inhibitors (mTKI), mammalian target of rapamycin inhibitors (mTORi), or peptide receptor radionuclide therapy (PRRT) [3, 4].
PRRT has been under development for over two decades as therapy for patients with advanced (metastatic) SSTR-positive tumors. PRRT is a form of systemic radiotherapy that relies on accumulation of a radiolabeled molecules in a cell. This local (internal) irradiation of cells will lead to irreversible damage to sensitive cellular structures, such as mitochondria or DNA, followed by apoptosis. Despite extensive data on disease control using PRRT in (retrospective) clinical cohorts, therapeutic efficacy over standard-of-care (SSA) was proven in the phase 3 randomized controlled trial, NETTER-1 [5•]. The benefit in QoL and progression-free survival after PRRT plus SSA over high-dose SSA-alone led to regulatory approvals and implementation of PRRT into earlier treatment lines in the recent guidelines [2, 6].
While various systemic therapies can be considered for patients with advanced (metastatic) NET, only limited phase 2–3 studies offer a head-to-head comparison of treatments. Additionally, clinical evidence on potential synergistic effects associated with combining treatments is scarce. This review will provide insights into the current role of PRRT in patients with advanced NET and an overview of possible strategies to combine PRRT with locoregional and systemic anticancer treatments that have been described in literature.
Peptide Targeted Radionuclide Therapies
Ideal for therapeutic radiopharmaceuticals is to have a cellular target that is highly overexpressed on malignant cells and with limited or absent expression in healthy tissues. For NETs, two molecules were initially developed: metaiodobenzylguanidine (mIBG; norepinephrine analog) and octreotide (one of the SSAs) [7,8,9,10]. The role of mIBG in patients with GEP-NET has subsided over the years due to its less favorable tumor targeting, toxicity profile and pharmacokinetic behavior, as compared to PRRT [11].
The first PRRT involved the use high-activity [111Indium]In-DTPA0-octreotide ([111In]In-pentetreotide) in 1994 [12, 13]. This radiopharmaceutical, widely utilized for diagnostic imaging, primarily has γ-emissions, but limited emission of (Auger/conversion-)electrons that can induce the therapeutic effects (see Table 1). Advances in the past decade led to the development of improved SSAs with increased affinity for specific subtypes of somatostatin receptors (SSTR), thereby improving tumor targeting and reducing accumulation in healthy tissues. Together with the introduction of novel bifunctional chelating agents (enabling stable binding of therapeutic radiometals; e.g., Yttrium-90 (90Y) or Lutetium-177 (177Lu)), this has led to the production of radiopharmaceuticals with superior characteristics (e.g. DOTATOC and DOTATATE). These radiopharmaceuticals had high tumor affinity, limited accumulation in non-target organs, high stability of the complex in vivo, and flexibility to label either diagnostic or therapeutic nuclides to similar targeting molecules [7].
[90Y]Y-DOTATOC should have improved tumor response compared to [111In]In-pentetreotide, since initial simulations showed that the median absorbed dose to tumors could be ten times higher compared to 111In. In addition, bone marrow toxicity should be less as the simulations showed substantially lower absorbed doses to the red marrow for [90Y]Y-DOTATOC (factor 3.5). The major benefit of 90Y is its high beta-energy (Emax 2.28 MeV) and long physical half-life (T1/2 64 h), allowing for longer penetration depths in tissue and longer irradiation, thus causing more cellular damage as compared to 111In [10, 14]. Despite being generally well tolerated, [90Y]Y-DOTATOC therapy’s increased penetration depth and significant retention in the proximal renal tubules may lead to considerable absorbed renal dose and an increased risk of developing (late) renal toxicities [15,16,17]. Unfortunately, phase-3 studies are still lacking.
The subsequent promising analog to be developed was 177Lutetium-labeled DOTATATE ([177Lu]Lu-DOTATATE), which has a six- to nine-fold higher affinity for SSTR2 and a longer tumor retention time compared to DOTATOC [18]. As a result, the radiation dose to kidneys and red marrow is far less for [177Lu]Lu-DOTATATE, with a median of 0.9–1 Gray per gigabecquerel administered activity (Gy/GBq) and 0.02–0.08 Gy/GBq, versus 1.7–6.1 Gy/GBq and 0.3–0.17 Gy/GBq for [90Y]Y-DOTATOC respectively [19, 20]. The absorbed dose to the tumor lesions shows similar results, 3.4–10 Gy/GBq for [177Lu]Lu-DOTATATE and 2.0–16.0 Gy/GBq for [90Y]Y-DOTATOC. Concurrent infusion of an amino-acid solution (e.g., combination of lysine and arginine) with PRRT nowadays reduces the absorbed kidney dose as this mixture limits retention in the proximal renal tubules [21,22,23].
The randomized-controlled phase III NETTER-1 trial proved the efficacy of [177Lu]Lu-DOTATATE plus long-acting SSA in patients with metastatic GEP-NETs, and its pivotal role in treatment was solidified. Patients with inoperable, well-differentiated midgut NETs (grade 1–2) with positive lesions on [111In]In-pentetreotide were randomized to receive either four cycles of 7.4 GBq [177Lu]Lu-DOTATATE every eight weeks plus concomitant long-acting octreotide (30 mg), versus high-dose of long-acting octreotide (60 mg) every four weeks. In the primary analysis, median progression-free survival after 20 months was significantly prolonged in patients treated with [177Lu]Lu-DOTATATE with a hazard ration (HR) of 0.18 (95% CI 0.11–0.29; p > 0.0001) [24]. Final analysis after 5 years (median follow-up > 76 months) showed a median survival of 48 months in the PRRT-group (n = 116) and 36.3 months in the control arm (n = 113), with a HR of 0.84 (95% CI 0.60–1.14; p = 0.30) [5•]. The longer follow-up did not translate into a significant overall survival difference, however, these outcomes are impacted by the high rate (36%) of crossover of patients from the control group towards the PRRT-group after disease progression [5•, 24]. Still, it can be concluded that PRRT with [177Lu]Lu-DOTATATE does yield clinical benefit with regard to controlling tumor volume and improving quality of life of patients, with a confirmed low risk of hematological and renal toxicities.
The NETTER-2 phase 3 trial, a continuation on the NETTER-1 trial, which compared [177Lu]Lu-DOTATATE plus SSA as a first-line treatment in patients with advanced, well-differentiated GEP-NET (grade 2–3) versus high-dose octreotide alone, has recently been presented at ASCO GI 2024 [25]. The median progression-free survival was significantly prolonged by ~ 14.3 months in the PRRT-group (HR 0.276; 95% CI 0.18–0.42; p < 0.0001). The response rate was 43% in the PRRT arm, compared to 9% in the control arm [26••]. While long-term follow-up data are awaited, this study can potentially change the clinical practice and further broaden the use of PRRT in patients with advanced NET, especially as an earlier treatment line.
The selection of patients eligible for PRRT is based on the so-called theranostic principle, which includes imaging of the same or comparable molecule target that will be used for PRRT [27]. In this way, only patients with sufficient SSTR-expression (e.g., higher than healthy liver uptake, known as the Krenning-score) on diagnostic imaging are selected [28, 29]. Additionally, patients should have ECOG performance status < 2 and sufficient bone marrow, renal, and liver function in order to safely receive four cycles of PRRT [6]. More details on clinical implementation of the treatment can be found in the recent guidelines [1, 23].
PRRT Combined with Transarterial Radioembolization
Majority of NET patients, up to 85%, will develop liver metastases [30]. As the foremost prognostic factor for survival and hormone-related symptoms, improved treatment of liver metastases may prolong survival but also reduce tumor related side-effects and consequently provide a better quality of life. In case of liver-only or liver-dominant metastatic disease, local radionuclide treatment using microspheres could be considered. Selective internal radiation therapy (SIRT), or transarterial radioembolization (TARE), is a safe and effective treatment in liver malignancies. It involves transarterial infusion of radioactive microspheres leading to localized irradiation [31]. NET liver lesions are generally hypervascular, causing preferential arterial flow towards tumors. This characteristic causes microspheres to lodge in tumor arterioles, ensuring high tumor accumulation of the infused microspheres, while relatively sparing normal liver parenchyma from irradiation [32]. Currently, three commercially available radioactive microspheres are used for radioembolization: 90Y-labeled glass spheres (Theraspheres, Boston Scientific), 90Y-labeled resin spheres (SirSpheres, SIRTex), and 166Holmium (166Ho)-labeled PLLA-MS spheres (QuiremSpheres, Quirem Medical). The entire work-up for radioembolization is beyond the scope of this review but has previously been explained [32].
SIRT is utilized in patients with NET either as a debulking treatment (independent of treatment line) or it is reserved as a salvage treatment following failure of other (systemic) therapies [30, 33]. SIRT is most effective when a patient-tailored dosing approach is applied (i.e., prospective dosimetry), as evident dose–response relationships are presented in literature [34, 35]. There is a difference to PRRT, where application of prospective dosimetry/patient-tailored dosing is not common. A tumor receiving a minimal mean absorbed-dose of 120 (166Ho)–150 (90Y glass) Gy has a high likelihood (> 80%) of an objective response according to RECIST 1.1. Reported local response rates for SIRT in patients with liver-only or liver-dominant disease vary between 23 and 64% (objective response as per RECIST 1.1) but are generally more profound compared to PRRT. These large variations in response rates are explained by patient, tumor, and dosing heterogeneities within studies [36].
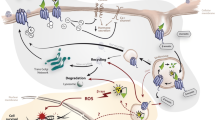
As illustrated by the post-hoc analysis of the NETTER-1 trial [37], progression free survival (PFS) following PRRT is significantly shorter in patients suffering from “bulky” liver disease (defined as having a lesion > 3 cm maximum diameter), which was present in approximately 70% of patients. Furthermore, patients can experience residual hormone related complaints following PRRT, assumed to be caused by liver metastases, bypassing the “filtration” of the liver. Therefore, combining systemic and local radionuclide treatments seems logical, gaining systemic control with PRRT whilst reducing prognostic significant liver disease (Fig. 1).
A 45-year-old, female patient with a grade 2 (ki67 7%) small intestinal NET (ileal origin), with a mesenteric mass, lymph node metastases and extensive bulky liver disease. Refractory to first-line SSA (continues flushing and diarrhea), referred for PRRT. A baseline [68 Ga]Ga-DOTATOC PET/CT. B [68 Ga]Ga-DOTATOC PET/CT 4 months after 4 cycles of PRRT, 7.4 GBq/cycle, with limited tumor reduction (RECIST 1.1 stable disease) and no improvement of complaints. 6 months after last PRRT cycle, received additional sequential whole liver 166Ho-SIRT. C [68 Ga]Ga-DOTATOC PET/CT 3 months after additional 166Ho-SIRT, additional tumor reduction (partial response according to RECIST 1.1) and resolution of flushing and diarrhea (with concurrent long-acting SSA, additional short-acting SSA was stopped). D [68 Ga]Ga-DOTATOC PET/CT 20 months after 166Ho-SIRT, showing durable response, with continues decrease of bulky liver disease (hepatomegaly has clearly been reduced). No signs of ‘pseudo-cirrhotic’ morphology on anatomical imaging were observed ever since
Based on retrospective data and results from the prospective phase 2 HEPAR PLuS study, it can be concluded that radioembolization and PRRT (4 cycles of 7.4 GBq [177Lu]Lu-DOTATATE) can safely be used in the same patient [38, 39]. In general toxicities were minor and transient, but the most commonly reported grade 3–4 toxicities at six months included abdominal pain (10%), lymphocytopenia (23%), and increased gamma-glutamyl transpeptidase (54%). In the HEPAR PLuS study, hepatic and patient-based response was over 40% at three months, which is high compared to the 18% achieved in the NETTER-1 study. Even with this prospective data, there is no broad clinical implementation of PRRT plus radioembolization, due to some fear of anecdotal reports of long-term hepatotoxicity when combining SIRT with systemic treatments [40]. However, evidence for late hepatotoxicity is scares, so long-term follow-up in these patients is needed.
PRRT Combined with Chemotherapy
The current role of chemotherapy in patients with grade 1–2 NETs is limited, because cytotoxic chemotherapies are most effective in malignancies that are more rapidly proliferating. According to ENETS guidelines, chemotherapy is recommended for progressive or advanced (bulky) pancreatic NET (pNET), grade 3 neuroendocrine neoplasms (NENs), and neuroendocrine carcinoma (NEC) [41]. Nevertheless, chemotherapy is widely used as a radiosensitizer during external-beam radiotherapy (EBRT) for various malignancies. Radiosensitizers like platinum, gemcitabine, and fluoropyrimidines are utilized to enhance radiation-induced cellular damage by suppressing radioprotective molecules, inhibiting DNA-repair mechanisms, or dysregulating the cell cycle [42].
Combinations of PRRT and chemotherapies are predominantly explored in phase 1 studies. A notable limitation in the existing literature lies in the diverse dosing regimens and chemotherapeutic agents, with most evidence focusing on capecitabine (CAP), temozolomide (TEM), and 5-fluorouracil (5-FU) combined with various PRRT-ligands. Claringbold et al. conducted a phase 2 study treating patients with advanced progressive pNETs with [177Lu]Lu-DOTATATE (four cycles ~ 7.9 GBq) combined with capecitabine (1500 mg/m2; 14 days) and temozolomide (200 mg/m2; 5 days) each cycle [43]. The overall response rate was 80%, with complete remission achieved in 13%; median overall survival (OS) has not yet been reached at 33 months follow-up. This combination was well tolerated, with the main toxicities being nausea (40%, grades 2–3) and hematological toxicity (10%, grades 2–3). In a prospective study by Nicolini et al. (2021), 37 patients with FDG- and SSTR-positive NETs were treated with five cycles of 5.5 GBq PRRT combined with capecitabine alone (1000–1500 mg/d) [44]. No significant (> grade 2) toxicities were observed during the median 30 months follow-up. A partial response was seen in 30% of patients, and median PFS reached 31.4 months. The effect of concomitant chemotherapy (600 mg/m2/d capecitabine and 75 mg/m2 temozolomide) with ~ 7.4 GBq [177Lu]Lu-DOTATATE on physiological uptake and tumor accumulation was assessed in 20 patients with advanced GEP-NET [45]. Those receiving combination treatments had comparable 177Lu-accumulation in tumor lesions (3.85 ± 1.74 mGy/MBq versus 5.6 ± 11.27 mGy/MBq) compared to the PRRT-alone group. Also, no difference in kidney, liver, spleen, and bone marrow uptake was observed.
While there is a growing body of research on this topic, it has yet to provide conclusive evidence supporting the observed synergistic effect, when combining radiotherapy and chemotherapy. Extensive literature reviews conducted by Chan et al. [46•] and Santo et al. [47] regarding this specific topic concluded have underscored the necessity for additional prospective data to delineate the efficacy and safety of PRRT in conjunction with chemotherapy regimens, before wide-spread clinical adaptation becomes feasible. Presently, several ongoing prospective (randomized controlled) clinical trials are investigating the effect of PRRT plus capecitabine (with or without temozolomide), additional details are outlined in Table 2.
PRRT Combined with Targeted Therapy
Everolimus is currently standard of care treatment for patients with advanced pNETs or progressing on prior lines of therapy. It is an inhibitor of mTOR, which stimulates cell growth, proliferation, and angiogenesis. Two phase I studies were published that analyzed safety and efficacy with a combination of everolimus and PRRT. The first study from 2015 included 16 patients with advanced unresectable progressive well-differentiated GEP-NETs [48]. They received four cycles of 7.8 GBq [177Lu]Lu-DOTATATE at eight-week interval and the dose of everolimus was escalated (5–7.5–10 mg daily for 24 weeks). They found an overall response rate of 44% and a maximum tolerated dose of 7.5 mg. The second trial treated three patients with 5 mg and the next eight patients with 10 mg everolimus daily [49]. The regular dosage of 10 mg everolimus daily was not tolerated, indicating that a lower dose is warranted in this combination therapy.
Sunitinib inhibits a variety of receptor tyrosine kinases involved in tumor growth, pathological angiogenesis and progression of cancer, and is one of the targeted therapies that provided new treatment opportunities in patients with pNETs. Although there is some evidence of a synergistic effect between radiotherapy and sunitinib in other malignancies [50], data in NETs remains limited. Another potential combination with PRRT is the targeted Poly (ADP-ribose) polymerase (PARP) inhibitor. PARP is an enzyme that plays a vital role in the repair of damaged DNA. Pre-clinical and theoretical work has shown the potential of combining PARP inhibitors and PRRT to enhance cell death and overall survival in NET cell-lines [51,52,53]. Human studies are still in the early stages. Ongoing clinical trials investigating the combination of targeted therapies with [177Lu]Lu-DOTATATE are also outlined in Table 2.
PRRT Combined with Immunotherapy
Radiation not only kills tumor cells, but it can also trigger some anticancer immune responses by increasing tumor antigen release and promoting immune cell infiltration. In contrast, immunotherapies work by directly activating the body’s immune system to recognize and attack cancer cells more effectively. The combination of these treatments may act synergistically to generate antitumor immunity and enhance overall therapeutic efficacy [54].
Immune-checkpoint-inhibitors, such as nivolumab and pembrolizumab (both anti-PD-1 antibodies), are considered groundbreaking in other solid tumors, but for NET limited data are available. The feasibility of combining nivolumab with [177Lu]Lu-DOTATATE was assessed in a phase I dose-finding study in nine patients with lung-NET [55]. Administered activities of 3.7 and 7.4 GBq [177Lu]Lu-DOTATATE every eight weeks (four cycles) were combined with four doses nivolumab (240 mg every two weeks). No dose-limiting toxicities were observed at 3.7 GBq, but at 7.4 GBq one patient developed a grade 3 rash. Other adverse events included lymphopenia (n = 7), thrombocytopenia (n = 4), anemia (n = 3), and nausea (n = 3). An overall response rate of 14.3% was found. Other clinical ongoing studies are outlined in Table 2.
The Role of PRRT Prior to Surgery
Several retrospective analyses suggest the potential efficacy of PRRT prior to surgery [56, 57]. Partelli et al. (2018) retrospectively compared two cohorts with pNET; one receiving PRRT before surgery, either [177Lu]Lu-DOTATATE or [90Y]Y-DOTATOC, and the other group underwent surgery without prior PRRT. Patients in the first group received various dose schedules of PRRT; either five cycles of 5.5 GBq (750 mCi total) or eight cycles of 3.7 GBq (800 mCi total) of [177Lu]Lu-DOTATATE, or either four cycles of 2.8 GBq (300 mCi total) or four cycles of 1.85 GBq (200 mCi total) of [90Y]Y-DOTATOC. Although the study was limited in size with 23 patients in each group, it demonstrated a reduced incidence of nodal metastases (9/23 vs 17/23; p < 0.02) and lower risk of pancreatic fistulas in the PRRT group (0/23 vs 4/23; p < 0.02). It did not find a significant difference in PFS (52 vs 37 months; p > 0.2) [58]. Similarly, Parghane et al. investigated the administration of four to five cycles 7.4 GBq [177Lu]Lu-DOTATATE prior to surgery in a heterogeneous population of 57 patients with GEP-NETs. They observed that 26% of patients achieved resectability of the primary tumor post-PRRT, with notable PFS rates at median follow-up of 24 months of 95% and 90%, either without (n = 23) or with liver metastases (n = 34), and 2 year OS of both groups combined was 92% [59].
The most recent study on PRRT before surgery by Minczeles et al. compared OS of 23 patients with pNETs with PRRT-only and 26 patients with PRRT followed by surgery [60]. They found an average decrease in pNET size of 26% (RECIST 1.1) and a reduction of vascular involvement. Total median OS was 8.5 years (95%, 4.5–12.5 years), with the cohort receiving surgery + PRRT exhibited a median OS of 14.7 years (95%, 5.9–23.6), compared to 5.5 years (95%, 4.5–6.5) for the PRRT-only group (p = 0.003). However, it is noteworthy that baseline comparability between the groups was not fully achieved, as the surgery cohort demonstrated a shorter interval from diagnosis to treatment initiation, alongside a notably higher proportion of grade 1 NETs. These findings underscore the potential benefits of presurgical PRRT in improving surgical outcomes and patient prognosis.
Future Directions
The theranostic approach is the foundation for PRRT, as imaging plays an eminent role in patient selection, treatment verification and follow-up. Prior to PRRT, SSTR-targeted imaging can help to assess SSTR-status of lesions, quantify tumor burden, and provide details on whole-body tracer distribution. Imaging during therapy can be used to measure the absorbed radiation dose in NET lesions and organs at risk (e.g., liver, bone marrow, and kidneys). While for radioembolization it has been proven that image-based treatment planning results in a more effective therapy with a lower chance of side-effects, this approach is not the standard for systemic PRRT. The goal of image-based planning is to estimate the therapeutic dose distribution, and modify the administered activity accordingly to increase the absorbed dose in the tumor, while controlling the absorbed dose in healthy tissues. This approach is an elaborate balance between the tumor control probability and normal tissue complication probability.
A dose–response relationship has been described for PRRT, but the scientific evidence is limited to observations in clinical cohorts and retrospective studies. Additionally, the limited implementation of personalized dosing is related to the fact that [177Lu]-DOTA-0-Tyr3-Octreotate (Lutathera®) are registered based on a fixed-dose posology. So any modifications in administered activity or treatment schedule could be considered off-label use. Exceptions are suggested in the product registration in cases where there is an increased risk of hematological toxicity (e.g., in patients with high skeletal metastatic burden), hepatotoxicity (e.g., in patients with high hepatic tumor load), or nephrotoxicity.
In the context of metastatic GEP-NET, therapeutic efficacy of PRRT as a mono-therapy could be improved by increasing the administered activity in the first two cycles, as the receptor density on tumors decreases over treatment cycles as a result of this therapy [61•, 62, 63]. More elaborate dose modifications in PRRT involve image-based treatment modelling either using the diagnostic SSTR-PET or therapeutic SPECT. The absorbed radiation dose to the tumor and organs-at-risk is calculated, and the administered activity for the subsequent cycles can then be adapted accordingly. Though image-based treatment planning for PRRT is feasible in a routine clinical practice, it is considered labor intensive by many and there is no consensus yet on effective absorbed dose levels.
In patients with low SSTR-expression whom are either considered ineligible for PRRT or respond poorly to [177Lu]Lu-DOTATATE, combinations with radiation sensitizers, DNA-repair inhibitors or immune-activating agents could improve treatment efficacy. There are also new molecular targets that may hold an even greater promise in NET and other malignancies. In NENs and related malignancies, fibroblast-activation protein (FAP) and urokinase plasminogen activator receptor (uPAR) are interesting targets for radionuclide therapy as they show expression in both low-grade and high-grade tumors. The first dose escalation studies to demonstrate the usability of Lutetium-labeled FAP-inhibitors in patients with NET are currently recruiting (NCT05432193, NCT04459273). In addition to SSTR-agonists, also antagonists have been opted as effective target.
In the search for more effective therapies, attention has shifted from beta-emitting towards alpha-emitting nuclides such as Actinium-225 (225Ac) and Lead-212 (212Pb). The advantage of alpha-radiation is that it has a high energy transfer upon interaction with tissues, and thus, has the ability to induce a lot of damage with a low range. At present, [225Ac]Ac-DOTATATE is the leading alpha-emitting radiopharmaceutical and mainly used in patients who progress after [177Lu]Lu-DOTATATE therapy. Though in certain cases remarkable responses have been described, the evidence for [225Ac]Ac-DOTATATE in patients with NET is still limited to small scale clinical studies in selected patient cohorts. Currently, after concluding its phase 1 part, the phase 3 randomized controlled trial ACTION-1 is recruiting patients in North America and Europe for patients with advanced NET, after initial [177Lu]Lu-DOTATATE therapy (NCT05477576). As with many developments in radionuclide therapies, most are implemented into “compasionate use” programs to gain the evidence needed to warrant subsequent phase I-II studies.
Availability of PRRT
As [177Lu]Lu-PRRT is nowadays considered a proven mono-therapy in patients with metastatic GEP-NET, the use of these therapies is increasing worldwide. Although [177Lu]Lu-DOTATATE was traditionally performed in specialized institutions, commercialization of PRRT has enabled smaller hospitals and day-clinics to also perform these therapies. This increased accessibility is considered an advantage for patients, but there are also concerns raised regarding their clinical benefit versus costs and general availability [33, 64]. Due to the increasing global demand, access to therapeutic radiopharmaceuticals is not always ascertained, so efforts are made to improve supply chains [65]. So, it has been postulated by ENETS and EANM that oncological radionuclide therapies should be mainly conducted, or at least coordinated, by specialized centers.
Conclusion
Following the publication of the NETTER-1 trial, which underscored the relevance of [177Lu]Lu-DOTATATE in the treatment of patients with advanced NET, extensive research has been conducted to further improve the treatment. This includes the potential synergistic combination of PRRT with other established treatments for patients with NET. Various treatments have been combined with PRRT, but evidence on the benefit of combined treatments is limited. Nonetheless, there are numerous ongoing clinical trials aiming to define the synergistic effect further.
Data Availability
No datasets were generated or analyzed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rinke A, Ambrosini V, Dromain C, Garcia-Carbonero R, Haji A, Koumarianou A, et al. European Neuroendocrine Tumor Society (ENETS) 2023 guidance paper for colorectal neuroendocrine tumours. J Neuroendocrinol. 2023;35:e13309.
Del Rivero J, Perez K, Kennedy EB, Mittra ES, Vijayvergia N, Arshad J, et al. Systemic therapy for tumor control in metastatic well-differentiated gastroenteropancreatic neuroendocrine tumors: ASCO guideline. JCO. 2023;41:5049–67.
Kaltsas GA, Papadogias D, Makras P, Grossman AB. Treatment of advanced neuroendocrine tumours with radiolabelled somatostatin analogues. Endocr Relat Cancer. 2005;12:683–99.
Kaderli RM, Spanjol M, Kollár A, Bütikofer L, Gloy V, Dumont RA, et al. Therapeutic options for neuroendocrine tumors: a systematic review and network meta-analysis. JAMA Oncol. 2019;5:480–9.
Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A, et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:1752–63. Final results of the NETTER-1 trial highlight the impact of PRRT as treatment for patients with advanced NETs.)
Bodei L, Mueller-Brand J, Baum RP, Pavel ME, Hörsch D, O’Dorisio MS, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40:800–16.
Eychenne R, Bouvry C, Bourgeois M, Loyer P, Benoist E, Lepareur N. Overview of radiolabeled somatostatin analogs for cancer imaging and therapy. Molecules. 2020;25:4012.
Wieland DM, Wu J, Brown LE, Mangner TJ, Swanson DP, Beierwaltes WH. Radiolabeled adrenergi neuron-blocking agents: adrenomedullary imaging with [131I]iodobenzylguanidine. J Nucl Med. 1980;21:349–53.
Krenning EP, Bakker WH, Breeman WA, Koper JW, Kooij PP, Ausema L, et al. Localisation of endocrine-related tumours with radioiodinated analogue of somatostatin. Lancet. 1989;1:242–4.
Bomanji JB, Papathanasiou ND. 111In-DTPA0-octreotide (Octreoscan), 131I-MIBG and other agents for radionuclide therapy of NETs. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S113-125.
Wiseman GA, Kvols LK. Therapy of neuroendocrine tumors with radiolabeled MIBG and somatostatin analogues. Semin Nucl Med. 1995;25:272–8.
Krenning EP, Kooij PP, Bakker WH, Breeman WA, Postema PT, Kwekkeboom DJ, et al. Radiotherapy with a radiolabeled somatostatin analogue, [111In-DTPA-D-Phe1]-octreotide. A case history. Ann N Y Acad Sci. 1994;733:496–506.
Chin R-I, Wu FS, Menda Y, Kim H. Radiopharmaceuticals for neuroendocrine tumors. Semin Radiat Oncol. 2021;31:60–70.
Barone R, Walrand S, Konijnenberg M, Valkema R, Kvols LK, Krenning EP, et al. Therapy using labelled somatostatin analogues: comparison of the absorbed doses with 111In-DTPA-D-Phe1-octreotide and yttrium-labelled DOTA-D-Phe1-Tyr3-octreotide. Nucl Med Commun. 2008;29:283–90.
Melis M, Krenning EP, Bernard BF, Barone R, Visser TJ, de Jong M. Localisation and mechanism of renal retention of radiolabelled somatostatin analogues. Eur J Nucl Med Mol Imaging. 2005;32:1136–43.
Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med. 1999;26:1439–47.
Paganelli G, Zoboli S, Cremonesi M, Bodei L, Ferrari M, Grana C, et al. Receptor-mediated radiotherapy with 90Y-DOTA-D-Phe1-Tyr3-octreotide. Eur J Nucl Med. 2001;28:426–34.
Kwekkeboom DJ, Bakker WH, Kam BL, Teunissen JJM, Kooij PPM, de Herder WW, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA(0), Tyr3]octreotate. Eur J Nucl Med Mol Imaging. 2003;30:417–22.
Cremonesi M, Ferrari ME, Bodei L, Chiesa C, Sarnelli A, Garibaldi C, et al. Correlation of dose with toxicity and tumour response to 90Y- and 177Lu-PRRT provides the basis for optimization through individualized treatment planning. Eur J Nucl Med Mol Imaging. 2018;45:2426–41.
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging. 2008;35:1847–56.
Jamar F, Barone R, Mathieu I, Walrand S, Labar D, Carlier P, et al. 86Y-DOTA0)-D-Phe1-Tyr3-octreotide (SMT487)–a phase 1 clinical study: pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion. Eur J Nucl Med Mol Imaging. 2003;30:510–8.
Rolleman EJ, Valkema R, de Jong M, Kooij PPM, Krenning EP. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging. 2003;30:9–15.
Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R, et al. ENETS Consensus Guidelines for the standards of care in neuroendocrine neoplasia: peptide receptor radionuclide therapy with radiolabeled somatostatin analogues. Neuroendocrinology. 2017;105:295–309.
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–35.
Myrehaug S, Singh S, Pavel M, Kunz P, De Herder W, Herrmann K, et al. 92: [177Lu]Lu-Dota-Tate as First-Line Therapy for Patients with Grade 2 and 3 Advanced Gastroenteropancreatic Neuroendocrine Tumours (GEP-NETS): The NETTER-2 Study. Radiother Oncol. 2022;174:S40–1.
Singh S, Halperin DM, Myrehaug S, Herrmann K, Pavel M, Kunz PL, et al. [177 Lu]Lu-DOTA-TATE in newly diagnosed patients with advanced grade 2 and grade 3, well-differentiated gastroenteropancreatic neuroendocrine tumors: Primary analysis of the phase 3 randomized NETTER-2 study. JCO. 2024;42:LBA588–LBA588. Preliminary results of the NETTER-2 study, presented at the ASCO GI 2024 that show the potential of PRRT as first-line therapy.
Rodrigues M, Svirydenka H, Virgolini I. Theragnostics in Neuroendocrine Tumors. PET Clin. 2021;16:365–73.
Krenning EP, de Jong M, Kooij PP, Breeman WA, Bakker WH, de Herder WW, et al. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol. 1999;10(Suppl 2):S23-29.
Ambrosini V, Kunikowska J, Baudin E, Bodei L, Bouvier C, Capdevila J, et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur J Cancer. 2021;146:56–73.
Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–76.
Anbari Y, Veerman FE, Keane G, Braat AJAT, Smits MLJ, Bruijnen RCG, et al. Current status of yttrium-90 microspheres radioembolization in primary and metastatic liver cancer. J Interv Med. 2023;6:153–9.
Ramdhani K, Braat AJAT. The evolving role of radioembolization in the treatment of neuroendocrine liver metastases. Cancers (Basel). 2022;14:3415.
Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844–60.
Chiesa C, Sjogreen-Gleisner K, Walrand S, Strigari L, Flux G, Gear J, et al. EANM dosimetry committee series on standard operational procedures: a unified methodology for 99mTc-MAA pre- and 90Y peri-therapy dosimetry in liver radioembolization with 90Y microspheres. EJNMMI Phys. 2021;8:77.
Ebbers SC, van Roekel C, Braat MNGJA, Barentsz MW, Lam MGEH, Braat AJAT. Dose-response relationship after yttrium-90-radioembolization with glass microspheres in patients with neuroendocrine tumor liver metastases. Eur J Nucl Med Mol Imaging. 2022;49:1700–10.
Lewandowski RJ, Toskich BB, Brown DB, El-Haddad G, Padia SA. Role of radioembolization in metastatic neuroendocrine tumors. Cardiovasc Intervent Radiol. 2022;45:1590–8.
Strosberg J, Kunz PL, Hendifar A, Yao J, Bushnell D, Kulke MH, et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with 177Lu-Dotatate: an analysis of the NETTER-1 study. Eur J Nucl Med Mol Imaging. 2020;47:2372–82.
Ezziddin S, Meyer C, Kahancova S, Haslerud T, Willinek W, Wilhelm K, et al. 90Y Radioembolization after radiation exposure from peptide receptor radionuclide therapy. J Nucl Med. 2012;53:1663–9.
Braat AJAT, Bruijnen RCG, van Rooij R, Braat MNGJA, Wessels FJ, van Leeuwaarde RS, et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (HEPAR PLuS): a single-centre, single-arm, open-label, phase 2 study. Lancet Oncol. 2020;21:561–70.
Ramdhani K, Smits MLJ, Lam MGEH, Braat AJAT. Combining selective internal radiation therapy with immunotherapy in treating hepatocellular carcinoma and hepatic colorectal metastases: a systematic review. Cancer Biother Radiopharm. 2023;38:216–24.
Garcia-Carbonero R, Rinke A, Valle JW, Fazio N, Caplin M, Gorbounova V, et al. ENETS Consensus Guidelines for the standards of care in neuroendocrine neoplasms: systemic therapy - chemotherapy. Neuroendocrinology. 2017;105:281–94.
Gong L, Zhang Y, Liu C, Zhang M, Han S. Application of radiosensitizers in cancer radiotherapy. Int J Nanomedicine. 2021;16:1083–102.
Claringbold PG, Turner JH. Pancreatic neuroendocrine tumor control: durable objective response to combination 177Lu-octreotate-capecitabine-temozolomide radiopeptide chemotherapy. Neuroendocrinology. 2016;103:432–9.
Nicolini S, Bodei L, Bongiovanni A, Sansovini M, Grassi I, Ibrahim T, et al. Combined use of 177Lu-DOTATATE and metronomic capecitabine (Lu-X) in FDG-positive gastro-entero-pancreatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2021;48:3260–7.
Thakral P, Sen I, Pant V, Gupta SK, Dureja S, Kumari J, et al. Dosimetric analysis of patients with gastro entero pancreatic neuroendocrine tumors (NETs) treated with PRCRT (peptide receptor chemo radionuclide therapy) using Lu-177 DOTATATE and capecitabine/temozolomide (CAP/TEM). BJR. 2018;91:20170172.
Chan DS, Kanagaratnam AL, Pavlakis N, Chan DL. Peptide receptor chemoradionuclide therapy for neuroendocrine neoplasms: a systematic review. J Neuroendocrinol. 2023;e13355. https://doi.org/10.1111/jne.13355. An extensive systematic review on combined PRRT and chemotherapy.
Di Santo G, Santo G, Sviridenko A, Virgolini I. Peptide receptor radionuclide therapy combinations for neuroendocrine tumours in ongoing clinical trials: status 2023. Theranostics. 2024;14:940–53.
Claringbold PG, Turner JH. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): a phase I study. Cancer Biother Radiopharm. 2015;30:261–9.
Aljubran A, Badran A, Alrowaily M, Raef H, Alzahrani AM, Almuhaideb A, et al. Efficacy of everolimus combined with 177Lu-dotatate in the treatment of neuroendocrine tumors. Cancer Biother Radiopharm. 2022;39(2):164–168. https://doi.org/10.1089/cbr.2022.0043.
Kleibeuker EA, ten Hooven MA, Verheul HM, Slotman BJ, Thijssen VL. Combining radiotherapy with sunitinib: lessons (to be) learned. Angiogenesis. 2015;18:385–95.
Nonnekens J, van Kranenburg M, Beerens CEMT, Suker M, Doukas M, van Eijck CHJ, et al. Potentiation of Peptide receptor radionuclide therapy by the PARP inhibitor olaparib. Theranostics. 2016;6:1821–32.
Hallqvist A, Svensson J, Hagmarker L, Marin I, Rydén T, Beauregard J-M, et al. Optimizing the schedule of PARP inhibitors in combination with 177Lu-DOTATATE: a dosimetry rationale. Biomedicines. 2021;9:1570.
Cullinane C, Waldeck K, Kirby L, Rogers BE, Eu P, Tothill RW, et al. Enhancing the anti-tumour activity of 177Lu-DOTA-octreotate radionuclide therapy in somatostatin receptor-2 expressing tumour models by targeting PARP. Sci Rep. 2020;10:10196.
Kleinendorst SC, Oosterwijk E, Bussink J, Westdorp H, Konijnenberg MW, Heskamp S. Combining targeted radionuclide therapy and immune checkpoint inhibition for cancer treatment. Clin Cancer Res. 2022;28:3652–7.
Kim C, Liu SV, Subramaniam DS, Torres T, Loda M, Esposito G, et al. Phase I study of the 177Lu-DOTA0-Tyr3-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J Immunother Cancer. 2020;8:e000980.
Barber TW, Hofman MS, Thomson BNJ, Hicks RJ. The potential for induction peptide receptor chemoradionuclide therapy to render inoperable pancreatic and duodenal neuroendocrine tumours resectable. Eur J Surg Oncol. 2012;38:64–71.
van Vliet EI, van Eijck CH, de Krijger RR, Nieveen van Dijkum EJ, Teunissen JJ, Kam BL, et al. Neoadjuvant treatment of nonfunctioning pancreatic neuroendocrine tumors with [177Lu-DOTA0, Tyr3]octreotate. J Nucl Med. 2015;56:1647–53.
Partelli S, Bertani E, Bartolomei M, Perali C, Muffatti F, Grana CM, et al. Peptide receptor radionuclide therapy as neoadjuvant therapy for resectable or potentially resectable pancreatic neuroendocrine neoplasms. Surgery. 2018;163:761–7.
Parghane RV, Bhandare M, Chaudhari V, Ostwal V, Ramaswamy A, Talole S, et al. Surgical feasibility, determinants, and overall efficacy of neoadjuvant 177Lu-DOTATATE PRRT for locally advanced unresectable gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2021;62:1558–63.
Minczeles NS, van Eijck CHJ, van Gils MJ, van Velthuysen M-LF, Nieveen van Dijkum EJM, Feelders RA, et al. Induction therapy with 177Lu-DOTATATE procures long-term survival in locally advanced or oligometastatic pancreatic neuroendocrine neoplasm patients. Eur J Nucl Med Mol Imaging. 2022;49:3203–14.
Siebinga H, Hendrikx JJMA, de Vries-Huizing DMV, Huitema ADR, de Wit-van der Veen BJ,. The cycle effect quantified: reduced tumour uptake in subsequent cycles of [177Lu]Lu-HA-DOTATATE during peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging. 2024;51:820–7. This study clearly quantifies the reduction in tumor uptake as treatment with PRRT continues. Highlighting the importance of the first two cycles of PRRT.
Roth D, Gustafsson J, Warfvinge CF, Sundlöv A, Åkesson A, Tennvall J, et al. Dosimetric quantities in neuroendocrine tumors over treatment cycles with 177 Lu-DOTATATE. J Nucl Med. 2022;63:399–405.
Jahn U, Ilan E, Sandström M, Lubberink M, Garske-Román U, Sundin A. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE; differences in tumor dosimetry, vascularity and lesion metrics in pancreatic and small intestinal neuroendocrine neoplasms. Cancers. 2021;13:962.
Fazio N, Falconi M, Foglia E, Bartolomei M, Berruti A, D’Onofrio M, et al. Optimising radioligand therapy for patients with gastro-entero-pancreatic neuroendocrine tumours: expert opinion from an Italian Multidisciplinary Group. Adv Ther. 2024;41:113–29.
Cutler CS, Bailey E, Kumar V, Schwarz SW, Bom H-S, Hatazawa J, et al. Global issues of radiopharmaceutical access and availability: a nuclear medicine global initiative project. J Nucl Med. 2021;62:422–30.
Author information
Authors and Affiliations
Contributions
D.S.H., B.J.W.V., D.M.V.H., and A.J.A.T.B. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
D.S. Hoogenkamp, B.J. de Wit–van der Veen, D.M.V. Huizing, M.E.T. Tesselaar, R.S. van Leeuwaarde, and M.P.M. Stokkel declare no conflict of interest. M.G.E.H. Lam has acted as a consultant for Boston Scientific and Terumo and receives research support from Novartis, Boston Scientific, and Terumo. A.J.A.T. Braat has acted as a consultant for Boston Scientific, GE Healthcare, and Terumo and receives research support from Ariceum Therapeutics. The UMC Utrecht receives research support and royalties from Terumo (Quiremspheres®).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoogenkamp, D.S., de Wit–van der Veen, L.J., Huizing, D.M.V. et al. Advances in Radionuclide Therapies for Patients with Neuro-endocrine Tumors. Curr Oncol Rep 26, 551–561 (2024). https://doi.org/10.1007/s11912-024-01521-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-024-01521-w