Abstract
Purpose of Review
Treatment of elderly patients with acute myeloid leukemia is a known challenge for hematologists due to patient diversity, heterogeneous disease biology, and a rapidly evolving treatment landscape. Here, we highlight the importance of determining fitness, review the latest therapeutic developments, and discuss clinical scenarios to provide guidance on individualized treatment for older AML patients.
Recent Findings
Several factors, like age, performance status, and comorbidities, play a role in fitness and are associated with outcome. Comorbidity scoring systems and geriatric assessments are tools to help physicians select the most appropriate treatment for each patient. The addition of venetoclax, targeted therapy with IDH1/2 and FLT3 inhibitors, and enhanced formulas of existing drugs like CPX-351 and oral azacitidine have improved responses and outcomes.
Summary
New drugs and combination therapies have increased the therapeutic options for elderly AML patients but determination of fitness and disease biology is essential to select patient-tailored treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is primarily a disease of the elderly with a median reported age at diagnosis of around 70 years [1]. It is estimated that in 2021, over 12,000 patients ≥ 65 years have been diagnosed with AML in the USA, and due to the aging population, this number will rise considerably in the next decades [2].
In contrast to younger patients, whose 5-year overall survival rates improved significantly since the 1970s (from 13 to 55%), survival in elderly patients remains poor with only slight improvements (from 8 to 17%) [3]. Several factors underlie this difference. For one, significant advances in allogeneic hematopoietic stem cell transplantation (HSCT) and optimized (support during) intensive chemotherapy (IC), that have improved outcomes in younger patients, are unfeasible in most elderly patients. Higher age comes with decreased performance status (PS) and the prevalence and severity of conditions that complicate intensive treatment, such as cardio-pulmonary disease, renal disease, and dementia, increase across cancer patients’ age spectrum [4]. Moreover, compared to younger patients, elderly patients more often present with moderate to severe comorbidities and irreversible end-organ disease which is associated with inferior overall survival (OS) in patients receiving IC for AML [4, 5].
Next to the differences in host factors between younger and elderly AML, there is a difference in disease biology. Elderly AML patients more often present with unfavorable cytogenetics or molecular abnormalities and a greater proportion has therapy-related (tAML) or secondary AML (sAML) than younger patients [6,7,8]. Frequently mutated genes include mutations in TP53 and chromatin–spliceosome genes, such as SRSF2 and ASXL1, which are independently and additively associated with a poor outcome [9]. In contrast, frequencies of favorable cytogenetics and mutations, like NPM1, are markedly lower in elderly AML [6, 10].
Together, host factors that limit the ability to receive IC and difficult-to-treat disease biology contribute to poorer outcome in elderly patients with AML. However, while this patient population is heterogeneous, elderly patients in general benefit from IC when deemed fit [11]. Registry data showed that more than 60% of patients aged 70–74 were considered fit for IC, and that this was still 45% in patients between 75 and 79 years of age [11]. For older, medically non-fit patients, hypomethylating agents (HMAs) are a relatively non-intensive treatment option which modestly improves survival rates compared to best supportive care (BSC) [12], but they must be considered a palliative option. Adequate assessment of fitness and characterizing the intrinsic properties of the disease is, therefore, crucial to direct therapy decision-making in older patients with AML.
Recently, the addition of venetoclax to HMA was shown to strongly improve outcome for older, medically non-fit AML patients, to such an extent that choosing the optimal treatment has become challenging [13••]. With the advent of eight other newly FDA-approved drugs for treatment of AML, the isocitrate dehydrogenase (IDH) inhibitors Ivosidenib and Enasidenib, the FMS-like tyrosine kinase 3 (FLT3) inhibitors Midostaurin and Gilteritinib, the anti-CD33 monoclonal antibody gemtuzumab ozogamicin (GO), CPX-351, the hedgehog signaling pathway inhibitor Glasdegib, and the oral HMA CC-486, the answer to the question how to treat elderly patients has become more complicated (Table 1).
This review highlights the options for assessing fitness and will discuss clinical scenarios in elderly AML to guide individualized treatment for older patients with AML.
Evaluating Fitness in the Older Patient
Many efforts have been undertaken to establish fitness and select suitable older AML patients for IC. Historically, older age has been seen as an important determinant of fitness and multiple large retrospective studies confirm age to be an independent prognostic factor for outcome in AML [14, 15, 16]. However, although age is clearly related to prognosis, factors associated with early treatment-related mortality (TRM) might be better fitness indicators. In that context, multicomponent models are more accurate than age alone [17]. Moreover, the elimination of age from these models only minimally affects their predictive accuracy, indicating that age is partly a surrogate for other covariates [17]. PS seems more predictive for early TRM than age [6, 17, 18] but there is a noticeable interaction. The likelihood of early death upon treatment with IC in patients with a PS of 3 increases significantly with age (0% in patients < 56 years vs. 82% in patients > 75 years), whereas the likelihood of comorbidities also increases with age [6]. Several risk score systems, like the hematopoietic stem cell transplantation-comorbidity index (HCT-CI), adult comorbidity evaluation 27 (ACE-27), and Charlson comorbidity index (CCI), have shown to be predictive of outcome in AML [5, 19, 20]. For example, a score of ≥ 3 on the HCT-CI in patients over 60 years is associated with an early mortality rate of 29% upon treatment with IC [19]. It is clear that comorbidities can limit treatment options and increase chances of toxicity but patients with well controlled comorbidities could still be candidates for IC. On the other hand, seemingly fit patients without relevant comorbidities can have considerable functional or cognitive impairment that is not necessarily directly noticeable during regular consultation. A structured geriatric assessment (GA) can help detect these impairments, initiate precautionary actions or early treatment, and discriminate fit from unfit patients. Pretreatment GA has predictive value for survival and other treatment-related outcomes of elderly patients with AML but can also be used during treatment, upon clinical improvement or decline, and guide care and decision-making [21, 22]. However, despite all tools available, the consensus and cut-off values to establish fitness remain rather arbitrary. Interestingly, clinical impression by the trained eye of physicians and nurses can estimate the patient’s clinical condition which is significantly associated with mortality and morbidity in hematology and oncology patients [23, 24]. We therefore use age, PS, comorbidity scores, and GA, all as tools to support the value of clinical impression of the treatment team to guide our treatment decisions.
Clinical Scenarios
The Fit Elderly Patient with Newly Diagnosed AML…
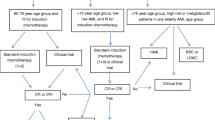
To date, despite all the recent advances, the only potential curative treatment for patients with AML is IC induction followed by either post-remission intermediate or high dose cytarabine courses, autologous HCT consolidation, or treatment with an allogeneic stem cell transplant. IC in fit elderly patients is known to be feasible and provides a valuable option associated with a better long-term survival in older AML patients compared to HMA [25]. However, IC remains more toxic than HMA in older AML patients and is associated with increased early mortality in retrospective studies. Whether induction with decitabine can provide comparable remission rates with less toxicity and offers a better bridge to transplant than IC, is currently investigated in the randomized EORTC-1301 trial (NCT02172872). Although these results are awaited with great interest, the landscape of AML therapy has already moved on. The current standard for the treatment of elderly (> 75 years) and unfit AML is the combination of HMA and venetoclax. It has demonstrated excellent activity with favorable safety, even in frail patients [13••, 26]. Studies suggest that complete remission (CR) rates attained by these combinations may approach those of IC and it is therefore often hypothesized that HMA with venetoclax should also be the preferred frontline treatment in elderly AML patients (60–75 years) who are fit for IC treatment. This is supported by propensity matching-based studies that suggest equivalence between the two treatment modalities [27, 28]. However, these studies use historical data with a high potential of selection bias and they should therefore not be viewed as prove for equivalent effectiveness. A trial randomizing between IC and HMA with venetoclax is enrolling (NCT04801797). Until these trials prove otherwise, we suggest to reserve HMA with venetoclax only for patients who match the inclusion criteria of the VIALE-A study and use IC for fit elderly patients without comorbidities. An algorithm for the treatment of elderly AML patients is purposed in Fig. 1.
Purposed treatment algorithm for elderly AML patients. CBF-AML, core-binding factor acute myeloid leukemia; FLT3m, FMS-like tyrosine kinase mutated; tAML, therapy-related AML; AML-MRC, AML with myelodysplasia-related changes; GO, gemtuzumab ozogamicin; HMA, hypomethylating agent; IDH1m, isocitrate dehydrogenase 1 mutated; IDH2m, isocitrate dehydrogenase 2 mutated; LDAC, low-dose cytarabine; BSC, best supportive care.
… and a Favorable or Intermediate Risk Profile
The choice between “classical” 7 + 3, newer intensive treatment options, like CPX-351, or the addition of targeted drugs strongly depends on the ELN risk classification and the molecular profile. For patients with a good (CEBPa double mutant, CEBPa-bZIP, or core-binding factor AML) or intermediate-risk profile, without FLT3 or IDH1/2 mutations, standard 7 + 3 induction treatment results in CR in 60–70% of patients and is therefore a valid treatment option [29]. The addition of gemutuzumab ozogamicin (GO) can enhance the outcome for patients within this AML risk group [30,31,31]. GO is a humanized antibody-drug conjugate composed of a monoclonal antibody targeting CD33, covalently linked to the cytotoxic drug N-acetyl-γ-calicheamicin. It is approved by the FDA for the treatment of relapsed/refractory (R/R) and newly diagnosed (ND-AML) CD33-positive AML and has EMA approval for de novo CD33-positive patients. A recent meta-analysis showed an improved survival benefit, especially for patients aged < 70 years, with de novo AML, with positive expression of CD33, with NPM1 mutation, without FLT3-ITD mutation, and with low-/intermediate-risk karyotypes [33]. Core-binding factor AML is another subtype that has shown improved survival with the use of GO in combination with IC although the toxicity of GO remains a concern especially in older patients. Reduction of the number of chemotherapy days when GO is combined with intensive treatment is advised for patients above the age of 60 [34]. Recently, GO was also investigated as a replacement for anthracycline (i.c. idarubicine) during induction of fit elderly patients in the randomized ALFA1401-Mylofrance 4 Study but this did not improve outcome and was associated with increased toxicity and a non-significant higher relapse incidence, shorter EFS, and shorter OS [35]. Although low- and intermediate-risk and CBF AML are less prevalent in elderly AML, and despite the concerns of toxicity, the addition of GO should be considered for fit elderly patients who present with these subtypes.
… with Myelodysplasia-Related Changes or tAML
In contrast, a significantly greater proportion of elderly patients present with sAML. This subgroup, representing AML that arises form a preceding myeloid disease or tAML, is generally associated with a poor prognosis [36]. Recently, CPX-351 (Vyxeos) received FDA and EMA approval for the treatment of ND tAML or AML with myelodysplasia-related changes (AML-MRC). CPX-351 is a liposomal formulation of cytarabine and daunorubicine at a fixed 5:1 molar ratio with improved uptake in leukemic cells in vitro and results in a long half-life in human plasma [37, 38]. In fit elderly patients with ND-AML, age 60 to 75 years, CPX-351 has been shown to increase response rates compared to conventional IC, resp. 66.7% vs. 51.2% [39]. Moreover, CPX-351 improved mOS (9.56 vs. 5.95 months) in a comparable phase III study in elderly high-risk ND-AML and sAML. A recent update of this study showed twice as many patients alive at 3 and 5 years with CPX-351 than with IC, although patient numbers were low [40•]. Interestingly, patients who went on to HSCT especially seemed to benefit. Likely explanations are that CPX-351 induces deeper remissions with higher rates of measurable residual disease (MRD) negativity or leads to better clinical condition pre-transplant due to less toxicity [41, 42]. Importantly, CPX-351 is generally well tolerated and associated with low frequency of alopecia and gastrointestinal toxicity but is associated with prolonged time to neutrophil and platelet count recovery [42]. Based on current knowledge, we intent to use CPX-351 for fit elderly patients between 60 and 75 years with ND tAML or AML-MRC, especially when there is an intent to consolidate with a HSCT. For patients with tAML or AML-MRC who present with comorbidity, or are fit but do not want to proceed to HSCT, we tend to use low-intensity regimens.
… with IDH1/2 Mutation
Patients who present with targetable mutations, like IDH1, IDH2, or FLT3, should preferably be treated in clinical trials investigating the addition of specific inhibitors combined with IC. Examples of such trials are the HOVON 150 (for IDH1/2 mutated AML, NCT03839771) and HOVON 156 (for FLT3 mutated AML, NCT04027309), in which fitness for intensive treatment, and not age itself, defines eligibility for these trials.
For IDH1/2 mutated AML, two inhibitors have been approved by the FDA. Ivosidenib is approved for ND-AML with an IDH1 mutation age 75 years or older, patients who are ineligible for IC, and R/R AML. Enasidenib is an IDH2 inhibitor and is approved for patients with R/R AML. Both inhibitors have shown variable results in the different treatment settings either as monotherapy or in combination with HMA [43,44,45,46,47••]. The studies supporting the use of these inhibitors in unfit patients are discussed in a different section of this paper. The combination of ivosidenib or enasidenib with IC induction has demonstrated encouraging responses and an acceptable safety profile in a phase I study [48]. Composite CR rates for ivosidenib and enasidenib in patients with de novo AML were 88% and 80%, respectively. The randomized phase III HOVON 150 trial investigating standard IC combined with either ivosidenib or enasidenib vs. placebo is currently ongoing.
… with FLT3 Mutation
Over the last years, multiple FLT3 inhibitors have been developed and entered clinical trials at various disease stages, either as mono- or in combination therapy. The first-generation FLT3 inhibitors include midostaurin, sorafenib, and lestaurtinib. As a single agent, these inhibitors have shown limited efficacy and variable tolerability due to the adverse effects derived from their multikinase inhibitory activities [49,50,51]. However, combined with standard IC, midostaurin improved overall survival of ND patients with FLT3 mutated AML, as shown in the RATIFY trial where a 7% higher probability of survival after 48 months was seen than in patients treated with IC only [52]. Although this study did not enroll patients over 60 years, midostaurin is FDA and EMA approved for the treatment of ND FLT3 mutated AML of all ages who undergo IC induction. Interim-analyses of the AMLSG 16-10 trial, which included patients up to 70 years, reported this combination to be feasible and equally effective in elderly patients, despite the more prevalent dose reductions compared to younger patients [53].
Second-generation FLT3 inhibitors include gilteritinib, quizartinib, and crenolanib. These newer inhibitors are more potent and selective than first-generation inhibitors, improving their tolerability and efficacy [54]. This was demonstrated by two phase III trials in which single-agent gilteritinib (ADMIRAL) and quizartinib (QUANTUM-R) improved remission rates and mOS compared to high- and low-intensive types of salvage chemotherapy in R/R AML [55, 56•]. Whether second-generation inhibitors also improve outcome of ND patients when combined with IC, is currently under investigation in multiple phase III trials (NCT02668653, NCT04027309). We recommend to include fit elderly FLT3 mutated patients in these trials.
… with Poor Risk Cytogenetics or TP53 Mutation
Elderly patients often present with unfavorable risk cytogenetics and poor-risk mutations. In particular, complex and monosomal karyotype and TP53 mutations confer very poor outcome, even in the context of HSCT [57, 58]. Attempts to improve outcomes in this subgroup thus far have been unsuccessful and also the addition of venetoclax failed to show clinical benefit in this context [59,60,61]. This raises the question whether to treat elderly TP53 mutated patients with intensive therapies that will reduce quality of life (QoL) without a reasonable chance of curation, or even, an acceptable improvement of disease free survival. We therefore tend to postpone treatment in elderly patients until cytogenetic and molecular results are available in order to make an informed treatment decision. Delaying treatment awaiting these results is not harmful in stable older AML patients [62,63,64]. In general, we do not commence treatment with IC in patients ≥ 65 years when extremely poor-risk features are found. Instead, we diverge to less toxic treatments with HMA and try to include these patients in specific clinical trials when available. Promising new therapies currently in clinical trials include the anti-CD47 antibody Magrolimab, the mutant p53 reactivator Eprenetapopt (APR-246), the dual-affinity molecule CD123 × CD3 Flotetuzumab, and the menin-inhibitor SNDX-5613 [65,66,67,68,69].
… Eligible for Allogeneic Stem Cell Transplant
Although new therapies have improved overall survival of patients with AML, curation cannot be achieved without high dose chemotherapy or HSCT. HSCT has been shown to effectively reduce relapse risk in all AML risk groups but improvement of survival is predominantly seen in intermediate and high-risk patients [70, 71]. Although older patients more often present with high-risk disease and have a higher chance of relapse, the use of HSCT, until recently, was limited to patients under 60 years, as older patients were more likely to experience complications and have higher TRM rates. However, reduced-intensity conditioning (RIC) regimens, improved immunosuppressive strategies, and better supportive care have made HSCT now also feasible for elderly patients [72]. Consolidation with HSCT has increased over the last decades and is now performed in approximately 26% of all elderly (60–75 years) AML patients in the Netherlands [73]. The importance of bringing eligible elderly patients to HSCT is demonstrated by multiple retrospective studies showing that transplants can reduce relapse and improve long-term overall survival compared to non-transplant strategies, even despite the fact that transplant is associated with an increased risk of (early) TRM [74,75,76]. Moreover, prospective data from the ECOG-ACRIN study, in which patients between 60–73 years of age received a HSCT in CR1, show comparable results with an encouraging 4-year OS rate of 43%. Interestingly, the NRM rate in patients > 65 years was similar compared to patients ≤ 65 years [77]. Therefore, we attempt to bring all eligible elderly patients ≥ 65 years, who achieve complete remission on intensive or low-intensity induction treatments, to HSCT.
… Non-eligible for Allogeneic Stem Cell Transplant
Unfortunately, HSCT is not feasible in all elderly patients. Patients who initially were fit at diagnosis can deteriorate due to treatment and complications and be deemed ineligible, or patients can decide to refrain from transplant based on personal preference. For these patients, low-intensity post-remission treatment to increase DFS and mOS is available. The Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) has investigated subcutaneous azacitidine maintenance after CR or CR with incomplete hematological recovery (CRi) after IC [78]. Maintenance with 50 mg/m2, for 5 days, every 4 weeks, was feasible and significantly improved DFS compared to observation, but OS did not differ between the groups.
CC-486 (ONUREG®) is an oral formulation of azacitidine. Compared to subcutaneously injected azacitidine, CC-486 has a different pharmacodynamic and pharmacokinetic profile, which allows for extended dosing schedules at lower dose, to prolong drug exposure with sustained epigenetic targeting [79]. CC-486 has received regulatory approval from the FDA and EMA as maintenance therapy for AML patients who have CR/CRi following IC with or without consolidation treatment and who are ineligible for, or choose not to proceed to, HSCT. Approval was based on results of the phase III, randomized, double-blind, placebo-controlled QUAZAR AML-001 trial (NCT01757535) [80••]. This trial investigated CC-486 maintenance versus placebo in patients aged ≥ 55 years with ND-AML, who were in remission after IC but not candidates for HSCT. Maintenance treatment with CC-486 resulted in improved mOS en mRFS compared to placebo (24.7 vs. 14.8 months and 10.2 vs. 4.8 months, respectively). Recently, updated survival analysis, with a median follow up of 51.7 months, showed a sustained, long-term OS benefit with CC-486 [81]. Long-term survival was associated with intermediate-risk cytogenetics and NPM1 mutations at diagnosis and absence of detectable MRD after induction. Gastrointestinal complaints are common during treatment with CC-486 which can decrease QoL and lead to treatment discontinuation. It is suggested that patients develop progressive tolerance with continued treatment but counseling and prophylactic treatment with anti-emetics, proton-pump inhibitors, laxatives, and/or anti-motility agentsare advised to increase treatment adherence [82].
The Unfit Elderly Patient with Newly Diagnosed AML…
For a large number of elderly patients, IC is deemed too toxic. For these patients, low-intensity treatments, such as HMA and low-dose cytarabine (LDAC), represent an effective alternative although the results remain unsatisfactory [12].
The addition of glasdegib, an oral smoothened inhibitor, in combination with LDAC was shown to improve response rates (CR/CRi 24.3% vs. 5.2%) and mOS (8.8 vs. 4.9 months) compared to LDAC alone [83]. Based on these results, glasdegib received FDA and EMA approval for the treatment of ND-AML in patients unfit for IC. However, response and survival rates are only modest and do not reach rates that are achieved with single-agent azacitidine.
There is a strong synergistic effect when venetoclax is combined with HMA [84]. The efficacy of the combination of venetoclax with azacitidine has been studied in the VIALE-A study [13••]. This randomized, double-blind, placebo-controlled, phase III trial showed improved OS in patients with ND-AML compared with azacitidine alone. Median OS increased from 9.6 to 14.7 months and the CR rate was significantly higher with the combination than with azacitidine alone, resp. 36.7% vs. 17.9%. The combination of venetoclax with decitabine has been investigated in several phase I/II studies and shows similar improvement in responses [26, 85, 86]. Combined with a 10-day decitabine regimen, an impressive CR/CRi rate of 84% in ND-AML could be achieved [87]. Improved responses and survival were also seen in patients treated with low-dose cytarabine when venetoclax was added, although responses overall were less than the combination with HMA and mOS (10.1 months) is more comparable to results obtained with HMA monotherapy [88]. Based on these studies, venetoclax has been granted approval by the FDA and EMA in combination with azacitidine, decitabine, or low-dose cytarabine for the treatment of ND-AML in adults ≥ 75 years, or who have comorbidities precluding IC induction.
In general, all combinations mentioned are well tolerated, but increased hematological toxicity and febrile neutropenia is common [86]. Compared to the treatment of CLL, tumor lysis syndrome with venetoclax in AML is infrequent. However, AML with NPM1 and/or IDH1/2 mutations may have increased risk due to their sensitivity. Outpatient treatment with venetoclax can be safe with careful monitoring but hospitalizations due to infectious complications are frequent awaiting hematologic recovery [89]. We therefore make an individualized decision and choose inpatient treatment for patients with high complication risk or poor social support networks.
… with IDH1/2 Mutation
As mentioned above, IDH1/2 mutated AML is particularly sensitive to azacitidine-venetoclax. Analysis from the VIALE-A study shows CR/CRi of 66% vs. 9% and 86% vs. 11% for IDH1 and IDH2 mutated AML, respectively, when compared to azacitidine-placebo [90]. Treatment with azacitidine-venetoclax therefore is a valid treatment option for these patients.
Another possibility is ivosidenib, that is FDA approved for the treatment of unfit elderly patients with IDH1 mutated AML. The drug induces lower responses (CR/CRi/CRp 41.2%) compared to azacitidine+venetoclax; however, it is well tolerated and seems to have lower rates of cytopenia and infectious complications [44]. The AGILE trial showed that the combination of ivosidinib and azacitidine had a higher rate of CR (47.2% vs. 14.9%) and a significantly improved mOS (24.0 vs. 7.9 months) compared to single-agent azacitidine treated patients [47••]. Notably, the combination was well tolerated and showed a reduced frequency of infections and a trend towards improved QoL and symptom burden.
In elderly IDH2 mutated ND-AML patients, treatment with enasidenib resulted in a CR rate of 18% [91]. The combination of enasidenib and azacitidine was studied in a large randomized phase II trial (AG221-AML-005) and significantly improved response (CR/CRi 57% vs. 18%) and duration of response (DOR) (24.1 vs. 9.9 months) compared to azacitidine monotherapy. Despite this improvement, the study failed to demonstrate a survival benefit, possibly due to post-study use of enasidenib in patients progressing after azacitidine [46].
Although ivosidenib and enasidenib are well tolerated, both are associated with differentiation syndrome. This potentially fatal adverse reaction is seen in around 19% of patients treated with IDH inhibitors and early recognition and treatment is critical to prevent severe complications and mortality [92, 93].
As IDH inhibitors are not globally available and responses are excellent, we tend to treat our IDH1/2 mutated unfit elderly patients with azacitidine-venetoclax. When available, monotherapy ivosidenib could be considered for the frailest patients or for those who do not want repeated hospital visits for azacitidine injections. Whether triplet regimens with azacitidine, venetoclax, and an IDH inhibitor induce even better responses is currently under investigation (NCT03471260) [94, 95].
… with FLT3 Mutation
Currently, the standard treatment of unfit elderly patients with a FLT3 mutation is azacitidine-venetoclax. This combination demonstrated a favorable response rate compared to azacitidine monotherapy (CR/CRi 67 vs. 36%), and longer mOS (12.5 months vs. 8.6 months) [13••, 96]. The addition of gilteritinib to azacitidine has been investigated in the LACEWING trial [97]. This randomized phase III study failed to demonstrate a survival benefit despite a significantly improved composite CR (CRc) rate compared to azacitidine monotherapy, 58.1% vs. 26.5%, respectively. The higher percentage of patients with an ECOG > 2 in the combination arm and the more frequent use of post-study TKI treatment in the control arm could have confounded the OS findings.
Whether triplet therapy, combining a FLT3 inhibitor, venetoclax, and a HMA could improve outcome is currently investigated in clinical trials. Maiti et al. reported on 12 elderly ND-AML patients treated with 10-day decitabine combined with venetoclax and various FLT3 inhibitors (gilteritinib, sorafinib, or midostaurin) [98]. The triplet demonstrated a high CRc rate of 92% and an 18-month progression free survival of 59%. Although the combination was well tolerated, triplet regimens seem to be more myelotoxic. Updated reports suggest dose modifications for gilteritinib and early bone marrow evaluation to evaluate marrow ablation and subsequent withholding of venetoclax in order to allow for neutrophil recovery.
… and a Poor Performance Status
Although low-intensity treatment can significantly improve survival compared to supportive care, not all elderly patients are able, or want, to undergo treatment. Prognosis is particularly poor in the eldest elderly (≥ 80 years) with a poor PS and in these patients, BSC can be a valuable option. Importantly, active AML can contribute to poor PS and, in some patients, treatment may improve performance and enhance the patient’s ability to tolerate and benefit from subsequent treatment. It is therefore important to carefully distinguish chronic comorbidities from transient, and potentially improvable, AML related complications. Hydroxyurea, transfusion, and antimicrobial prophylaxis can be applied to patients who are only candidates for BSC. Importantly, specialty palliative care is recommended to improve QoL, psychological distress, and end of life care [99].
The Elderly Patient with Relapsed AML
Unfortunately, recurrence of AML after CR is frequently seen in elderly AML and prognosis is then extremely poor with an mOS of at highest 6 months [100, 101]. Management of these patients is highly dependent on the clinical context, disease biology, and presence of targetable mutations, and therefore, treatment strategies range from reinduction with salvage chemotherapy to BSC. Treatment with curative intent may be attempted for the “younger elderly” patients who have the possibility to undergo HSCT or donor lymphocyte infusion after achievement of a second CR. Salvage regimens with IC should only be considered for exceptionally fit patients with late relapses (> 1 year) without poor-risk features and targetable mutations. For patients with IDH1/2 or FLT3 mutations, targeted treatment with ivosidenib, enasidenib, or gilteritinib has shown to be effective and less toxic than IC. When targetable mutations are absent, less intensive treatment with HMA, when possible combined with venetoclax, is the preferred option for less-fit patients who have not been pretreated with these agents before [102, 103]. However, for most patients, especially the eldest elderly or patients with poor-risk features and/or early relapse, treatment is mainly palliative. Enrollment in clinical trials is encouraged for all patients as new therapies and combinations are under investigation that are desperately needed to improve outcome for these patients.
Conclusion
Treatment of the elderly patient with AML is challenging due to the heterogeneity between aging patients and their diverse disease biology. Outcome remains poor, especially for those who are not candidates for HSCT. Introduction of new drugs has now increased treatment options for both fit and unfit elderly patients. Especially in elderly patients, factors as age, PS, comorbidity, cognitive and physical functioning, social network, and diseases biology must be weighed against the various treatment options with different response rates and side effects. Individualized and tailored medicine is therefore needed to select the best fitting therapy for each patient.
Abbreviations
- ACE-27:
-
adult comorbidity evaluation 27
- AML:
-
acute myeloid leukemia
- BSC:
-
best supportive care
- CBF :
-
core-binding factor
- CCI:
-
Charlson comorbidity index
- CR:
-
complete remission
- CRc:
-
composite complete remission
- CRi:
-
complete remission with incomplete hematological recovery
- DFS:
-
disease-free survival
- FLT3:
-
FMS-like tyrosine kinase 3
- GA:
-
geriatric assessment
- GO:
-
gemtuzumab ozogamicin
- HCT-CI:
-
hematopoietic stem cell transplantation-comorbidity index
- HMA:
-
hypomethylating agents
- HOVON:
-
the Dutch-Belgian Hemato-Oncology Cooperative Group
- HSCT:
-
allogeneic hematopoietic stem cell transplantation
- IC:
-
intensive chemotherapy
- IDH:
-
isocitrate dehydrogenase
- LDAC:
-
low-dose cytarabine
- mOS:
-
median overall survival
- MRC:
-
myelodysplasia-related changes
- MRD:
-
measurable residual disease
- ND :
-
newly diagnosed
- NRM:
-
non-relapse mortality
- OS:
-
overall survival
- PS:
-
performance status
- QoL:
-
quality of life
- R/R :
-
relapsed/refractory
- RFS:
-
relapse-free survival
- RIC:
-
reduced-intensity conditioning
- sAML:
-
secondary AML
- tAML:
-
therapy-related AML
- TRM:
-
treatment-related mortality
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cancer stat facts: leukemia—acute myeloid leukemia (AML) derived via https://seer.cancer.gov/statfacts/html/amyl.html.
Vespa J, Medina L, Armstrong DM. Demographic turning points for the United States: population projections for 2020 to 2060. 2020.
Kantarjian H, Kadia T, DiNardo C, Daver N, Borthakur G, Jabbour E, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11(2):41.
Piccirillo JF, Vlahiotis A, Barrett LB, Flood KL, Spitznagel EL, Steyerberg EW. The changing prevalence of comorbidity across the age spectrum. Crit Rev Oncol Hematol. 2008;67(2):124–32.
Wass M, Hitz F, Schaffrath J, Muller-Tidow C, Muller LP. Value of different comorbidity indices for predicting outcome in patients with acute myeloid leukemia. PLoS One. 2016;11(10):e0164587.
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–5.
Rossi G, Pelizzari AM, Bellotti D, Tonelli M, Barlati S. Cytogenetic analogy between myelodysplastic syndrome and acute myeloid leukemia of elderly patients. Leukemia. 2000;14(4):636–41.
Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1312–20.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209–21.
Schneider F, Hoster E, Schneider S, Dufour A, Benthaus T, Kakadia PM, et al. Age-dependent frequencies of NPM1 mutations and FLT3-ITD in patients with normal karyotype AML (NK-AML). Ann Hematol. 2012;91(1):9–18.
Juliusson G. Older patients with acute myeloid leukemia benefit from intensive chemotherapy: an update from the Swedish Acute Leukemia Registry. Clin Lymphoma Myeloma Leuk. 2011;11(Suppl 1):S54–9.
Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–9.
•• DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–29. This phase III trail randomized elderly and unfit AML patients to azacitidine and venetoclax versus azacitidine and placebo. Patients treated with venetoclax had significantly improved response rates and median overall survival.
Buchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Muller-Tidow C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol. 2009;27(1):61–9.
Kantarjian H, Ravandi F, O’Brien S, Cortes J, Faderl S, Garcia-Manero G, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116(22):4422–9.
Wheatley K, Brookes CL, Howman AJ, Goldstone AH, Milligan DW, Prentice AG, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145(5):598–605.
Walter RB, Othus M, Borthakur G, Ravandi F, Cortes JE, Pierce SA, et al. Prediction of early death after induction therapy for newly diagnosed acute myeloid leukemia with pretreatment risk scores: a novel paradigm for treatment assignment. J Clin Oncol. 2011;29(33):4417–23.
Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L, Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179–87.
Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136(4):624–7.
Dhakal P, Shostrom V, Al-Kadhimi ZS, Maness LJ, Gundabolu K, Bhatt VR. Usefulness of Charlson comorbidity index to predict early mortality and overall survival in older patients with acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2020;20(12):804–12 e8.
Klepin HD, Geiger AM, Tooze JA, Kritchevsky SB, Williamson JD, Pardee TS, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121(21):4287–94.
Sherman AE, Motyckova G, Fega KR, Deangelo DJ, Abel GA, Steensma D, et al. Geriatric assessment in older patients with acute myeloid leukemia: a retrospective study of associated treatment and outcomes. Leuk Res. 2013;37(9):998–1003.
Moss AH, Lunney JR, Culp S, Auber M, Kurian S, Rogers J, et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13(7):837–40.
Halbe L, Gerlach C, Hess G, Wehler T, Theobald M, Weber M. “Would I be surprised if this patient died in the next year?” — prognostic significance of the “surprise” question in a university hematology and oncology outpatients clinic. Basel: Jahrestagung der Deutschen, Österreichischen und Schweizerischen Gesellschaften für Hämatologie und Medizinische Onkologie; 2015. p. P484.
Recher C, Rollig C, Berard E, Bertoli S, Dumas PY, Tavitian S, et al. Long-term survival after intensive chemotherapy or hypomethylating agents in AML patients aged 70 years and older: a large patient data set study from European registries. Leukemia. 2021.
DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
Cherry E, Abbott D, Amaya M, McMahon C, Schwartz M, Rosser J, et al. Venetoclax and azacitidine compared to induction chemotherapy for newly diagnosed patients with acute myeloid leukemia. Blood Adv. 2021.
Zeidan AM, Pollyea DA, Borate U, Vasconcelos A, Potluri R, Rotter D, et al. Venetoclax plus azacitidine (VEN-AZA) vs. intensive chemotherapy (IC) as induction for patients with acute myeloid leukemia (AML): retrospective analysis of an electronic medical records (EMR) database in the United States. Blood. 2021;138(Supplement 1):277.
Eisfeld AK, Kohlschmidt J, Mrozek K, Blachly JS, Walker CJ, Nicolet D, et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: an analysis of Alliance studies. Leukemia. 2018;32(6):1338–48.
Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369–77.
Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30(32):3924–31.
Castaigne S, Pautas C, Terré C, Renneville A, Gardin C, Suarez F, et al. Final analysis of the ALFA 0701 study. Blood. 2014;124(21):376.
Xu Q, He S, Yu L. Clinical benefits and safety of gemtuzumab ozogamicin in treating acute myeloid leukemia in various subgroups: an updated systematic review, meta-analysis, and network meta-analysis. Front Immunol. 2021;12:683595.
Kantarjian HM, Kadia TM, DiNardo CD, Welch MA, Ravandi F. Acute myeloid leukemia: treatment and research outlook for 2021 and the MD Anderson approach. Cancer. 2021;127(8):1186–207.
Lambert J, Lambert J, Lemasle E, Joris M, Chantepie S, Pautas C, et al. Replacing the anthracycline by gemtuzumab ozogamicin in older patients with de novo standard-risk acute myeloid leukemia treated intensively — results of the randomized ALFA1401-mylofrance 4 study. Blood. 2021;138(Supplement 1):31.
Boddu P, Kantarjian HM, Garcia-Manero G, Ravandi F, Verstovsek S, Jabbour E, et al. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Adv. 2017;1(17):1312–23.
Feldman EJ, Kolitz JE, Trang JM, Liboiron BD, Swenson CE, Chiarella MT, et al. Pharmacokinetics of CPX-351; a nano-scale liposomal fixed molar ratio formulation of cytarabine:daunorubicin, in patients with advanced leukemia. Leuk Res. 2012;36(10):1283–9.
Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF, et al. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29(8):979–85.
Lancet JE, Cortes JE, Hogge DE, Tallman MS, Kovacsovics TJ, Damon LE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123(21):3239–46.
• Lancet JE, Uy GL, Newell LF, Lin TL, Ritchie EK, Stuart RK, et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481–e91. This abstract reported the long-term outcome of the phase III trial that randomized elderly patients with tAML or AML-MRC between CPX-351 and conventional intensive chemotherapy. Results show that the imporved overall surival of CPX-351 is maintained and can contribute to long-term remission.
Rautenberg C, Stolzel F, Rollig C, Stelljes M, Gaidzik V, Lauseker M, et al. Real-world experience of CPX-351 as first-line treatment for patients with acute myeloid leukemia. Blood Cancer J. 2021;11(10):164.
Chiche E, Rahmé R, Bertoli S, Dumas P-Y, Micol J-B, Hicheri Y, et al. Real-life experience with CPX-351 and impact on the outcome of high-risk AML patients: a multicentric French cohort. Blood Advances. 2021;5(1):176–84.
Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–31.
DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–98.
DiNardo P, CM, A. Schuh, C. Papayannidis, P. Vyas, A. Wei, et al. A phase 3 study of enasidenib (ENA) versus conventional care regimens (CCR) in older patients with late-stage mutant-IDH2 (MIDH2) relapsed/refractory acute myeloid leukemia (R/R AML). EHA 2021; virtual: HemaSphere; 2021. p. 188-189.
DiNardo CD, Schuh AC, Stein EM, Montesinos P, Wei AH, de Botton S, et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021;22(11):1597–608.
•• Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, et al. AGILE: a global, randomized, double-blind, phase 3 study of ivosidenib + azacitidine versus placebo + azacitidine in patients with newly diagnosed acute myeloid leukemia with an IDH1 mutation. Blood. 2021;138(Supplement 1):697. This phase III trail randomized IDH1 mutated AML patients to ivosidenib and azacitidine versus azacitidine and placebo. Patients who received ivosidenib had significantly imporved response rates and better median overall survival.
Stein EM, DiNardo CD, Fathi AT, Mims AS, Pratz KW, Savona MR, et al. Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: a phase 1 study. Blood. 2021;137(13):1792–803.
Knapper S, Burnett AK, Littlewood T, Kell WJ, Agrawal S, Chopra R, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108(10):3262–70.
Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96(1):62–8.
Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28(28):4339–45.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–64.
Schlenk RF, Fiedler W, Salih HR, Wulf G, Thol F, Kündgen A, et al. Impact of age and midostaurin-dose on response and outcome in acute myeloid leukemia with FLT3-ITD: interim-analyses of the AMLSG 16-10 trial. Blood. 2016;128(22):449.
Staudt D, Murray HC, McLachlan T, Alvaro F, Enjeti AK, Verrills NM, et al. Targeting oncogenic signaling in mutant FLT3 acute myeloid leukemia: the path to least resistance. Int J Mol Sci. 2018;19(10).
Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):984–97.
• Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–40. This phase III trail randomized relapsed/refractory FLT3 mutated AML patients between gilteritinib and conventional salvage therapy. Patients treated with gilteritinib had higher remissin rates and a imporved median overall survival.
Poire X, Labopin M, Maertens J, Yakoub-Agha I, Blaise D, Ifrah N, et al. Allogeneic stem cell transplantation in adult patients with acute myeloid leukaemia and 17p abnormalities in first complete remission: a study from the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). J Hematol Oncol. 2017;10(1):20.
Grob T, Al Hinai AS, Sanders MA, Kavelaars F, Rijken M, Gradowska P, et al. Molecular characterization of mutant Tp53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2022.
Kim K, Maiti A, Loghavi S, Pourebrahim R, Kadia TM, Rausch CR, et al. Outcomes of TP53-mutant acute myeloid leukemia with decitabine and venetoclax. Cancer. 2021;127(20):3772–81.
Short NJ, Montalban-Bravo G, Hwang H, Ning J, Franquiz MJ, Kanagal-Shamanna R, et al. Prognostic and therapeutic impacts of mutant TP53 variant allelic frequency in newly diagnosed acute myeloid leukemia. Blood Advances. 2020;4(22):5681–9.
DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135(11):791–803.
Sekeres MA, Elson P, Kalaycio ME, Advani AS, Copelan EA, Faderl S, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28–36.
Juliusson G, Hagberg O, Lazarevic VL, Lehmann S, Höglund M. Registry ftSA. Impact of treatment delay in acute myeloid leukemia revisited. Blood. Advances. 2021;5(3):787–90.
Röllig C, Kramer M, Schliemann C, Mikesch J-H, Steffen B, Krämer A, et al. Does time from diagnosis to treatment affect the prognosis of patients with newly diagnosed acute myeloid leukemia? Blood. 2020;136(7):823–30.
Sallman D, AA, Kambhampati S, Al Malki M, Zeidner J, Donnellan W, et al. AML-196: the first-in-class anti-CD47 antibody magrolimab in combination with azacitidine is well tolerated and effective in AML patients: phase 1b results. Clinical Lymphoma Myeloma and Leukemia. 2021;21:s290.
Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA, et al. Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol. 2021;39(14):1584–94.
David A, Sallman RSK, De Zern AE, Sebert M, Garcia-Manero G, Rahmé R, Steensma DP, et al. Long term follow-up and combined phase 2 results of eprenetapopt (APR-246) and azacitidine (AZA) in patients with TP53 mutant myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia (AML). Blood. 2021;138:246.
Uy GL, Aldoss I, Foster MC, Sayre PH, Wieduwilt MJ, Advani AS, et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood. 2021;137(6):751–62.
Stein EM, Aldoss I, DiPersio JF, Stone RM, Arellano ML, Rosen G, et al. Safety and efficacy of menin inhibition in patients (Pts) with MLL-rearranged and NPM1 mutant acute leukemia: a phase (Ph) 1, first-in-human study of SNDX-5613 (AUGMENT 101). Blood. 2021;138(Supplement 1):699.
Ossenkoppele GJ, Janssen JJ, van de Loosdrecht AA. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica. 2016;101(1):20–5.
Devillier R, Forcade E, Garnier A, Guenounou S, Thepot S, Guillerm G, et al. In-depth time-dependent analysis of the benefit of allo-HSCT for elderly patients with CR1 AML: a FILO study. Blood Advances. 2022;6(6):1804–12.
Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017;130(9):1156–64.
Kaplan ZLR, van Leeuwen N, Posthuma EFM, Visser O, Huls G, van de Loosdrecht AA, et al. Improved relative survival in older patients with acute myeloid leukemia over a 30-year period in the Netherlands: a long haul is needed to change nothing into something. Leukemia. 2022;36(2):596–8.
Ustun C, Le-Rademacher J, Wang HL, Othus M, Sun Z, Major B, et al. Allogeneic hematopoietic cell transplantation compared to chemotherapy consolidation in older acute myeloid leukemia (AML) patients 60-75 years in first complete remission (CR1): an alliance (A151509), SWOG, ECOG-ACRIN, and CIBMTR study. Leukemia. 2019;33(11):2599–609.
Hilberink J, Hazenberg C, van den Berg E, Mulder A, Schuringa JJ, van der Helm L, et al. Not type of induction therapy but consolidation with allogeneic hematopoietic cell transplantation determines outcome in older AML patients: a single center experience of 355 consecutive patients. Leuk Res. 2019;80:33–9.
Versluis J, Hazenberg CL, Passweg JR, van Putten WL, Maertens J, Biemond BJ, et al. Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: a time-dependent analysis. Lancet Haematol. 2015;2(10):e427–36.
Ofran Y, Sun Z, Rowe JM, Claxton DF, Tallman MS, Arber DA, et al. Allogeneic transplantation in fit older adults is feasible and encouragingly efficacious. post remission data from the prospective ECOG-ACRIN (E2906) clinical study. Blood. 2021;138(Supplement 1):413.
Huls G, Chitu DA, Havelange V, Jongen-Lavrencic M, van de Loosdrecht AA, Biemond BJ, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457–64.
Laille E, Shi T, Garcia-Manero G, Cogle CR, Gore SD, Hetzer J, et al. Pharmacokinetics and pharmacodynamics with extended dosing of CC-486 in patients with hematologic malignancies. PLoS One. 2015;10(8):e0135520.
•• Wei AH, Dohner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al. Oral azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526–37. This phase III trail randomized AML patients unfit for HSCT between maintanaince with oral azacitidine or placebo. Patients treated with oral azacitidine had improved relapse free and overall survival.
Wei AH, Döhner H, Sayar H, Ravandi F, Montesinos P, Dombret H, et al. Long-Term Overall survival (OS) with oral azacitidine (oral-AZA) in patients with acute myeloid leukemia (AML) in first remission after intensive chemotherapy (IC): updated results from the phase 3 QUAZAR AML-001 trial. Blood. 2021;138(Supplement 1):871.
Ravandi F, Roboz GJ, Wei AH, Dohner H, Pocock C, Selleslag D, et al. Management of adverse events in patients with acute myeloid leukemia in remission receiving oral azacitidine: experience from the phase 3 randomized QUAZAR AML-001 trial. J Hematol Oncol. 2021;14(1):133.
Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–89.
Tsao T, Shi Y, Kornblau S, Lu H, Konoplev S, Antony A, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol. 2012;91(12):1861–70.
Aldoss I, Yang D, Aribi A, Ali H, Sandhu K, Al Malki MM, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica. 2018;103(9):e404–e7.
Pollyea DA, Pratz K, Letai A, Jonas BA, Wei AH, Pullarkat V, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol. 2021;96(2):208–17.
DiNardo CD, Maiti A, Rausch CR, Pemmaraju N, Naqvi K, Daver NG, et al. 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol. 2020;7(10):e724–e36.
Wei AH, Strickland SA Jr, Hou JZ, Fiedler W, Lin TL, Walter RB, et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277–84.
Pelcovits A, Moore J, Bakow B, Niroula R, Egan P, Reagan JL. Tumor lysis syndrome risk in outpatient versus inpatient administration of venetoclax and hypomethlators for acute myeloid leukemia. Support Care Cancer. 2021;29(9):5323–7.
Pollyea DA, Dinardo CD, Arellano ML, Pigneux A, Fiedler W, Konopleva M, et al. Results of venetoclax and azacitidine combination in chemotherapy ineligible untreated patients with acute myeloid leukemia with IDH 1/2 mutations. Blood. 2020;136(Supplement 1):5–7.
Pollyea DA, Tallman MS, de Botton S, Kantarjian HM, Collins R, Stein AS, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019;33(11):2575–84.
Norsworthy KJ, Mulkey F, Scott EC, Ward AF, Przepiorka D, Charlab R, et al. Differentiation syndrome with ivosidenib and enasidenib treatment in patients with relapsed or refractory IDH-mutated AML: a U.S. Food and Drug administration systematic analysis. Clin Cancer Res. 2020;26(16):4280–8.
Zeidner JF. Differentiating the differentiation syndrome associated with IDH inhibitors in AML. Clin Cancer Res. 2020;26(16):4174–6.
Jasra S, Kazemi M, Shah N, Chen J, Fehn K, Wang Y, et al. Case report of combination therapy with azacytidine, enasidenib and venetoclax in primary refractory AML. Exp Hematol Oncol. 2021;10(1):1.
Lachowiez CA, Borthakur G, Loghavi S, Zeng Z, Kadia TM, Masarova L, et al. A phase Ib/II study of ivosidenib with venetoclax +/- azacitidine in IDH1-mutated myeloid malignancies. Journal of Clinical Oncology. 2021;39(15_suppl):7012.
Konopleva M, Thirman MJ, Pratz KW, Garcia JS, Recher C, Pullarkat V, et al. Impact of F LT3 mutation on outcomes after venetoclax and azacitidine for patients with treatment-naive acute myeloid leukemia. Clin Cancer Res. 2022.
Wang ES, Montesinos P, Minden MD, Lee J-H, Heuser M, Naoe T, et al. Phase 3, open-label, randomized study of gilteritinib and azacitidine vs azacitidine for newly diagnosed FLT3-mutated acute myeloid leukemia in patients ineligible for intensive induction chemotherapy. Blood. 2021;138(Supplement 1):700.
Maiti A, DiNardo CD, Daver NG, Rausch CR, Ravandi F, Kadia TM, et al. Triplet therapy with venetoclax, FLT3 inhibitor and decitabine for FLT3-mutated acute myeloid leukemia. Blood Cancer J. 2021;11(2):25.
El-Jawahri A, LeBlanc TW, Kavanaugh A, Webb JA, Jackson VA, Campbell TC, et al. Effectiveness of integrated palliative and oncology care for patients with acute myeloid leukemia: a randomized clinical trial. JAMA Oncol. 2021;7(2):238–45.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–47.
Ferrara F, Lessi F, Vitagliano O, Birkenghi E, Rossi G. Current therapeutic results and treatment options for older patients with relapsed acute myeloid leukemia. Cancers (Basel). 2019;11(2).
Stahl M, DeVeaux M, Montesinos P, Itzykson R, Ritchie EK, Sekeres MA, et al. Hypomethylating agents in relapsed and refractory AML: outcomes and their predictors in a large international patient cohort. Blood Adv. 2018;2(8):923–32.
Bewersdorf JP, Giri S, Wang R, Williams RT, Tallman MS, Zeidan AM, et al. Venetoclax as monotherapy and in combination with hypomethylating agents or low dose cytarabine in relapsed and treatment refractory acute myeloid leukemia: a systematic review and meta-analysis. Haematologica. 2020;105(11):2659–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. de Leeuw has received honoraria for advisory boards for Servier and Takeda and lectures for Servier and Roche. Dr. Ossenkoppele has received honoraria for advisory boards for AbbVie, Roche, Astellas, Bristol Myers Squibb, Celgene, Gilead, Servier, JAZZ, and Novartis. Dr. Janssen has received research support from Novartis, Bristol Myers Squibb, Apps for Care and Science and non-profit foundation support by Amgen, Astelllas, Daiichi-Sankyo, Janssen, Olympus, Incyte, BMS, Sanofi Genzyme, Servier, Jazz, Takeda and honoraria for advisory boards for Abbvie, Novartis, Pfizer, and Incyte.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Geriatric Oncology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Leeuw, D.C., Ossenkoppele, G.J. & Janssen, J.J.W.M. Older Patients with Acute Myeloid Leukemia Deserve Individualized Treatment. Curr Oncol Rep 24, 1387–1400 (2022). https://doi.org/10.1007/s11912-022-01299-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-022-01299-9