Abstract
Purpose of Review
Due to advancements in oncologic treatment strategies and techniques, the number of survivors who have undergone hematopoetic stem cell transplant (HCT) continues to increase in the United States; this number is projected to reach 502,000 by the year 2030. There is significant interest within the field of cardio-oncology to identify cardiotoxicity and cardiovascular disease in the HCT population. Epidemiologic studies analyzing both short- and long-term cardiovascular effects, risk stratification modeling, cardioprotective strategies, and expert consensus documents for cardiotoxicity surveillance recommendations are reviewed.
Recent Findings
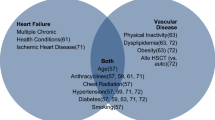
Patients who have undergone HCT are at increased risk of cardiovascular events and mortality compared to matched controls. The type of cardiotoxicity and the incidence rates vary based on specific therapeutic regimens and pre-existing cardiovascular risk factors. Life-threatening cardiotoxicity can present during HCT as acute heart failure, arrhythmias, pericardial tamponade, or cardiac arrest; or it can present late after treatment as cardiomyopathy, ischemic heart disease, vascular disease, stroke, or comorbid conditions, such as hypertension and diabetes mellitus that are associated with cardiac events.
Summary
HCT is associated with excess cardiovascular risk partially due to exposure to cardiotoxic chemotherapy and radiation, as well as indirect and direct detrimental effects on cardiovascular reserve. This review discusses the epidemiology and the known cardiotoxic effects of historical chemoradiation agents in addition to newer targeted therapies. Recent expert consensus statements from cardiology and hematology/oncology societies are reviewed in regard to risk stratification of the cancer patient based on the type of treatments. Finally, gaps in knowledge are identified with proposed avenues of research that will allow for more accurate risk assessment, prediction, and potential treatment of the HCT patient in attenuating the risk of developing both short- and long-term cardiovascular comorbidities.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–26.
Majhail NS, Tao L, Bredeson C, Davies S, Dehn J, Gajewski JL, et al. Prevalence of hematopoietic cell transplant survivors in the United States. Biol Blood Marrow Transpl. 2013;19(10):1498–501.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15(12):1628–33.
Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2012;18(3):348–71.
Murdych TWD. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977-1997. Bone Marrow Transpl. 2001;28(3):283–7.
Hertenstein B, Stefanic M, Schmeiser T, Scholz M, Göller V, Clausen M, et al. Cardiac toxicity of bone marrow transplantation: predictive value of cardiologic evaluation before transplant. J Clin Oncol. 1994;12(5):998–1004.
Kupari M, Volin L, Suokas A, Hekali PRT. Cardiac involvement in bone marrow transplantation: serial changes in left ventricular size, mass and performance. J Intern Med. 1990;227(4):256–66.
Fujimaki K, Maruta A, Yoshida M, Sakai R, Tanabe J, Koharazawa H, et al. Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone Marrow Transpl. 2001;27(3):307–10.
Tonorezos ES, Stillwell EE, Calloway JJ, Glew T, Wessler JD, Rebolledo BJ, et al. Arrhythmias in the setting of hematopoietic cell transplants. Bone Marrow Transpl. 2015;50(9):1212–6.
Chow EJ, Mueller BA, Baker KS, Cushing-Haugen KL, Flowers ME, Martin PJ, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155(1):21–32.
Armenian SH, Sun CL, Francisco L, Steinberger J, Kurian S, Wong FL, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26(34):5537–43.
Armenian SH, Sun CL, Shannon T, Mills G, Francisco L, Venkataraman K, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118(23):6023–9.
•• Bosch X, Rovira M, Sitges M, Domènech A, Ortiz-Pérez JT, de Caralt TM, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted t). J Am Coll Cardiol. 2013;61(23):2355–62. One of the only randomized control trials focusing on the bone marrow transplantation population showing a decreased incidence of congestive heart failure with initiation of carvedilol and enalapril.
Tichelli A, Bucher C, Rovó A, Stussi G, Stern M, Paulussen M, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110(9):3463–71.
Armenian SHCE. Cardiovascular disease in survivors of hematopoietic cell transplantation. Cancer. 2014;120(4):469–79.
Tichelli A, Passweg J, Wójcik D, Rovó A, Harousseau JL, Masszi T, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the late effects working party of the European group for blood and marrow transplantation. Haematologica. 2008;93(8):1203–10.
Bhatia S, Robison LL, Francisco L, Carter A, Liu Y, Grant M, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215–22.
Bhatia S, Francisco L, Carter A, Sun CL, Baker KS, Gurney JG, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–92.
Goldberg MA, Antin JH, Guinan ECRJ. Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor. Blood. 1986;68(5):1114–8.
Gottdiener JS, Appelbaum FR, Ferrans VJ, Deisseroth AZJ. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med. 1981;141(6):758–63.
Morandi P, Ruffini PA, Benvenuto GM, Raimondi RFV. Cardiac toxicity of high-dose chemotherapy. Bone Marrow Transpl. 2005;35(4):323–34.
Slavin RE, Millan JCMG. Pathology of high dose intermittent cyclophosphamide therapy. Hum Pathol. 1975;6(6):693–709.
Weinberger A, Pinkhas J, Sandbank U, de VA SM. Endocardial fibrosis following busulfan treatment. JAMA. 1975;231(5):495.
Yanamandra U, Gupta S, Khadwal AMP. Melphalan-induced cardiotoxicity: ventricular arrhythmias. BMJ Case Rep. 2016.
Quezado ZM, Wilson WH, Cunnion RE, Parker MM, Reda D, Bryant GOF. High-dose ifosfamide is associated with severe, reversible cardiac dysfunction. Ann Intern Med. 1993;118(1):31–6.
Kandylis K, Vassilomanolakis M, Tsoussis SEA. Ifosfamide cardiotoxicity in humans. Cancer Chemother Pharmacol. 1989;24(6):395–6.
Renu K, A VG, TP PB. Molecular mechanism of doxorubicin-induced cardiomyopathy—an update. Eur J Pharmacol. 2018;818:241–53.
Godown J, Deal AM, Riley K, Bailliard F, Julie Blatt M. Worsening bradycardia following antithymocyte globulin treatment of severe aplastic anemia. J Pediatr Pharmacol Ther. 2011;16(3):218–21.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375:740–53.
C RJ. Monoclonal antibodies in the treatment of cancer, part 1. Am J Health Syst Pharm. 2003;60(15):1531–48.
C RJ. Monoclonal antibodies in the treatment of cancer, part 2. Am J Health Syst Pharm. 2003;60(16):1631–41.
Talcott JAHT. Acute ischemic vascular events and cisplatin. Ann Intern Med. 1987;107(1):121–2.
Reykdal S, Sham RKP. Cytarabine-induced pericarditis: a case report and review of the literature of the cardio-pulmonary complications of cytarabine therapy. Leuk Res. 1995;19(2):141–4.
Yeh ETBC. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53(24):2231–47.
Van Besien K, Devine S, Wickrema A, Jessop E, Amin K, Yassine M, et al. Regimen-related toxicity after fludarabine-melphalan conditioning: a prospective study of 31 patients with hematologic malignancies. Bone Marrow Transpl. 2003;32(5):471–6.
Martino R, Pérez-Simón JA, Moreno E, Queraltó JM, Caballero D, Mateos M, et al. Reduced-intensity conditioning allogeneic blood stem cell transplantation with fludarabine and oral busulfan with or without pharmacokinetically targeted busulfan dosing in patients with myeloid leukemia ineligible for conventional conditioning. Biol Blood Marrow Transpl. 2005;11(6):437–47.
Kettunen R, Huikuri HV, Oikarinen ATJ. Methotrexate-linked ventricular arrhythmias. Acta Derm Venereol. 1995;75(5):391–2.
Perez-Verdia A, Angulo F, Hardwicke FLNK. Acute cardiac toxicity associated with high-dose intravenous methotrexate therapy: case report and review of the literature. Pharmacotherapy. 2005;25(9):1271–6.
Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47.
Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–16.
Sander M, Lyson T, Thomas GDVR. Sympathetic neural mechanisms of cyclosporine-induced hypertension. Am J Hypertens. 1996;9(11):121S–38S.
Ong CS, Pollock CA, Caterson RJ, Mahony JF, Waugh DAIL. Hyperlipidemia in renal transplant recipients: natural history and response to treatment. Medicine. 1994;73(4):215–23.
Textor SC, Canzanello VJ, Taler SJ, Schwartz LAJ. Hypertension after liver transplantation. Liver Transpl Surg. 1995;1(5 Suppl 1):20–8.
Dehghani SM, Haghighat M, Imanieh MH, Zahmatkeshan M, Borzooei M, Amoozegar H, et al. Tacrolimus related hypertrophic cardiomyopathy in liver transplant recipients. Arch Iran Med. 2010;13(2):116–9.
Curtis JJ, Galla JH, Woodford SY, Lucas BALR. Effect of alternate-day prednisone on plasma lipids in renal transplant recipients. Kidney Int. 1982;22(1):42–7.
Florescu M, Mircea Cinteza D, Vinereanu. Chemotherapy-induced cardiotoxicity. Maedica (Buchar). 2013;8(1):59–67.
Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–92.
Strati P, Kantarjian H, Ravandi F, Nazha A, Borthakur G, Daver N, et al. Phase I/II trial of the combination of Midostaurin (PKC412) and 5-Azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am J Hematol. 2015;90(4):276–81.
San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–206.
Newman M, Malla MGI. Azacitidine-induced pericarditis: a case series. Pharmacotherapy. 2016;36(4):443–8.
Bibault JE, Cambier N, Lemahieu JM, Quesnel B, Auffret MRC. Acute myocarditis induced by hypomethylating agents. J Clin Oncol. 2011;14:e411–2.
Gov. UK. Lenalidomide: risk of thrombosis and thromboembolism. https://www.gov.uk/drug-safety-update/lenalidomide-risk-of-thrombosis-and-thromboembolism. Accessed November 12, 2018.
Force T, Krause DSVER. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7(5):332–44.
van der SL HN. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57(4):748–57.
M LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transpl. 2002;2(9):807–18.
Salma Alam C, Yuk Ting Ma PP. Gemcitabine-induced cardiotoxicity in patients receiving adjuvant chemotherapy for pancreatic cancer: a case series. Case Rep Oncol. 2018;11:221–7.
Tomirotti M, Riundi R, Pulici S, Ungaro A, Pedretti D, Villa SSA. Ischemic cardiopathy from cis-diamminedichloroplatinum (CDDP). Tumori. 1984;70(3):235–6.
Mortimer JE, Crowley J, Eyre H, Weiden P, Eltringham JSW. A phase II randomized study comparing sequential and combined intraarterial cisplatin and radiation therapy in primary brain tumors. A Southwest Oncology Group study. Cancer. 1992;69(5):1220–3.
Dugbartey GJ, de GI PLJ. An integrative view of cisplatin-induced renal and cardiac toxicities: molecular mechanisms, current treatment challenges and potential protective measures. Toxicology. 2016;371:58–66.
Grandin EW, Ky B, Cornell RF, Carver JLD. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. J Card Fail. 2015;21(2):138–44.
Atrash S, Tullos A, Panozzo S, Bhutani M, Van Rhee F, Barlogie BUS. Cardiac complications in relapsed and refractory multiple myeloma patients treated with carfilzomib. Blood Cancer J. 2015;5:e272.
Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–17.
Montani D, Bergot E, Günther S, Savale L, Bergeron A, Bourdin A, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation. 2012;125(17):2128–37.
Chen MH, Kerkelä RFT. Mechanisms of cardiac dysfunction associated with tyrosine kinase inhibitor cancer therapeutics. Circulation. 2008;118(1):84–95.
Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(4):2542–51.
Miller GD, Bruno BJLC. Resistant mutations in CML and Ph(+)ALL—role of ponatinib. Biologics. 2014;8:243–54.
Mandel EM, Lewinski UDM. Vincristine-induced myocardial infarction. Cancer. 1975;36(6):1979–82.
Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DCPM. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005;23(30):7685–96.
Armenian SH, Sun CL, Vase T, Ness KK, Blum E, Francisco L, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120(23):4505–12.
Sakata-Yanagimoto M, Kanda Y, Nakagawa M, Asano-Mori Y, Kandabashi K, Izutsu K, et al. Predictors for severe cardiac complications after hematopoietic stem cell transplantation. Bone Marrow Transpl. 2004;33(10):1043–7.
Peres E, Levine JE, Khaled YA, Ibrahim RB, Braun TM, Krijanovski OI, et al. Cardiac complications in patients undergoing a reduced-intensity conditioning hematopoietic stem cell transplantation. Bone Marrow Transpl. 2010;45(1):149–52.
Heidenreich PAKJ. Radiation induced heart disease: systemic disorders in heart disease. Heart. 2009;95:252–8.
Mulrooney DA, Nunnery SE, Armstrong GT, Ness KK, Srivastava D, Donovan FD, et al. Coronary artery disease detected by coronary computed tomography angiography in adult survivors of childhood Hodgkin lymphoma. Cancer. 2014;120(22):3536–44.
Armenian SH, Sun CL, Mills G, Teh JB, Francisco L, Durand JB, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transpl. 2010;16(8):1138–44.
S C. Acute life-threatening toxicity of cancer treatment. Crit Care Clin. 2001;17(3):483–502.
Braverman AC, Antin JH, Plappert MT, Cook EFLR. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol. 1991;9(7):1215–23.
Mesa RA, Verstovsek S, Gupta V, Mascarenhas JO, Atallah E, Burn T, et al. Effects of ruxolitinib treatment on metabolic and nutritional parameters in patients with myelofibrosis from COMFORT-I. Clin Lymphoma Myeloma Leuk. 2015;15(4):214–21.
Tichelli AGA. Vascular endothelium as “novel” target of graft-versus-host disease. Best Pr Res Clin Haematol. 2008;21(2):139–48.
Ferreira DC, de Oliveira JS, Parísio KRF. Pericardial effusion and cardiac tamponade: clinical manifestation of chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Rev Bras Hematol Hemoter. 2014;36(2):159–61.
Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–72.
•• Armenian SH, Yang D, Teh JB, Atencio LC, Gonzales A, Wong FL, et al. Prediction of cardiovascular disease among hematopoietic cell transplantation survivors. Blood Adv. 2018;2(14):1756–64. A recent proposed risk stratification model in hematopoetic cell transplantations in predicting long term cardiovascular risk.
•• Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39. Expert consensus document from the American Society of Echocardiography and European Association of Cardiovascular Imaging discussing the various modern cardiovascular imaging modalities and recommendations for screening for cardiotoxicity, based on cancer treatment agents used.
• Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Hear J Cardiovasc Imaging. 2013;14(8):721–40. Expert consensus statement from the European Association of Cardiovascular Imaging and American Society of Echocardiography reviewing different modern cardiovascular imaging modalities in screening and evaluating for long term sequelae of radiation treatments.
Chow EJ, Anderson L, Baker KS, Bhatia S, Guilcher GM, Huang JT, et al. Late effects surveillance recommendations among survivors of childhood hematopoietic cell transplantation: a Children’s Oncology Group report. Biol Blood Marrow Transpl. 2016;22(5):782–95.
•• Children’s Oncology Group long-term follow-up guidelines. Version 4.0, October 2013. http://www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf. Accessed September 15, 2018. An all encompassing document reviewing multiorgan short and long term toxicities in childhood cancer survivors.
•• Armenian SH, Lacchetti CLD, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline summary. J Clin Oncol. 2017;35(8):893–911. Expert Consensus document by the American Society of Clinical Oncology that, amongst other recommendations, demarcates the "high risk" threshold for cardiotoxicity with a total cumulative dose of doxorubicin of >/= 250 mg/m2.
•• Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123–36. An expert consensus statement unifying the varying expert consensus opinions on cardiotoxicity screening frequency and type in childhood cancer survivors.
• Squires RW, Shultz AM, Herrmann J. Exercise training and cardiovascular health in cancer patients. Curr Oncol Rep. 2018;20(3):27. State of the art review evaluating the potential role of exercise therapy in cancer patients, as well as cardiopulmonary exercise effects in cancer patients.
Armenian SH, Horak D, Scott JM, Mills G, Siyahian A, Berano Teh J, et al. Cardiovascular function in long-term hematopoietic cell transplantation survivors. Biol Blood Marrow Transpl. 2017;23(4):700–5.
Abo S, Ritchie D, Denehy L, Panek-Hudson Y, Irving LGC. A hospital and home-based exercise program to address functional decline in people following allogeneic stem cell transplantation. Support Care Cancer. 2018;26(6):1727–36.
Yildiz Kabak V, Duger TUCD. Investigation of the effects of an exercise program on physical functions and activities of daily life in pediatric hematopoietic stem cell transplantation. Pediatr Blood Cancer. 2016;63(9):1643–8.
Keen C, Skilbeck J, Ross H, Smith L, Collins K, Dixey J, et al. Is it feasible to conduct a randomised controlled trial of pretransplant exercise (prehabilitation) for patients with multiple myeloma awaiting autologous haematopoietic stem cell transplantation? Protocol for the PREeMPT study. BMJ Open. 2018;8(3):e021333.
Keats MR, Grandy SA, Giacomantonio N, MacDonald D, Rajda M, TY. EXercise to prevent AnthrCycline-based Cardio-Toxicity (EXACT) in individuals with breast or hematological cancers: a feasibility study protocol. Pilot Feasibility Study. 2016;2(44).
Pituskin E, Haykowsky M, McNeely M, Mackey J, Chua NPI. Rationale and design of the multidisciplinary team IntervenTion in cArdio-oNcology study (TITAN). BMC Cancer. 2016;16(1):733.
Chang VY, W J. Pharmacogenetics of chemotherapy-induced cardiotoxicity. Curr Oncol Rep. 2018;20(7):52.
van Dalen EC, Caron HN, Dickinson HOKL. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev 2012. 2008;2:CD003917.
Asselin BL, Devidas M, Chen L, Franco VI, Pullen J, Borowitz MJ, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children’s Oncology Group randomized trial pediatric oncology group. J Clin Oncol. 2016;34(8):854–62.
Blaes AH, Thavendiranathan P, Moslehi J. Cardiac toxicities in the era of precision medicine: underlying risk factors, targeted therapies, and cardiac biomarkers. ASCO Educ B. 2018;(38).
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31.
Sheng CC, Amiri-Kordestani L, Palmby T, Force T, Hong CC, Wu JC, et al. 21st Century cardio-oncology: identifying cardiac safety signals in the era of personalized medicine. JACC Basic Transl Sci. 2016;1(5):386–98.
Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RPEB. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9–16.
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98.
• Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–21. Genetic study linking a clonal somatic mutations, known as “CHIP”, with possible links to both hematologic malignancies and cardiovascular disease.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mirela Tuzovic, Monica Mead, Patricia A. Young, Gary Schiller, and Eric H. Yang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-oncology
Rights and permissions
About this article
Cite this article
Tuzovic, M., Mead, M., Young, P.A. et al. Cardiac Complications in the Adult Bone Marrow Transplant Patient. Curr Oncol Rep 21, 28 (2019). https://doi.org/10.1007/s11912-019-0774-6
Published:
DOI: https://doi.org/10.1007/s11912-019-0774-6