Abstract
Purpose of Review
To review the literature on visual dysfunction in dementia with Lewy bodies (DLB), including its mechanisms and clinical implications.
Recent Findings
Recent studies have explored novel aspects of visual dysfunction in DLB, including visual texture agnosia, mental rotation of 3-dimensional drawn objects, and reading fragmented letters. Recent studies have shown parietal and occipital hypoperfusion correlating with impaired visuoconstruction performance. While visual dysfunction in clinically manifest DLB is well recognized, recent work has focused on prodromal or mild cognitive impairment (MCI) due to Lewy body pathology with mixed results. Advances in retinal imaging have recently led to the identification of abnormalities such as parafoveal thinning in DLB.
Summary
Patients with DLB experience impairment in color perception, form and object identification, space and motion perception, visuoconstruction tasks, and illusions in association with visual cortex and network dysfunction. These symptoms are associated with visual hallucinations, driving impairment, falls, and other negative outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia with Lewy bodies (DLB) is the second-most common neurodegenerative cause of dementia after Alzheimer disease (AD) and is defined pathologically by the presence of synuclein-positive Lewy bodies [1,2,3]. In addition to progressive cognitive decline leading to dementia, DLB is characterized by four cardinal symptoms: fluctuating cognition, recurrent visual hallucinations, rapid eye movement (REM) sleep behavior disorder (RBD), and parkinsonism [4] (Table 1). Cognitive impairment in DLB is most prominent in the executive, attention and visuospatial domains, with relative sparing of memory earlier in the disease [4,5,6]. Prominent visuoperceptive dysfunction and visual hallucinations figure prominently in the most recent diagnostic criteria for DLB (Table 1) [4], In this article, we review the patterns, causes, and clinical implications of visual dysfunction in DLB.
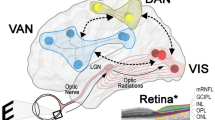
Overview of the central visual pathways
The retina converts light into signals that are relayed by white matter tracts to the lateral geniculate nucleus and ultimately the visual cortex. Cortical visual processing begins in the primary visual cortex (V1) and secondary visual cortex (V2) before extending to large areas of the occipital, parietal, and temporal lobes. Higher order visual processing can be conceptually divided into two broad domains: the ventral “what” and dorsal “where” streams. The ventral “what” stream involves the inferior occipital and temporal lobes and is important for generating perceptual representations from visual input, including processing color, form, shape, and recognizing faces and objects. The dorsal “where” stream is located in the superior occipital and parietal lobes and is important for perceiving spatial localization and motion, including motor planning and execution in response to visual inputs [7].
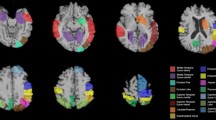
While visual acuity is typically preserved in DLB [8], the functions of the dorsal and ventral streams are both prominently affected, resulting in broad impairments in visuoperception and visuospatial function (Table 2). Consistent with these symptoms, studies have demonstrated hypometabolism, hypoperfusion, abnormal connectivity, reduced gray matter thickness, and white matter disruption within the occipital lobes [4, 9,10,11,12,13,14,15,16,17,18,19]. However, pathological studies have found that the occipital lobe is not disproportionately affected by Lewy body density or neuronal loss compared to other cortical regions in DLB [20,21,22,23,24]. The occipital cortex has reduced PET markers of cortical synaptic density in DLB and PDD, but this is not of sufficient amplitude to account for the degree of occipital hypometabolism seen [25]. Rather, subcortical structures with projections to the visual cortex such as the pulvinar nucleus and superior colliculus have significant pathological involvement, which may contribute to functional impairment in visual pathways and regions [26,27,28].
Visual networks in DLB
A number of studies have examined alterations in network connectivity in DLB. As recently systematically reviewed by Habich et al., visual networks, along with frontoparietal networks and the default mode network, are together the most prominently and consistently altered networks across studies and modalities (structural, fMRI, PET, and EEG) [10], concordant with the hallmark clinical deficits in visuoperception, attention, and executive function seen in DLB. Alterations in white matter tracts connecting different regions are associated with decreased activity in associated cortical regions, and may contribute to this impaired network connectivity [29].
These network changes correlate directly with performance on neuropsychological tests of visuospatial function [9, 18, 30, 31]. For example, one study found that performance on a visual search attentive matrix task correlated with primary visual network connectivity as measured using FDG-PET connectivity, and the Raven’s colored progressive matrix task (a test of visuospatial reasoning) correlated with executive prefrontal cortex network connectivity [32]. Network alterations figure prominently in a number of visual symptoms in DLB, especially visual hallucinations, as discussed below.
Contrast and Color
Aspects of visual function that involve perceiving shades of contrast or color may be comparatively simple compared to other higher order tasks, but the anatomy of these functions is nevertheless complex. Contrast sensitivity [33] and color vision [33,34,35] have both been shown to be impaired in DLB compared to controls and AD. Color vision is especially promising as a potential biomarker of DLB given its impairment in prodromal DLB and in patients with isolated RBD who later develop parkinsonism-dementia syndromes [35, 36]. To our knowledge, the prevalence of visual field defects in DLB has not been reported. Homonymous visual field defects can occur in posterior cortical atrophy, but this syndrome is more often caused by AD than DLB pathology.
Contrast sensitivity and color vision are prominently affected in retinal and optic nerve disease. As such, while research has historically focused on the brain as the primary source of visual dysfunction in DLB, there is growing evidence that the retina may also be affected. Pale retinal inclusions have been identified at autopsy in a single patient with DLB [37], and electroretinogram abnormalities have also been reported [38]. Retinal phosphorylated α-synuclein was previously reported in a subset of patients with PD (7/9) and DLB (1/3) [39], and a more recent study found retinal and optic nerve head α-synuclein aggregates and inclusions in 5/5 patients with DLB and 16/21 patients with PD [40].
Optical coherence tomography (OCT) analysis of patients with DLB has demonstrated thinning of the ganglion cell layer, but this is associated with both age and occipital lobe volume in the general population, so it was initially unclear if this represents primary retinal pathology or is a marker of age and global neurodegeneration [41,42,43]. However, a recent study did show significant OCT differences between age-matched groups of patients with DLB and healthy controls, including thinner ganglion cell layer and altered microvasculature [44]. In general, the retina has been better studied in PD than DLB [45,46,47,48,49,50]. More work utilizing OCT and related measures on larger DLB cohorts would be helpful for expanding on these early findings.
Object and Form Recognition
Perception of an image’s size, shape, form, and orientation involves aspects of the dorsal and ventral streams, both of which are affected in DLB. As a result, a number of impairments in object recognition have been identified in DLB. Visuoperceptual impairments in DLB can be elicited through the use of images with distorted or missing components (such as fragmented letters), which require reconstructing an image from incomplete information. A number of studies have found impairment in object and form recognition tasks in DLB, including identifying overlapping figures, silhouettes, rotated figures, incomplete figures, and figures from illusory contours (Table 3) [33, 51,52,53,54,55,56]. The fragmented letters task may be particularly sensitive for differentiating DLB from AD [55, 57, 58]. Patients with DLB have also been shown to have impairments in tasks requiring assessment of shape features, such as matching polygons [51], analyzing the number of cubes in a shape [33, 55], and visual search for target features in an array of shapes [61, 62].
A particular type of visuoperceptual impairment is known as visual agnosia, which refers to the inability to recognize an object despite being able to see all elements of the image. While commonly tested using 2-dimensional or 3-dimensional images of everyday household objects or representations of familiar faces, recent studies have examined the ability to recognize object textures (visual texture agnosia), finding DLB patients have impaired recognition of both real and computer-generated image textures, with particular difficulty in recognizing ceramic textures, independently of contrast sensitivity and color vision [33].
People with DLB show abnormal electrographic responses and connectivity in response to visual stimuli, including checkerboard patterns and faces. Findings include delayed visual evoked potentials, prolonged late visual responses and reduced theta activation in response to visual stimuli but not auditory stimuli, indicating that this disruption specifically involves vision-related networks [63,64,65]. Using resting state EEG, occipital visual networks have also been shown to be reduced in DLB, with decreased occipital alpha correlating with impaired visual shape discrimination [66]. Further work is needed to elucidate the causative role of these network disruptions in object recognition.
Spatial Orientation and Motion
Impairment in perception of space and motion are also broadly impaired in DLB. Examples of visuospatial impairment in DLB include difficulty with mental rotation of complex images [8, 60], assessment of position in space [33, 53], and motion detection [51, 53, 59] This impairment varies with the task in question: for example, assessment of the orientation of individual lines has been found to be preserved in DLB, but determining the orientation of a 3-dimensional object is impaired [8]. Of note, visuospatial impairment in DLB is associated with executive dysfunction, cognitive fluctuations, and hallucinations, but is independent of memory impairment or global measures of cognition such as the Mini Mental Status Exam (MMSE) [60]. This suggests that visuospatial dysfunction is a manifestation of DLB-specific cognitive impairment rather than simply a reflection of global cognitive impairment.
Task-specific fMRI studies during visual motion tasks show that patients with DLB have less activation of the middle temporal visual area (V5/MT), which is involved in motion detection, compared to AD and healthy controls [67, 68]. In contrast, fMRI responses in other regions to other stimuli were not significantly different in patients with DLB, including to color, face, and pattern stimuli. This suggests the impaired activation of V5/MT may play a role in impaired motion detection in DLB, but the underlying mechanism behind this requires further study.
Figure Copying and Other Constructional Tasks
Figure copying relies heavily on both the ventral and dorsal visual streams. The ventral “what” stream is required for perception of the features of the object to be copied, and the dorsal “where” stream is required to determine the spatial relationship between those features and to guide the subject’s movements to create that representation. However, unlike perceptual tasks which rely solely on the encoding and interpretation of a visual image, constructional tasks additionally require executive function to plan the drawing and motor function to draw it. Given that executive and motor impairment are also prominently affected in DLB, these tasks are less precise assessments of visual function. However, visuoconstruction tasks such as figure copying and clock drawing are frequently used as part of office-based cognitive screening exams such as the Mini-Mental Status Exam (MMSE), Montreal Cognitive Assessment (MOCA), and Mini-Cog, providing the practical advantage of easy administration and widespread availability.
Clock drawing in particular has consistently been shown to be impaired in DLB, both when drawing from memory and when copying the image of a clock [6, 69,70,71], and poorer clock drawing performance has been shown to predict more rapid progression of dementia [72]. Clock drawing impairment is associated with hypometabolism in the precuneus, middle frontal gyrus, and temporoparietal region including the angular gyrus after controlling for MMSE score [73], which reflects the aspects of visuospatial, motor, and executive function required for this task.
The pentagon copy task, which is part of the MMSE, has also been shown to be impaired in DLB [74,75,76,77], and errors in the number of angles drawn are common in mild cognitive impairment (MCI) that later evolves into DLB [78, 79]. Pentagon copying impairment in DLB correlates with hypometabolism throughout the occipital cortex as well as posterior temporo-parietal cortex and small regions of frontal cortex, while in AD there is comparable correlation with temporo-parietal and frontal hypometabolism but no correlation with occipital hypometabolism [80].
Other tests of figure copying including the Bender Gestalt test [81, 82] and Rey-Osterrieth complex figure (ROCF) copying [83] have also been shown to be impaired in DLB, and this impairment has correlates with occipital cortex hypometabolism [84, 85]. A recent study applying both FDG and dopamine transporter (DAT) PET to DLB patients found that while both occipital and lateral parietal hypometabolism and caudate DAT signal correlated with ROCF copy performance, path analysis suggested that the cortical hypometabolism alone causally mediated the relationship [85].
Visual Impairment in prodromal DLB
Studies of visual perception in prodromal DLB or MCI due to presumed Lewy body pathology (MCI-LB) have yielded inconsistent results. Several recent studies found mild abnormalities in subtests of the Visual Object and Space Perception (VOSP) battery in DLB compared to other mild cognitive impairment groups [79, 86, 87], but others have not [88,89,90]. Errors in figure copying have also been found in patients with isolated REM behavior disorder up to a year prior to DLB diagnosis [91]. Overall, this is an emerging area in need of further research, and these discrepancies may be due to differences in the specific populations and how prodromal DLB-related states were defined.
Visual hallucinations and illusions
Visual hallucinations (VH) are one of the core clinical features of DLB (Table 1) and a common presenting symptom [92,93,94], with one key study estimating 72% of DLB patients to have visual hallucinations [95]. Visual hallucinations are much more common than auditory hallucinations, in contrast to the pattern seen in primary psychotic disorders [96]. Patients with DLB can experience complex visual hallucinations of people and animals as well as other visual phenomena, such as visual illusions and brief passage hallucinations [4]. Visual hallucinations have been reported to resolve with eye closure in DLB [97], suggesting that activation of the primary visual cortex is a necessary prerequisite for hallucination formation. The importance of the visual system in visual hallucinations is exemplified by the Charles Bonnet syndrome, a phenomenon in which patients with vision loss due to ophthalmic disease develop visual hallucinations in the absence of neurologic or psychiatric disease. Visual hallucinations in the Charles Bonnet syndrome and DLB have several features in common, although hallucinations due to eye disease are more likely to be simpler phenomena than the complex hallucinations often seen in DLB [98]. Eye disease has been associated with an increased risk of hallucinations in PD [99, 100], suggesting that ophthalmic disease may represents an opportunity to prevent or reduce symptom burden. However, further work is needed to elucidate the relationship between anterior visual pathway impairment and hallucinations in DLB specifically.
Formed visual hallucinations (e.g. seeing faces, animals, or people) in DLB are associated with deficits in visuoperception, abstraction, and attention [52, 101,102,103,104,105,106]. However, one study found that only verbal memory impairment (and not visual impairment) was predictive of non-hallucinating DLB patients developing new visual hallucinations in the future [104]. Interestingly, while a recent study of patients with DLB and PDD showed complex visual hallucinations correlating with this multidomain cognitive impairment, minor hallucinations (illusions, passage hallucinations, presence hallucinations) were not correlated with cognitive impairment in any domain [102]. They also found that while complex hallucinations were associated with multiple visual and non-visual network disruptions (ventral visual stream, salience network, and default mode network), minor visual hallucinations were associated with decreased connectivity between primary visual areas, ventral visual stream, and brainstem. This raises the hypothesis that disruption in visual networks may be the driver of minor visual hallucinations, but that complex hallucinations require broader disruption involving these other cortical networks.
Perfusion SPECT studies in DLB with and without visual hallucinations have consistently found that visual hallucinations correlate with occipital hypoperfusion, with some studies also showing hypoperfusion in anterior cingulate, orbitofrontal, parietal, and superior temporal cortical regions [107,108,109,110,111]. Metabolic (FDG-PET) and functional (fMRI) connectivity analyses found that patients with DLB who have visual hallucinations show decreased connectivity in both visual and attention systems [32, 112,113,114]. However, this is not universal, as one functional connectivity analysis showed DLB patients with visual hallucinations had altered connectivity in attention but not visual networks, as well as associated white matter structural abnormalities in association tracts [115]. Structural-electrophysiological connectivity network analysis has found that DLB and PDD patients with hallucinations have both reduced electrophysiological connectivity in the ventral visual network and decreased structural connectivity between visual cortex and subcortical structures (thalamus and nucleus basalis) [28]. Overall, this suggests that dysfunction in visual systems is one driver of visual hallucinations, but other regions and systems, including attention networks and subcortical structures, appear to play key roles as well.
Pareidolia, an illusion wherein an image of one object is misinterpreted as another (for example, mistaking a coat rack for a person), is a symptom that frequently co-occurs with hallucinations in DLB. This can be tested using complex visual scenes (scene pareidolia), or by assessing whether patients perceive faces or objects in a black-and-white noise pattern (noise pareidolia). Of the two, noise pareidolia is more strongly associated with visual hallucinations and neuropsychological impairment in DLB [116, 117]. However, a recent study found that noise pareidolia impairment in DLB did not correlate with occipital cortex hypoperfusion but instead weakly correlated with frontal, parietal, and cingulate cortical hypoperfusion [118]. A recent study using factor analysis to assess relationships between scene pareidolia and other behavioral factors seen in DLB found distinct associations with both 1) visual hallucinations and cognitive fluctuations and 2) visual processing, suggesting both factors may be independently playing a role in pareidolic illusions [119].
Phosphenes can be induced by transcranial magnetic stimulation (TMS), and this has been used as a model to manipulate the brain to produce false visual perceptions analogous to hallucinations. However, there was no difference in phosphene threshold elicited by TMS to the visual cortex in patients with DLB compared to healthy controls, and only a weak relationship between phosphene threshold and hallucination severity in DLB [120, 121]. There was also no significant difference in electrographic cortical response to visual cortex TMS in patients with DLB or PDD, either with or without visual hallucinations [122]. However, patients with DLB who had visual hallucinations showed less activation of the dorsal attention network (DAN) in response to TMS network stimulation, raising the possibility that impairment in this attention network may play a role in visual hallucinations in DLB [122].
Overall, this evidence suggests that visual hallucinations in DLB may result from interplay between visual system dysfunction and other systems, including attention, association, abstract reasoning, and the default mode network.
Outcomes and quality of life associated with visual dysfunction in DLB
While a considerable amount of research has focused on describing visual dysfunction in DLB and identifying its functional and anatomic correlates, the effects of visual dysfunction on patient-reported outcomes and quality of life have not been well studied. Patients frequently complain of misjudging objects, even in MCI due to presumed Lewy body disease that has not yet progressed to dementia [123,124,125]. Validated vision-related quality of life instruments such as the VFQ-25 have not been used, though they do not capture visual dysfunction due to neurologic disease as they were developed primarily for patients with reduced visual acuity due to ophthalmic disease (e.g. macular degeneration, cataracts). A 10-item neurological supplement to the VFQ-25 has been developed and validated in other conditions, but this has not been applied to DLB [126].
In the general older adult population, visual impairment is associated with an increased risk of falls through multiple mechanisms, including effects on maintaining posture and navigating stairs. Patients with DLB are already at risk of falls due to parkinsonism and postural instability, and because spatial perception and navigation are also affected, this may further increase the risk of falls. Notably, performance on figure copying tests is associated with fall risk in DLB even when adjusting for other fall risk factors such as visual hallucinations, parkinsonism, and cognitive fluctuations [127].
Driving is another key area where visual dysfunction may have major implications. In a systematic review of the relationship between cognitive assessments and driving in dementia in the general population [128], the Visual Object and Space Perception battery was consistently associated with poor performance in driving simulations across studies [129,130,131]. A study of simulated driving performance in mild DLB found a strong correlation between driving rating score and performance on the visuospatial but not object subsets of the VOSP [132]. In on-road driving assessments, nearly one in three patients with DLB or PDD failed their driving assessment, primarily due to difficulties with positioning on the road, merging with traffic, and turning left [133, 134].
Treatment
Evidence-based treatments for visual dysfunction in DLB, especially to improve quality of life and prevent falls and injury, are lacking. The cholinergic neurotransmitter system is prominently affected in DLB, including alterations in cholinergic networks, and acetylcholine transmission is particularly reduced in the occipital lobes [135,136,137,138,139,140,141]. However, while cholinesterase inhibitors such as donepezil may reduce visual hallucinations, improve global cognitive function, and even increase occipital lobe perfusion [142], other visual symptoms do not appear responsive to these changes. Specifically, tests of object recognition and visual agnosia did not improve in a randomized controlled trial of donepezil [143]. Trials of galantamine and rivastigmine [144, 145] did not report on primary visuoperceptual measures.
Because of the known relationship between visual function and hallucinations, it has been hypothesized that improving visual acuity through ophthalmologic interventions such as refraction or cataract surgery may reduce hallucinations. Preliminary support for this comes from small case series of patients with non-DLB dementias [146], but more extensive studies are lacking. Physical and occupational therapy interventions to prevent falls commonly include recommendations to maximize contrast and lighting in addition to clearing pathways of obstacles. Further data is needed to clarify how clinicians should best approach driving safety in patients with DLB who have visuoperceptual deficits, especially since standard office-based cognitive assessments do not predict driving performance well. Possibilities to consider for stratifying driving risk include on-road driving assessments, comprehensive neuropsychological assessments, and ride-alongs from support persons to assess driving safety.
Comparison to Alzheimer disease
DLB is the second most common neurodegenerative cause of dementia after AD. Distinguishing between the two can be challenging, especially in earlier stages of disease when parkinsonism and hallucinations may be mild, but this differential is important especially as amyloid-specific therapies for AD become available. In these cases, testing visuoperception and visuoconstruction can be helpful for clarifying the diagnosis. Compared to AD, DLB is characterized by greater visuospatial impairment and less memory impairment, especially early in disease [5, 6, 71, 147, 148]. In one study, a combination of worse performance on trail-making and the Rey-Osterrieth Complex Figure copy and better performance on the Boston Naming and Auditory Verbal Learning tests best differentiated DLB from AD [83]. A more recent clinical-pathological study found that the Fragmented Letters test was the single best test to distinguish autopsy-confirmed DLB from AD, and that Fragmented Letters test score correlated with the density of Lewy bodies on histopathology [57]. Comparing figure copying and delayed figure recall can also be helpful. AD patients may have difficulty drawing a clock from memory, but this improves when asked to copy a clock from an image they can actively see image. In contrast, DLB patients have difficulty with both copying and spontaneous recall of images [6, 69,70,71].
Conclusion
Visual dysfunction is a core feature of DLB, including impairments in contrast and color discrimination, object and form recognition, and spatial orientation and motion. Patients frequently report misjudging objects and distances, and deficits can be illustrated through simple constructional tasks such as figure copying. While treatment options are limited, addressing visuospatial impairment is an important part of a multidisciplinary approach to fall prevention, and improving vision may reduce the burden of hallucinations and other adverse outcomes in DLB, though further research is needed.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, et al. The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology. 1990;40(1):1–8.
Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree--a new disease? Clin Neuropathol. 1984;3(5):185–92.
Lewis AJ, Gawel MJ. Diffuse lewy body disease with dementia and oculomotor dysfunction. Mov Disord. 1990;5(2):143–7.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100.
Connor DJ, Salmon DP, Sandy TJ, Galasko D, Hansen LA, Thal LJ. Cognitive Profiles of Autopsy-Confirmed Lewy Body Variant vs Pure Alzheimer Disease. Arch Neurol. 1998;55(7):994–1000.
Galasko D, Katzman R, Salmon DP, Hansen L. Clinical and Neuropathological Findings in Lewy Body Dementias. Brain Cogn. 1996;31(2):166–75.
Milner AD, Goodale MA. Two visual systems re-viewed. Neuropsychologia. 2008;46(3):774–85.
Metzler-Baddeley C, Baddeley RJ, Lovell PG, Laffan A, Jones RW. Visual impairments in dementia with Lewy bodies and posterior cortical atrophy. Neuropsychology. 2010;24(1):35.
Bozzali M, Falini A, Cercignani M, Baglio F, Farina E, Alberoni M, et al. Brain tissue damage in dementia with Lewy bodies: an in vivo diffusion tensor MRI study. Brain. 2005;128(7):1595–604.
Habich A, Wahlund LO, Westman E, Dierks T, Ferreira D. (Dis-)Connected Dots in Dementia with Lewy Bodies—A Systematic Review of Connectivity Studies. Mov Disord. 2023;38(1):4–15.
Kantarci K, Ferman TJ, Boeve BF, Weigand SD, Przybelski S, Vemuri P, et al. Focal atrophy on MRI and neuropathologic classification of dementia with Lewy bodies. Neurology. 2012;79(6):553–60.
Khadhraoui E, Müller SJ, Hansen N, Riedel CH, Langer P, Timäeus C, et al. Manual and automated analysis of atrophy patterns in dementia with Lewy bodies on MRI. BMC Neurol. 2022;22(1):114.
Lee JE, Park HJ, Park B, Song SK, Sohn YH, Lee JD, et al. A comparative analysis of cognitive profiles and white-matter alterations using voxel-based diffusion tensor imaging between patients with Parkinson’s disease dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2010;81(3):320–6.
Middelkoop HAM, Van Der Flier WM, Burton EJ, Lloyd AJ, Paling S, Barber R, et al. Dementia with Lewy bodies and AD are not associated with occipital lobe atrophy on MRI. Neurology. 2001;57(11):2117–20.
Pizzi SD, Franciotti R, Tartaro A, Caulo M, Thomas A, Onofrj M, et al. Structural Alteration of the Dorsal Visual Network in DLB Patients with Visual Hallucinations: A Cortical Thickness MRI Study. PLoS One. 2014;9(1):e86624.
Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, et al. Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord. 2010;25(5):615–22.
Vemuri P, Simon G, Kantarci K, Whitwell JL, Senjem ML, Przybelski SA, et al. Antemortem differential diagnosis of dementia pathology using structural MRI: Differential-STAND. NeuroImage. 2011;55(2):522–31.
Watson R, Blamire AM, Colloby SJ, Wood JS, Barber R, He J, et al. Characterizing dementia with Lewy bodies by means of diffusion tensor imaging. Neurology. 2012;79(9):906–14.
Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130(3):708–19.
Armstrong RA. Visual signs and symptoms of dementia with Lewy bodies. Clin Exp Optom. 2012;95(6):621–30.
Gomez-Tortosa E, Newell K, Irizarry MC, Albert M, Growdon JH, Hyman BT. Clinical and quantitative pathologic correlates of dementia with Lewy bodies. Neurology. 1999;53(6):1284–4.
Khundakar AA, Hanson PS, Erskine D, Lax NZ, Roscamp J, Karyka E, et al. Analysis of primary visual cortex in dementia with Lewy bodies indicates GABAergic involvement associated with recurrent complex visual hallucinations. Acta Neuropathol Commun. 2016;4(1):1–18.
Yamamoto R, Iseki E, Murayama N, Minegishi M, Marui W, Togo T, et al. Investigation of Lewy pathology in the visual pathway of brains of dementia with Lewy bodies. J Neurol Sci. 2006;246(1):95–101.
Patterson L, Firbank MJ, Colloby SJ, Attems J, Thomas AJ, Morris CM. Neuropathological Changes in Dementia With Lewy Bodies and the Cingulate Island Sign. J Neuropathol Exp Neurol. 2019;78(8):717–24.
Andersen KB, Hansen AK, Schacht AC, Horsager J, Gottrup H, Klit H, et al. Synaptic Density and Glucose Consumption in Patients with Lewy Body Diseases : An [ 11 C ] UCB-J and [ 18 F ] FDG PET Study. Mov Disord. 2023;38(5):796–805.
Erskine D, Thomas AJ, Attems J, Taylor JP, McKeith IG, Morris CM, et al. Specific patterns of neuronal loss in the pulvinar nucleus in dementia with lewy bodies. Mov Disord. 2017;32(3):414–22.
Erskine D, Thomas AJ, Taylor JP, Savage MA, Attems J, McKeith IG, et al. Neuronal Loss and Α-Synuclein Pathology in the Superior Colliculus and Its Relationship to Visual Hallucinations in Dementia with Lewy Bodies. Am J Geriatr Psychiatry. 2017;25(6):595–604.
Mehraram R, Peraza LR, Murphy NRE, Cromarty RA, Graziadio S, O’Brien JT, et al. Functional and structural brain network correlates of visual hallucinations in Lewy body dementia. Brain J Neurol. 2022;145(6):2190–205.
Nedelska Z, Schwarz CG, Boeve BF, Lowe VJ, Reid RI, Przybelski SA, et al. White matter integrity in dementia with Lewy bodies: a voxel-based analysis of diffusion tensor imaging. Neurobiol Aging. 2015;36(6):2010–7.
Babiloni C, Del Percio C, Lizio R, Noce G, Lopez S, Soricelli A, et al. Abnormalities of resting-state functional cortical connectivity in patients with dementia due to Alzheimer’s and Lewy body diseases: an EEG study. Neurobiol Aging. 2018;65:18–40.
van Dellen E, de Waal H, van der Flier WM, Lemstra AW, Slooter AJC, Smits LL, et al. Loss of EEG Network Efficiency Is Related to Cognitive Impairment in Dementia With Lewy Bodies. Mov Disord. 2015;30(13):1785–93.
Sala A, Caminiti SP, Iaccarino L, Beretta L, Iannaccone S, Magnani G, et al. Vulnerability of multiple large-scale brain networks in dementia with Lewy bodies. Hum Brain Mapp. 2019;40(15):4537–50.
• Oishi Y, Imamura T, Shimomura T, Suzuki K. Visual texture agnosia in dementia with Lewy bodies and Alzheimer’s disease. Cortex. 2018;103:277–90. Study that showed visual texture agnosia in DLB, as subjects showed impaired ability to recognize textures when controlling for contrast sensitivity and color vision impairments.
Flanigan PM, Khosravi MA, Leverenz JB, Tousi B. Color Vision Impairment Differentiates Alzheimer Dementia From Dementia With Lewy Bodies. J Geriatr Psychiatry Neurol. 2018;31(2):97–102.
Unger R, Flanigan P, Khosravi M, Tousi B. Color Vision Impairment in Prodromal and Dementia Stages of Lewy Body Disease versus Alzheimer’s Disease (P4.1-027). Neurology [Internet]. 2019 Apr 9 [cited 2023 Apr 6];92(15 Supplement). Available from: https://n.neurology.org/content/92/15_Supplement/P4.1-027
Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol. 2011;69(5):811–8.
Maurage CA, Ruchoux MM, De Vos R, Surguchov A, Destee A. Retinal involvement in dementia with Lewy bodies: A clue to hallucinations? Ann Neurol. 2003;54(4):542–7.
Devos D, Tir M, Maurage CA, Waucquier N, Defebvre L, Defoort-Dhellemmes S, et al. ERG and anatomical abnormalities suggesting retinopathy in dementia with Lewy bodies. Neurology. 2005;65(7):1107–10.
Beach TG, Carew J, Serrano G, Adler CH, Shill HA, Sue LI, et al. Phosphorylated α-synuclein-immunoreactive retinal neuronal elements in Parkinson’s disease subjects. Neurosci Lett. 2014;571:34–8.
• Hart de Ruyter FJ, Morrema THJ, den Haan J, Gase G, Twisk JWR, de Boer JF, et al. α-Synuclein pathology in post-mortem retina and optic nerve is specific for α-synucleinopathies. Npj Park Dis. 2023;9(1):1–9. Pathological study showing α-synuclein aggregates and inclusions to be present in the retina and optic nerve of 5/5 DLB patients studied, as well as 16/21 PD patients, but not found in any patients with absent brain synuclein pathology.
Fragiacomo F, Miante S, Pengo M, Bussè C, Zorzi G, Ermani M, et al. Clinical and cognitive correlates of selective regional retinal thinning in dementia with lewy bodies. Mov Disord. 2019;34(10):1582–3.
Murueta-Goyena A, Del Pino R, Galdós M, Arana B, Acera M, Carmona-Abellán M, et al. Retinal Thickness Predicts the Risk of Cognitive Decline in Parkinson Disease. Ann Neurol. 2021;89(1):165–76.
Murueta-Goyena A, del Pino R, Reyero P, Galdós M, Arana B, Lucas-Jiménez O, et al. Parafoveal thinning of inner retina is associated with visual dysfunction in Lewy body diseases. Mov Disord. 2019;34(9):1315–24.
Joseph S, Robbins CB, Allen A, Haystead A, Hemesath A, Kundu A, et al. Differences in Retinal and Choroidal Microvasculature and Structure in Dementia With Lewy Bodies Compared With Normal Cognition. J Vitreoretin Dis. 2024;8(1):67–74.
Ahn J, Lee JY, Kim TW, Yoon EJ, Oh S, Kim YK, et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology. 2018;91(11):e1003–12.
Archibald NK, Clarke MP, Mosimann UP, Burn DJ. The retina in Parkinson’s disease. Brain. 2009;132(5):1128–45.
Lee JY, Ahn J, Oh S, Shin JY, Kim YK, Nam H, et al. Retina Thickness as a Marker of Neurodegeneration in Prodromal Lewy Body Disease. Mov Disord. 2020;35(2):349–54.
Ma LJ, Xu LL, Jie MC, Fu YT, Ji XY, Shen Y, et al. Progressive Changes in the Retinal Structure of Patients with Parkinson’s Disease. J Parkinsons Dis. 2018;8(1):85–92.
Ortuño-Lizarán I, Sánchez-Sáez X, Lax P, Serrano GE, Beach TG, Adler CH, et al. Dopaminergic Retinal Cell Loss and Visual Dysfunction in Parkinson Disease. Ann Neurol. 2020;88(5):893–906.
Ortuño-Lizarán I, Beach TG, Serrano GE, Walker DG, Adler CH, Cuenca N. PHOSPHORYLATED α-SYNUCLEIN IN THE RETINA IS A BIOMARKER OF PARKINSON’S DISEASE PATHOLOGY SEVERITY. Mov Disord Off J Mov Disord Soc. 2018;33(8):1315–24.
Wood JS, Firbank MJ, Mosimann UP, Watson R, Barber R, Blamire AM, et al. Testing Visual Perception in Dementia with Lewy Bodies and Alzheimer Disease. Am J Geriatr Psychiatry. 2013;21(6):501–8.
Mori E, Shimomura T, Fujimori M, Hirono N, Imamura T, Hashimoto M, et al. Visuoperceptual Impairment in Dementia With Lewy Bodies. Arch Neurol. 2000;57(4):489–93.
Mosimann UP, Mather G, Wesnes KA, O’Brien JT, Burn DJ, McKeith IG. Visual perception in Parkinson disease dementia and dementia with Lewy bodies. Neurology. 2004;63(11):2091–6.
Ota K, Murayama N, Kasanuki K, Kondo D, Fujishiro H, Arai H, et al. Visuoperceptual Assessments for Differentiating Dementia with Lewy Bodies and Alzheimer’s Disease: Illusory Contours and Other Neuropsychological Examinations. Arch Clin Neuropsychol. 2015;30(3):256–63.
Calderon J, Perry RJ, Erzinclioglu SW, Berrios GE, Dening TR, Hodges JR. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;70(2):157–64.
Mitolo M, Hamilton JM, Landy KM, Hansen LA, Galasko D, Pazzaglia F, et al. Visual Perceptual Organization Ability in Autopsy-Verified Dementia with Lewy Bodies and Alzheimer’s Disease. J Int Neuropsychol Soc. 2016;22(6):609–19.
•• Salmon DP, Smirnov DS, Coughlin DG, Hamilton JM, Landy KM, Filoteo JV, et al. Perception of Fragmented Letters by Patients With Pathologically Confirmed Dementia With Lewy Bodies or Alzheimer Disease. Neurology. 2022;26:10.1212/WNL.0000000000201068. Clinical-pathological study that showed the Fragmented Letters test could effectively differentiate pathologic DLB from AD, and that worse test performance correlated with more severe Lewy body pathology.
Martini A, Weis L, Schifano R, Pistonesi F, Fiorenzato E, Antonini A, et al. Differences in cognitive profiles between Lewy body and Parkinson’s disease dementia. J Neural Transm. 2020;127(3):323–30.
Landy KM, Salmon DP, Galasko D, Filoteo JV, Festa EK, Heindel WC, et al. Motion discrimination in dementia with Lewy bodies and Alzheimer disease. Neurology. 2015;85(16):1376–82.
• Phillips JR, Matar E, Ehgoetz Martens KA, Moustafa AA, Halliday GM, Lewis SJG. An Adaptive Measure of Visuospatial Impairment in Dementia with Lewy Bodies. Mov Disord Clin Pract. 2022;9(5):619–27. Study that showed that DLB but not PD patients had impaired performance in a mental rotation task assessing 3-dimensional visuospatial skills, correlating with other visuospatial measures but independent of MMSE score and memory.
Cormack F, Gray A, Ballard C, Tovée MJ. A failure of ‘Pop-Out’ in visual search tasks in dementia with Lewy Bodies as compared to Alzheimer’s and Parkinson’s disease. Int J Geriatr Psychiatry. 2004;19(8):763–72.
Landy KM, Salmon DP, Filoteo JV, Heindel WC, Galasko D, Hamilton JM. Visual search in Dementia with Lewy Bodies and Alzheimer’s disease. Cortex. 2015;73:228–39.
Carrarini C, Russo M, Pagliaccio G, Dono F, Franciotti R, Deluca G, et al. Visual evoked potential abnormalities in dementia with Lewy bodies. Neurophysiol Clin. 2021;51(5):425–31.
Kurita A, Murakami M, Takagi S, Matsushima M, Suzuki M. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disord. 2010;25(2):167–71.
Rosenblum Y, Maidan I, Fahoum F, Giladi N, Bregman N, Shiner T, et al. Differential changes in visual and auditory event-related oscillations in dementia with Lewy bodies. Clin Neurophysiol. 2020;131(10):2357–66.
Aoki Y, Kazui H, Pascal-Marqui RD, Ishii R, Yoshiyama K, Kanemoto H, et al. EEG Resting-State Networks in Dementia with Lewy Bodies Associated with Clinical Symptoms. Neuropsychobiology. 2019;77(4):206–18.
Sauer J, Ffytche DH, Ballard C, Brown RG, Howard R. Differences between Alzheimer’s disease and dementia with Lewy bodies: an fMRI study of task-related brain activity. Brain. 2006;129(7):1780–8.
Taylor JP, Firbank MJ, He J, Barnett N, Pearce S, Livingstone A, et al. Visual cortex in dementia with Lewy bodies: Magnetic resonance imaging study. Br J Psychiatry. 2012;200(6):491–8.
Gnanalingham KK, Byrne EJ, Thornton A. Clock-face drawing to differentiate Lewy body and Alzheimer type dementia syndromes. Lancet. 1996;347(9002):696–7.
Gnanalingham KK, Byrne EJ, Thornton A, Sambrook MA, Bannister P. Motor and cognitive function in Lewy body dementia: comparison with Alzheimer’s and Parkinson’s diseases. J Neurol Neurosurg Psychiatry. 1997;62(3):243–52.
Salmon DP, Galasko D, Hansen LA, Masliah E, Butters N, Thal LJ, et al. Neuropsychological Deficits Associated with Diffuse Lewy Body Disease. Brain Cogn. 1996;31(2):148–65.
Hamilton JM, Salmon DP, Galasko D, Raman R, Emond J, Hansen LA, et al. Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology. 2008;22(6):729–37.
Perneczky R, Drzezga A, Boecker H, Ceballos-Baumann AO, Valet M, Feurer R, et al. Metabolic alterations associated with impaired clock drawing in Lewy body dementia. Psychiatry Res Neuroimaging. 2010;181(2):85–9.
Ala TA, Hughes LF, Kyrouac GA, Ghobrial MW, Elble RJ. Pentagon copying is more impaired in dementia with Lewy bodies than in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;70(4):483–8.
Caffarra P, Gardini S, Dieci F, Copelli S, Maset L, Concari L, et al. The qualitative scoring MMSE pentagon test (QSPT): A new method for differentiating dementia with Lewy Body from Alzheimer’s disease. Behav Neurol. 2013;27(2):213–20.
Cormack F, Aarsland D, Ballard C, Tovée MJ. Pentagon drawing and neuropsychological performance in Dementia with Lewy Bodies, Alzheimer’s disease, Parkinson’s disease and Parkinson’s disease with dementia. Int J Geriatr Psychiatry. 2004;19(4):371–7.
Mitolo M, Salmon DP, Gardini S, Galasko D, Grossi E, Caffarra P. The New Qualitative Scoring MMSE Pentagon Test (QSPT) as a Valid Screening Tool between Autopsy-Confirmed Dementia with Lewy Bodies and Alzheimer’s Disease. J Alzheimers Dis. 2014;39(4):823–32.
Cagnin A, Bussè C, Jelcic N, Gnoato F, Mitolo M, Caffarra P. High specificity of MMSE pentagon scoring for diagnosis of prodromal dementia with Lewy bodies. Parkinsonism Relat Disord. 2015;21(3):303–5.
Cagnin A, Bussè C, Gardini S, Jelcic N, Guzzo C, Gnoato F, et al. Clinical and Cognitive Phenotype of Mild Cognitive Impairment Evolving to Dementia with Lewy Bodies. Dement Geriatr Cogn Disord Extra. 2015;5(3):442–9.
Beretta L, Caminiti SP, Santangelo R, Magnani G, Ferrari-Pellegrini F, Caffarra P, et al. Two distinct pathological substrates associated with MMSE-pentagons item deficit in DLB and AD. Neuropsychologia. 2019;133:107174.
Murayama N, Iseki E, Yamamoto R, Kimura M, Eto K, Arai H. Utility of the Bender Gestalt Test for differentiation of dementia with Lewy bodies from Alzheimer’s disease in patients showing mild to moderate dementia. Dement Geriatr Cogn Disord. 2007;23(4):258–63.
Murayama N, Ota K, Iseki E. The Bender Gestalt Test is useful for clinically diagnosing dementia with Lewy bodies: Analysis of its sensitivity, specificity, and clinical characteristics of the figure copy. Appl Neuropsychol Adult. 2022;19:1–6.
Ferman TJ, Smith GE, Boeve BF, Graff-Radford NR, Lucas JA, Knopman DS, et al. Neuropsychological Differentiation of Dementia with Lewy Bodies from Normal Aging and Alzheimer’s Disease. Clin Neuropsychol. 2006;20(4):623–36.
• Beretta L, Carli G, Caffarra P, Perani D. Distinct brain dysfunctions underlying visuo-constructive deficit in DLB and AD. Brain Imaging Behav. 2022;16(1):532–7. Study correlating ROCF copy performance and FDG-PET in DLB and AD, showing worse performance correlated with visual cortex hypometabolism in DLB but not AD.
• Yoo HS, Jeong SH, Oh KT, Lee S, Sohn YH, Ye BS, et al. Interrelation of striatal dopamine, brain metabolism and cognition in dementia with Lewy bodies. Brain. 2022;145(12):4448–58. Study correlating ROCF copy performance in DLB with multiple PET modalities that found correlation between worse test performance and occipital and posterior parietal hypometabolism independent of DaT scan co-correlation.
Blanc F, Bouteloup V, Paquet C, Chupin M, Pasquier F, Gabelle A, et al. Prodromal characteristics of dementia with Lewy bodies: baseline results of the MEMENTO memory clinics nationwide cohort. Alzheimers Res Ther. 2022;14(1):96.
van de Beek M, van Steenoven I, van der Zande JJ, Barkhof F, Teunissen CE, van der Flier WM, et al. Prodromal Dementia With Lewy Bodies: Clinical Characterization and Predictors of Progression. Mov Disord. 2020;35(5):859–67.
Ciafone J, Thomas A, Durcan R, Donaghy PC, Hamilton CA, Lawley S, et al. Neuropsychological Impairments and Their Cognitive Architecture in Mild Cognitive Impairment (MCI) with Lewy Bodies and MCI-Alzheimer’s Disease. J Int Neuropsychol Soc. 2022;28(9):963–73.
Donaghy PC, Ciafone J, Durcan R, Hamilton CA, Barker S, Lloyd J, et al. Mild cognitive impairment with Lewy bodies: neuropsychiatric supportive symptoms and cognitive profile. Psychol Med. 2022;52(6):1147–55.
Kemp J, Philippi N, Phillipps C, Demuynck C, Albasser T, Martin-Hunyadi C, et al. Cognitive profile in prodromal dementia with Lewy bodies. Alzheimers Res Ther. 2017;9(1):1–10.
Génier Marchand D, Postuma RB, Escudier F, De Roy J, Pelletier A, Montplaisir J, et al. How does dementia with Lewy bodies start? prodromal cognitive changes in REM sleep behavior disorder. Ann Neurol. 2018;83(5):1016–26.
Auning E, Rongve A, Fladby T, Booij J, Hortobágyi T, Siepel FJ, et al. Early and presenting symptoms of dementia with lewy bodies. Dement Geriatr Cogn Disord. 2011;32(3):202–8.
Donaghy PC, McKeith IG. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther. 2014;6(4):46.
Sim J, Li H, Hameed S, Ting SKS. Clinical Manifestations of Early-Onset Dementia With Lewy Bodies Compared With Late-Onset Dementia With Lewy Bodies and Early-Onset Alzheimer Disease. JAMA Neurol. 2022;79(7):702–9.
Aarsland D, Ballard C, Larsen JP, McKeith I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int J Geriatr Psychiatry. 2001;16(5):528–36.
Waters F, Fernyhough C. Hallucinations: A Systematic Review of Points of Similarity and Difference Across Diagnostic Classes. Schizophr Bull. 2017;43(1):32–43.
Collerton D, Perry E. Thalamocortical dysfunction and complex visual hallucinations in brain disease – Are the primary disturbances in the cerebral cortex? Behav Brain Sci. 2004;27(6):789–90.
Urwyler P, Nef T, Müri R, Archibald N, Makin SM, Collerton D, et al. Visual Hallucinations in Eye Disease and Lewy Body Disease. Am J Geriatr Psychiatry. 2016;24(5):350–8.
Marques A, Beze S, Pereira B, Chassain C, Monneyron N, Delaby L, et al. Visual hallucinations and illusions in Parkinson’s disease: the role of ocular pathology. J Neurol. 2020;267(10):2829–41.
de Maindreville AD, Fénelon G, Mahieux F. Hallucinations in Parkinson’s disease: a follow-up study. Mov Disord Off J Mov Disord Soc. 2005;20(2):212–7.
Cagnin A, Gnoato F, Jelcic N, Favaretto S, Zarantonello G, Ermani M, et al. Clinical and cognitive correlates of visual hallucinations in dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2013;84(5):505–10.
• D’Antonio F, Boccia M, Di Vita A, Suppa A, Fabbrini A, Canevelli M, et al. Visual hallucinations in Lewy body disease: pathophysiological insights from phenomenology. J Neurol. 2022;269(7):3636–52. Study showing that in DLB complex visual hallucinations but not minor visual phenomena correlated with cognitive deficits in visuoperception, abstraction and attention.
Hamilton JM, Landy KM, Salmon DP, Hansen LA, Masliah E, Galasko D. Early Visuospatial Deficits Predict the Occurrence of Visual Hallucinations in Autopsy-Confirmed Dementia With Lewy Bodies. Am J Geriatr Psychiatry. 2012;20(9):773–81.
Pezzoli S, Cagnin A, Bussè C, Zorzi G, Fragiacomo F, Bandmann O, et al. Cognitive correlates and baseline predictors of future development of visual hallucinations in dementia with Lewy bodies. Cortex. 2021;142:74–83.
Pezzoli S, Cagnin A, Antonini A, Venneri A. Frontal and subcortical contribution to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease. Postgrad Med. 2019;131(7):509–22.
Rosenblum Y, Bregman N, Giladi N, Mirelman A, Shiner T. Associations between visual hallucinations and impaired visuo-spatial abilities in dementia with Lewy bodies. Neuropsychology. 2021;35:276–84.
Firbank MJ, Lloyd J, O’Brien JT. The relationship between hallucinations and FDG-PET in dementia with Lewy bodies. Brain Imaging Behav. 2016;10(3):636–9.
Heitz C, Noblet V, Cretin B, Philippi N, Kremer L, Stackfleth M, et al. Neural correlates of visual hallucinations in dementia with Lewy bodies. Alzheimers Res Ther. 2015;7(1):1–8.
Murayama T, Kobayashi S, Ishida T, Utsumi K, Kawanishi C. Associations Between Regional Cerebral Blood Flow and Psychiatric Symptoms in Dementia With Lewy Bodies Without Parkinsonism. Am J Alzheimers Dis Dementias®. 2022;37:15333175221075109.
Nagahama Y, Okina T, Suzuki N, Matsuda M. Neural correlates of psychotic symptoms in dementia with Lewy bodies. Brain. 2010;133(2):557–67.
Pasquier J, Michel BF, Brenot-Rossi I, Hassan-Sebbag N, Sauvan R, Gastaut J. Value of 99mTc-ECD SPET for the diagnosis of dementia with Lewy bodies. Eur J Nucl Med Mol Imaging. 2002;29(10):1342–8.
Firbank MJ, Collerton D, da Silva MK, Schumacher J, Donaghy PC, O’Brien JT, et al. Functional connectivity in Lewy body disease with visual hallucinations. Eur J Neurol. 2024;31(2):e16115.
Iaccarino L, Sala A, Caminiti SP, Santangelo R, Iannaccone S, Magnani G, et al. The brain metabolic signature of visual hallucinations in dementia with Lewy bodies. Cortex. 2018;108:13–24.
Zorzi G, Cecchin D, Bussè C, Perini G, Corbetta M, Cagnin A. Changes of Metabolic Connectivity in Dementia with Lewy Bodies with Visual Hallucinations: A 18F-Fluorodeoxyglucose Positron Emission Tomography/Magnetic Resonance Study. Brain Connect. 2021;11(7):518–28.
Zorzi G, Thiebaut de Schotten M, Manara R, Bussè C, Corbetta M, Cagnin A. White matter abnormalities of right hemisphere attention networks contribute to visual hallucinations in dementia with Lewy bodies. Cortex. 2021;139:86–98.
Uchiyama M, Nishio Y, Yokoi K, Hirayama K, Imamura T, Shimomura T, et al. Pareidolias: complex visual illusions in dementia with Lewy bodies. Brain J Neurol. 2012;135(Pt 8):2458–69.
Yokoi K, Nishio Y, Uchiyama M, Shimomura T, Iizuka O, Mori E. Hallucinators find meaning in noises: Pareidolic illusions in dementia with Lewy bodies. Neuropsychologia. 2014;56:245–54.
Nakata T, Shimada K, Iba A, Oda H, Terashima A, Koide Y, et al. Correlation between noise pareidolia test scores for visual hallucinations and regional cerebral blood flow in dementia with Lewy bodies. Ann Nucl Med. 2022;36(4):384–92.
Watanabe H, Uchiyama M, Yokoi K, Mamiya Y, Narita W, Iizuka O, et al. Behavioral and neural correlates of pareidolic illusions in dementia with Lewy bodies. Parkinsonism Relat Disord. 2023;113:105513.
Taylor JP, Firbank M, O’Brien JT. Visual cortical excitability in dementia with Lewy bodies. Br J Psychiatry. 2016;208(5):497–8.
Taylor JP, Firbank M, Barnett N, Pearce S, Livingstone A, Mosimann U, et al. Visual hallucinations in dementia with Lewy bodies: transcranial magnetic stimulation study. Br J Psychiatry. 2011;199(6):492–500.
Leodori G, Fabbrini A, Suppa A, Mancuso M, Tikoo S, Belvisi D, et al. Effective connectivity abnormalities in Lewy body disease with visual hallucinations. Clin Neurophysiol. 2023;156:156–65.
•• Donaghy PC, Hamilton C, Durcan R, Lawley S, Barker S, Ciafone J, et al. Clinical symptoms in mild cognitive impairment with Lewy bodies: Frequency, time of onset, and discriminant ability. Eur J Neurol. 2023;30(6):1585–93. Study expanding on survey work by the same group showing that MCI-LB patients reported frequent subjective complaints of misjudging objects, significantly more than in MCI-AD where it was similar to controls.
Donaghy PC, Barnett N, Olsen K, Taylor JP, McKeith IG, O’Brien JT, et al. Symptoms associated with Lewy body disease in mild cognitive impairment. Int J Geriatr Psychiatry. 2017;32(11):1163–71.
Jefferis JM, Clarke MP, Mosimann UP, O’Brien JT, Taylor JP. The influence of two common dementia types on visual symptoms. Acta Ophthalmol. 2013;91(2):e159–60.
Raphael BA, Galetta KM, Jacobs DA, Markowitz CE, Liu GT, Nano-Schiavi ML, et al. Validation and test characteristics of a 10-item neuro-ophthalmic supplement to the NEI-VFQ-25. Am J Ophthalmol. 2006;142(6):1026–35.
Kudo Y, Imamura T, Sato A, Endo N. Risk Factors for Falls in Community-Dwelling Patients with Alzheimer’s Disease and Dementia with Lewy Bodies: Walking with Visuocognitive Impairment May Cause a Fall. Dement Geriatr Cogn Disord. 2009;27(2):139–46.
Bennett JM, Chekaluk E, Batchelor J. Cognitive Tests and Determining Fitness to Drive in Dementia: A Systematic Review. J Am Geriatr Soc. 2016;64(9):1904–17.
Harvey RJ, Fraser D, Bonner D, Warnes A, Warrington E, Rossor M. Dementia and driving: Results of a semi-realistic simulator study. Int J Geriatr Psychiatry. 1995;10(10):859–64.
Lincoln NB, Taylor JL, Vella K, Bouman WP, Radford KA. A prospective study of cognitive tests to predict performance on a standardised road test in people with dementia. Int J Geriatr Psychiatry. 2010;25(5):489–96.
Lincoln NB, Radford KA, Lee E, Reay AC. The assessment of fitness to drive in people with dementia. Int J Geriatr Psychiatry. 2006;21(11):1044–51.
Yamin S, Stinchcombe A, Gagnon S. Driving Competence in Mild Dementia with Lewy Bodies: In Search of Cognitive Predictors Using Driving Simulation. Int J Alzheimers Dis. 2015;2015:e806024.
• Fuermaier ABM, Piersma D, de Waard D, Davidse RJ, de Groot J, Doumen MJA, et al. Driving Difficulties Among Patients with Alzheimer’s Disease and Other Neurodegenerative Disorders. J Alzheimers Dis. 2019;69(4):1019–30. Study with on-road driving tests in dementia patients with valid drivers licenses and desire to continue driving, including a small subset of DLB patients, which showed high fail rates in DLB.
Piersma D, Fuermaier ABM, De Waard D, Davidse RJ, De Groot J, Doumen MJA, et al. Assessing Fitness to Drive in Patients With Different Types of Dementia. Alzheimer Dis Assoc Disord. 2018;32(1):70.
Carli G, Caminiti SP, Sala A, Galbiati A, Pilotto A, Ferini-Strambi L, et al. Impaired metabolic brain networks associated with neurotransmission systems in the α-synuclein spectrum. Parkinsonism Relat Disord. 2020;81:113–22.
Colloby SJ, Nathan PJ, McKeith IG, Bakker G, O’Brien JT, Taylor JP. Cholinergic muscarinic M1/M4 receptor networks in dementia with Lewy bodies. Brain Commun. 2020;2(2):fcaa098.
Colloby SJ, Pakrasi S, Firbank MJ, Perry EK, Piggott MA, Owens J, et al. In vivo SPECT imaging of muscarinic acetylcholine receptors using (R,R) 123I-QNB in dementia with Lewy bodies and Parkinson’s disease dementia. NeuroImage. 2006;33(2):423–9.
Kanel P, Müller MLTM, van der Zee S, Sanchez-Catasus CA, Koeppe RA, Frey KA, et al. Topography of Cholinergic Changes in Dementia With Lewy Bodies and Key Neural Network Hubs. J Neuropsychiatr Clin Neurosci. 2020;32(4):370–5.
Mukaetova-Ladinska EB, Andras A, Milne J, Abdel-All Z, Borr I, Jaros E, et al. Synaptic Proteins and Choline Acetyltransferase Loss in Visual Cortex in Dementia With Lewy Bodies. J Neuropathol Exp Neurol. 2013;72(1):53–60.
O’Brien JT, Colloby SJ, Pakrasi S, Perry EK, Pimlott SL, Wyper DJ, et al. Nicotinic α4β2 receptor binding in dementia with Lewy bodies using 123I-5IA-85380 SPECT demonstrates a link between occipital changes and visual hallucinations. NeuroImage. 2008;40(3):1056–63.
Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology. 2009;73(4):273–8.
Mori T, Ikeda M, Fukuhara R, Nestor PJ, Tanabe H. Correlation of visual hallucinations with occipital rCBF changes by donepezil in DLB. Neurology. 2006;66(6):935–7.
Mori E, Ikeda M, Kosaka K. Donepezil for Dementia with Lewy Bodies: A Randomized. Placebo-Controlled Trial Ann Neurol. 2012;72:41–52.
McKeith I, Del Ser T, Spano P, Emre M, Wesnes K, Anand R, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031–6.
Edwards K, Royall D, Hershey L, Lichter D, Hake A, Farlow M, et al. Efficacy and safety of galantamine in patients with dementia with Lewy bodies: a 24-week open-label study. Dement Geriatr Cogn Disord. 2007;23(6):401–5.
Pankow L, Pliskin N, Luchins D. An optical intervention for visual hallucinations associated with visual impairment and dementia in elderly patients. J Neuropsychiatr Clin Neurosci. 1996;8(1):88–92.
Shimomura T, Mori E, Yamashita H, Imamura T, Hirono N, Hashimoto M, et al. Cognitive Loss in Dementia With Lewy Bodies and Alzheimer Disease. Arch Neurol. 1998;55(12):1547–52.
Walker Z, Allen RL, Shergill S, Katona CLE. Neuropsychological performance in Lewy body dementia and Alzheimer’s disease. Br J Psychiatry. 1997;170(2):156–8.
Author information
Authors and Affiliations
Contributions
R.D. wrote the main manuscript text and prepared the table. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Key Concepts
- DLB patients show impairment in a broad range of visual domains, including contrast and color discrimination, object recognition, spatial orientation, and motion perception.
- Visual dysfunction in DLB is associated with functional and anatomical changes in the occipital lobes, white matter tracts, and retina and the disruption of visual and attention networks.
- Visual dysfunction is more prominent in DLB than AD, and tasks such as figure copying and perception of fragmented letters may help distinguish DLB from AD.
- Visual hallucinations are highly prevalent in DLB and associated with visual dysfunction network disruption.
- DLB patients frequently complain of misjudging objects, and visual impairment may play roles in fall risk and driving safety.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Devenyi, R.A., Hamedani, A.G. Visual dysfunction in dementia with Lewy bodies. Curr Neurol Neurosci Rep (2024). https://doi.org/10.1007/s11910-024-01349-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s11910-024-01349-8