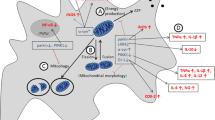
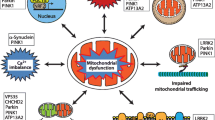
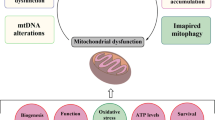
Abstract
Purpose of Review
Overwhelming evidence indicates that mitochondrial dysfunction is a central factor in Parkinson’s disease (PD) pathophysiology. This paper aims to review the latest literature published, focusing on genetic defects and expression alterations affecting mitochondria-associated genes, in support of their key role in PD pathogenesis.
Recent Findings
Thanks to the use of new omics approaches, a growing number of studies are discovering alterations affecting genes with mitochondrial functions in patients with PD and parkinsonisms. These genetic alterations include pathogenic single-nucleotide variants, polymorphisms acting as risk factors, and transcriptome modifications, affecting both nuclear and mitochondrial genes.
Summary
We will focus on alterations of mitochondria-associated genes described by studies conducted on patients or on animal/cellular models of PD or parkinsonisms. We will comment how these findings can be taken into consideration for improving the diagnostic procedures or for deepening our knowledge on the role of mitochondrial dysfunctions in PD.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40.
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A, Lang AE. Parkinson disease Nat Rev Dis Primers. 2017;23(3):17013.
Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76.
Keener A, Bordelon Y. Parkinsonism. Semin Neurol. 2016;36:330–4.
Davis GC, Williams AC, Markey SP, Ebert MH, Caine ED, Reichert CM, Kopin IJ. Chronic parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979;1:249–54.
Schapira AH. Mitochondrial complex I deficiency in Parkinson’s disease. Adv Neurol. 1993;60:288–91.
Gu G, Reyes PE, Golden GT, Woltjer RL, Hulette C, Montine TJ, Zhang J. Mitochondrial DNA deletions/rearrangements in Parkinson disease and related neurodegenerative disorders. J Neuropathol Exp Neurol. 2002;61:634–9.
Tiangyou W, Hudson G, Ghezzi D, Ferrari G, Zeviani M, Burn DJ, Chinnery PF. POLG1 in idiopathic Parkinson disease. Neurology. 2006;67:1698–700.
Anheim M, Elbaz A, Lesage S, Durr A, Condroyer C, Viallet F, Pollak P, Bonaïti B, Bonaïti-Pellié C, Brice A, French Parkinson Disease Genetic Group. Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology. 2012;78(6):417–20.
Nicoletti A, Luca A, Baschi R, Cicero CE, Mostile G, Davì M, La Bianca G, Restivo V, Zappia M, Monastero R. Vascular risk factors, white matter lesions and cognitive impairment in Parkinson’s disease: the PACOS longitudinal study. J Neurol. 2021;268(2):549–58.
Hauser DN, Hastings TG. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol Dis. 2013;51:35–42.
Lin MT, Cantuti-Castelvetri I, Zheng K, Jackson KE, Tan YB, Arzberger T, Lees AJ, Betensky RA, Beal MF, Simon DK. Somatic mitochondrial DNA mutations in early Parkinson and incidental Lewy body disease. Ann Neurol. 2012;71(6):850–4.
Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38(5):515.
Dölle C, Flønes I, Nido GS, Miletic H, Osuagwu N, Kristoffersen S, Lilleng PK, Larsen JP, Tysnes OB, Haugarvoll K. Defective mitochondrial DNA homeostasis in the substantia nigra in Parkinson disease. Nat Commun. 2016;7:13548.
•• Borsche M, König IR, Delcambre S, Petrucci S, Balck A, Brüggemann N, Zimprich A, Wasner K, Pereira SL, Avenali M, Deuschle C, Badanjak K, Ghelfi J, Gasser T, Kasten M, Rosenstiel P, Lohmann K, Brockmann K, Valente EM, Youle RJ, Grünewald A, Klein C. Mitochondrial damage-associated inflammation highlights biomarkers in PRKN/PINK1 parkinsonism. Brain. 2020;143(10):3041–3051. This study shows how, in individuals carrying pathogenic variants in PRKN/PINK1, circulating cell-free mtDNA levels may serve as marker for PD state and progression.
Herrmann JM, Longen S, Weckbecker D, Depuydt M. Biogenesis of mitochondrial proteins. Adv Exp Med Biol. 2012;748:41–64.
Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44(D1):D1251–7.
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–7.
Funayama M, Nishioka K, Li Y, Hattori N. Molecular genetics of Parkinson’s disease: contributions and global trends. J Hum Genet. 2022.
Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett. 2010;486(3):235–9.
Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601–7.
Hsieh CH, Shaltouki A, Gonzalez AE, Bettencourt da Cruz A, Burbulla LF, St Lawrence E, Schüle B, Krainc D, Palmer TD, Wang X. Functional impairment in Miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease. Cell Stem Cell. 2016;19(6):709–724.
• Ouzren N, Delcambre S, Ghelfi J, Seibler P, Farrer MJ, Konig IR, Aasly JO, Trinh J, Klein C, Grunewald A. Mitochondrial DNA deletions discriminate affected from unaffected LRRK2 mutation carriers. Ann Neurol. 2019;86(2):324–326. This study found increased mtDNA deletions in LRRK2+/PD+ compared to LRRK2+/PD− individuals, supporting a link between increased levels of reactive oxygen species and mtDNA damage in manifesting G2019S carriers.
Trinh J, Zeldenrust FMJ, Huang J, Kasten M, Schaake S, Petkovic S, Madoev H, Grunewald A, Almuammar S, Konig IR, Lill CM, Lohmann K, Klein C, Marras C. Genotype-phenotype relations for the Parkinson’s disease genes SNCA, LRRK2, VPS35: MDSGene systematic review. Mov Disord. 2018;33(12):1857–70.
Kasten M, Hartmann C, Hampf J, Schaake S, Westenberger A, Vollstedt EJ, Balck A, Domingo A, Vulinovic F, Dulovic M, Zorn I, Madoev H, Zehnle H, Lembeck CM, Schawe L, Reginold J, Huang J, Konig IR, Bertram L, Marras C, Lohmann K, Lill CM, Klein C. Genotype-phenotype relations for the Parkinson’s disease genes Parkin, PINK1, DJ1: MDSGene systematic review. Mov Disord. 2018;33(5):730–41.
Wang W, Wang X, Fujioka H, Hoppel C, Whone AL, Caldwell MA, Cullen PJ, Liu J, Zhu X. Parkinson’s disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat Med. 22:54–63.
Truban D, Hou X, Caulfield TR, Fiesel FC, Springer W. PINK1, Parkin, and mitochondrial quality control: what can we learn about Parkinson’s disease pathobiology? J Parkinsons Dis. 2017;7(1):13–29.
Leites EP, Morais VA. The PINK1-mediated crosstalk between neural cells and the underlying link to Parkinson’s disease. Cells. 2021;10(6):1395.
Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–9.
Thomas KJ, McCoy MK, Blackinton J, Beilina A, van der Brug M, Sandebring A, Miller D, Maric D, Cedazo-Minguez A, Cookson MR. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet. 2011;20(1):40–50.
Müller-Nedebock AC, van der Westhuizen FH, Kõks S, Bardien S. Nuclear genes associated with mitochondrial DNA processes as contributors to Parkinson’s disease risk. Mov Disord. 2021;36(4):815–31.
Van Maldergem L, Besse A, De Paepe B, Blakely EL, Appadurai V, Humble MM, Piard J, Craig K, He L, Hella P, Debray FG, Martin JJ, Gaussen M, Laloux P, Stevanin G, Van Coster R, Taylor RW, Copeland WC, Mormont E, Bonnen PE. POLG2 deficiency causes adult-onset syndromic sensory neuropathy, ataxia and parkinsonism. Ann Clin Transl Neurol. 2016;4(1):4–14.
Percetti M, Franco G, Monfrini E, Caporali L, Minardi R, La Morgia C, Valentino ML, Liguori R, Palmieri I, Ottaviani D, Vizziello M, Ronchi D, Di Berardino F, Cocco A, Macao B, Falkenberg M, Comi GP, Albanese A, Giometto B, Valente EM, Carelli V, Di Fonzo A. TWNK in Parkinson’s disease: a movement disorder and mitochondrial disease center perspective study. Mov Disord. 2022;37(9):1938–43.
Nogueira C, Almeida LS, Nesti C, Pezzini I, Videira A, Vilarinho L, Santorelli FM. Syndromes associated with mitochondrial DNA depletion. Ital J Pediatr. 2014;40:34.
Caporali L, Bello L, Tagliavini F, La Morgia C, Maresca A, Di Vito L, Liguori R, Valentino ML, Cecchin D, Pegoraro E, Carelli V. DGUOK recessive mutations in patients with CPEO, mitochondrial myopathy, parkinsonism and mtDNA deletions. Brain. 2018;141(1):e3.
Ronchi D, Caporali L, Manenti GF, Meneri M, Mohamed S, Bordoni A, Tagliavini F, Contin M, Piga D, Sciacco M, Saetti C, Carelli V, Comi GP. TYMP variants result in late-onset mitochondrial myopathy with altered muscle mitochondrial DNA homeostasis. Front Genet. 2020;5(11):860.
Garone C, Rubio JC, Calvo SE, Naini A, Tanji K, Dimauro S, Mootha VK, Hirano M. MPV17 Mutations causing adult-onset multisystemic disorder with multiple mitochondrial DNA deletions. Arch Neurol. 2012;69(12):1648–51.
Carelli V, Musumeci O, Caporali L, et al. Syndromic parkinsonism and dementia associated with OPA1 missense mutations. Ann Neurol. 2015;78:21–38.
Lynch DS, Loh SHY, Harley J, et al. Nonsyndromic Parkinson disease in a family with autosomal dominant optic atrophy due to OPA1 mutations. Neurol Genet. 2017;3(5):e188.
Bitetto G, Malaguti MC, Ceravolo R, Monfrini E, Straniero L, Morini A, Di Giacopo R, Frosini D, Palermo G, Biella F, Ronchi D, Duga S, Taroni F, Corti S, Comi GP, Bresolin N, Giometto B, Di Fonzo A. SLC25A46 mutations in patients with Parkinson’s disease and optic atrophy. Parkinsonism Relat Disord. 2020;74:1–5.
Bhattacharjee S, Noushad M, Sadler M. Early onset degenerative parkinsonism - consider SPG7 mutation. Neurol India. 2021;69(4):1051–1052.
De la Casa-Fages B, Fernández-Eulate G, Gamez J, Barahona-Hernando R, Morís G, García-Barcina M, Infante J, Zulaica M, Fernández-Pelayo U, Muñoz-Oreja M, Urtasun M, Olaskoaga A, Zelaya V, Jericó I, Saez-Villaverde R, Catalina I, Sola E, Martínez-Sáez E, Pujol A, Ruiz M, Schlüter A, Spinazzola A, Muñoz-Blanco JL, Grandas F, Holt I, Álvarez V, López de Munaín A. Parkinsonism and spastic paraplegia type 7: expanding the spectrum of mitochondrial parkinsonism. Mov Disord. 2019;34(10):1547–1561.
Pedroso JL, Vale TC, Bueno FL, Marussi VHR, Amaral LLFD, França MC Jr, Barsottini OG. SPG7 with parkinsonism responsive to levodopa and dopaminergic deficit. Parkinsonism Relat Disord. 2018;47:88–90.
Bellini G, Del Prete E, Unti E, Frosini D, Siciliano G, Ceravolo R. Positive DAT-SCAN in SPG7: a case report mimicking possible MSA-C. BMC Neurol. 2021;21(1):328.
Magri S, Fracasso V, Plumari M, Alfei E, Ghezzi D, Gellera C, Rusmini P, Poletti A, Di Bella D, Elia AE, Pantaleoni C, Taroni F. Concurrent AFG3L2 and SPG7 mutations associated with syndromic parkinsonism and optic atrophy with aberrant OPA1 processing and mitochondrial network fragmentation. Hum Mutat. 2018;39(12):2060–71.
Nishioka K, Vilariño-Güell C, Cobb SA, Kachergus JM, Ross OA, Hentati E, Hentati F, Farrer MJ. Genetic variation of the mitochondrial complex I subunit NDUFV2 and Parkinson’s disease. Parkinsonism Relat Disord. 2010;16(10):686–7.
Eis PS, Huang N, Langston JW, Hatchwell E, Schüle B. Loss-of-function NUBPL mutation may link Parkinson’s disease to recessive complex I deficiency. Front Neurol. 2020;29(11):555961.
Mahale RR, Arunachal G, Gautam J, Dutta D, Kovoor J, Mailankody P, Padmanabha H, Mathuranath PS. Parkinsonism, olivary hypertrophy and cerebellar atrophy with TTC19 gene mutation. Ann Indian Acad Neurol. 2021;24(6):991–993.
Lin CH, Tsai PI, Lin HY, Hattori N, Funayama M, Jeon B, Sato K, Abe K, Mukai Y, Takahashi Y, Li Y, Nishioka K, Yoshino H, Daida K, Chen ML, Cheng J, Huang CY, Tzeng SR, Wu YS, Lai HJ, Tsai HH, Yen RF, Lee NC, Lo WC, Hung YC, Chan CC, Ke YC, Chao CC, Hsieh ST, Farrer M, Wu RM. Mitochondrial UQCRC1 mutations cause autosomal dominant parkinsonism with polyneuropathy. Brain. 2020;143(11):3352–73.
Yang X, Xi J, Zhao Q, Jia H, An R, Liu Z, Xu Y. Association of the COQ2 V393A variant with Parkinson’s disease: a case-control study and meta-analysis. PLoS One. 2015;10(6):e0130970.
Porto KJ, Hirano M, Mitsui J, Chikada A, Matsukawa T, Ishiura H; Japan Multiple System Atrophy Registry Consortium, Toda T, Kusunoki S, Tsuji S. COQ2 V393A confers high risk susceptibility for multiple system atrophy in East Asian population. J Neurol Sci. 2021;429:117623.
Mikasa M, Kanai K, Li Y, Yoshino H, Mogushi K, Hayashida A, Ikeda A, Kawajiri S, Okuma Y, Kashihara K, Sato T, Kondo H, Funayama M, Nishioka K, Hattori N. COQ2 variants in Parkinson’s disease and multiple system atrophy. J Neural Transm (Vienna). 2018;125(6):937–44.
Multiple-System Atrophy Research Collaboration. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369(3):233–44.
Baba Y, Uitti RJ, Boylan KB, Uehara Y, Yamada T, Farrer MJ, Couchon E, Batish SD, Wszolek ZK. Aprataxin (APTX) gene mutations resembling multiple system atrophy. Parkinsonism Relat Disord. 2007;13(3):139–42.
Ortez C, Jou C, Cortès-Saladelafont E, Moreno J, Pérez A, Ormazábal A, Pérez-Cerdá C, Pérez B, Artuch R, Cusi V, García-Cazorla A. Infantile parkinsonism and GABAergic hypotransmission in a patient with pyruvate carboxylase deficiency. Gene. 2013;532(2):302–6.
Olgiati S, Skorvanek M, Quadri M, Minneboo M, Graafland J, Breedveld GJ, Bonte R, Ozgur Z, van den Hout MC, Schoonderwoerd K, Verheijen FW, van IJcken WF, Chien HF, Barbosa ER, Chang HC, Lai SC, Yeh TH, Lu CS, Wu-Chou YH, Kievit AJ, Han V, Gdovinova Z, Jech R, Hofstra RM, Ruijter GJ, Mandemakers W, Bonifati V. Paroxysmal exercise-induced dystonia within the phenotypic spectrum of ECHS1 deficiency. Mov Disord. 2016;31(7):1041–8.
Erro R, Stamelou M, Ganos C, Skorvanek M, Han V, Batla A, Bhatia KP. The clinical syndrome of paroxysmal exercise-induced dystonia: diagnostic outcomes and an algorithm. Mov Disord Clin Pract. 2014;1(1):57–61.
De Michele G, Galatolo D, Lieto M, Maione L, Cocozza S, Santorelli FM, Filla A. New AARS2 mutations in two siblings with tremor, downbeat nystagmus, and primary amenorrhea: a benign phenotype without leukoencephalopathy. Mov Disord Clin Pract. 2020;7(6):684–7.
Burke EA, Frucht SJ, Thompson K, Wolfe LA, Yokoyama T, Bertoni M, Huang Y, Sincan M, Adams DR, Taylor RW, Gahl WA, Toro C, Malicdan MCV. Biallelic mutations in mitochondrial tryptophanyl-tRNA synthetase cause Levodopa-responsive infantile-onset Parkinsonism. Clin Genet. 2018;93(3):712–8.
Vantroys E, Smet J, Vanlander AV, Vergult S, De Bruyne R, Roels F, Stepman H, Roeyers H, Menten B, Van Coster R. Severe hepatopathy and neurological deterioration after start of valproate treatment in a 6-year-old child with mitochondrial tryptophanyl-tRNA synthetase deficiency. Orphanet J Rare Dis. 2018;13(1):80.
Martinelli S, Cordeddu V, Galosi S, Lanzo A, Palma E, Pannone L, Ciolfi A, Di Nottia M, Rizza T, Bocchinfuso G, Traversa A, Caputo V, Farrotti A, Carducci C, Bernardini L, Cogo S, Paglione M, Venditti M, Bentivoglio A, Ng J, Kurian MA, Civiero L, Greggio E, Stella L, Trettel F, Sciaccaluga M, Roseti C, Carrozzo R, Fucile S, Limatola C, Di Schiavi E, Tartaglia M, Leuzzi V. Co-occurring WARS2 and CHRNA6 mutations in a child with a severe form of infantile parkinsonism. Parkinsonism Relat Disord. 2020;72:75–9.
Skorvanek M, Baloghova J, Kulcsarova K, Winkelmann J, Jech R, Ostrozovicova M, Zech M. Adult-onset neurodegeneration in nucleotide excision repair disorders: more common than expected. Mov Disord. 2022.
Hemelsoet DM, Vanlander AV, Smet J, Vantroys E, Acou M, Goethals I, Sante T, Seneca S, Menten B, Van Coster R. Leigh syndrome followed by parkinsonism in an adult with homozygous c.626C>T mutation in MTFMT. Neurol Genet. 2018;4(6):e298.
Ma J, Wang L, Yang YM, Mao CH, Wan XH. Novel SERAC1 mutations in a Chinese patient presenting with parkinsonism and dystonia. Neurol Sci. 2018;39(5):967–9.
Sharma VD, Buetefisch CM, Kendall FD, Gross RE, DeLong MR, Juncos JL. Secondary dystonia in a novel mitochondriopathy responsive to deep brain stimulation therapy. Mov Disord Clin Pract. 2020;8(1):135–8.
Fecto F, Yan J, Vemula SP, Liu E, Yang Y, Chen W, Zheng JG, Shi Y, Siddique N, Arrat H, Donkervoort S, Ajroud-Driss S, Sufit RL, Heller SL, Deng HX, Siddique T. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch Neurol. 2011;68(11):1440–6.
Zhang M, Xi Z, Zinman L, Bruni AC, Maletta RG, Curcio SA, Rainero I, Rubino E, Pinessi L, Nacmias B, Sorbi S, Galimberti D, Lang AE, Fox S, Surace EI, Ghani M, Guo J, Sato C, Moreno D, Liang Y, Keith J, Traynor BJ, St George-Hyslop P, Rogaeva E. Mutation analysis of CHCHD10 in different neurodegenerative diseases. Brain. 2015;138(Pt 9):e380.
Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Müller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Krüger R. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet. 2005;14(15):2099–111.
Unal Gulsuner H, Gulsuner S, Mercan FN, Onat OE, Walsh T, Shahin H, Lee MK, Dogu O, Kansu T, Topaloglu H, Elibol B, Akbostanci C, King MC, Ozcelik T, Tekinay AB. Mitochondrial serine protease HTRA2 p.G399S in a kindred with essential tremor and Parkinson disease. Proc Natl Acad Sci U S A. 2014;111(51):18285–90.
Yoo D, Lee W, Lee SJ, Sung JJ, Jeon GS, Ban JJ, Shin C, Kim J, Kim HS, Ahn TB. A novel TFG mutation in a Korean family with α-synucleinopathy and amyotrophic lateral sclerosis. Mov Disord. 2022;37(2):384–91.
Rodrigo LM, Nyholt DR. Imputation and reanalysis of ExomeChip data identifies novel, conditional and joint genetic effects on Parkinson’s disease risk. Genes (Basel). 2021;12(5):689.
Butcher NJ, Merico D, Zarrei M, Ogura L, Marshall CR, Chow EWC, Lang AE, Scherer SW, Bassett AS. Whole-genome sequencing suggests mechanisms for 22q11.2 deletion-associated Parkinson’s disease. PLoS One. 2017;12(4):e0173944.
Strafella C, Caputo V, Termine A, Assogna F, Pellicano C, Pontieri FE, Macchiusi L, Minozzi G, Gambardella S, Centonze D, Bossù P, Spalletta G, Caltagirone C, Giardina E, Cascella R. Immune system and neuroinflammation in idiopathic Parkinson’s disease: association analysis of genetic variants and miRNAs interactions. Front Genet. 2021;3(12):651971.
Gatt AP, Jones EL, Francis PT, Ballard C, Bateman JM. Association of a polymorphism in mitochondrial transcription factor A (TFAM) with Parkinson’s disease dementia but not dementia with Lewy bodies. Neurosci Lett. 2013;557 Pt B:177–80.
Pickrell JK, Berisa T, Liu JZ, Ségurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48(7):709–17.
Shadrina M, Nikopensius T, Slominsky P, Illarioshkin S, Bagyeva G, Markova E, Ivanova-Smolenskaia I, Kurg A, Limborska S, Metspalu A. Association study of sporadic Parkinson’s disease genetic risk factors in patients from Russia by APEX technology. Neurosci Lett. 2006;405(3):212–6.
Londono C, Osorio C, Gama V, Alzate O. Mortalin, apoptosis, and neurodegeneration. Biomolecules. 2012;2(1):143–64.
De Mena L, Coto E, Sánchez-Ferrero E, Ribacoba R, Guisasola LM, Salvador C, Blázquez M, Alvarez V. Mutational screening of the mortalin gene (HSPA9) in Parkinson’s disease. J Neural Transm (Vienna). 2009;116(10):1289–93.
Fang HS, Wang CC, Chao CY, Fan WL, Su SC, Wu YR. Association of ITPKB, IL1R2 and COQ7 with Parkinson’s disease in Taiwan. J Formos Med Assoc. 2022;121(3):679–86.
Wallace DC. Mitochondrial DNA variation in human radiation and disease. Cell. 2015;163(1):33–8.
Chinnery PF, Johnson MA, Wardell TM, Singh-Kler R, Hayes C, Brown DT, Taylor RW, Bindoff LA, Turnbull DM: Epidemiology of pathogenic mitochondrial DNA mutations. Ann Neurol 2000, 48:188e193
Gorman GS, Schaefer AM, Ng Y, Gomez N, Blakely EL, Alston CL, Feeney C, Horvath R, Yu-Wai-Man P, Chinnery PF, Taylor RW, Turnbull DM, McFarland R: Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol 2015;77:753e759
Horvath R, Kley RA, Lochmüller H, Vorgerd M. Parkinson syndrome, neuropathy, and myopathy caused by the mutation A8344G (MERRF) in tRNALys. Neurology. 2007;68(1):56–8.
Simon DK, Pulst SM, Sutton JP, Browne SE, Beal MF, Johns DR. Familial multisystem degeneration with parkinsonism associated with the 11778 mitochondrial DNA mutation. Neurology. 1999;53(8):1787–93.
Martín-Jiménez R, Lurette O, Hebert-Chatelain E. Damage in mitochondrial DNA associated with Parkinson’s disease. DNA Cell Biol. 2020;39(8):1421–30.
Ghezzi D, Marelli C, Achilli A, Goldwurm S, Pezzoli G, Barone P, Pellecchia MT, Stanzione P, Brusa L, Bentivoglio AR, Bonuccelli U, Petrozzi L, Abbruzzese G, Marchese R, Cortelli P, Grimaldi D, Martinelli P, Ferrarese C, Garavaglia B, Sangiorgi S, Carelli V, Torroni A, Albanese A, Zeviani M. Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson’s disease in Italians. Eur J Hum Genet. 2005;13(6):748–52.
•• Müller-Nedebock AC, Pfaff AL, Pienaar IS, Kõks S, van der Westhuizen FH, Elson JL, Bardien S. Mitochondrial DNA variation in Parkinson’s disease: analysis of “out-of-place” population variants as a risk factor. Front Aging Neurosci. 2022 Jul 14;14:921412. This study tested the hypothesis that haplogroup-defining mtDNA variants, in genes encoding components of the oxidative phosphorylation complexes, may have pathogenic potential in PD if they occur “out-of-place” on a different maternal lineage.
Thomas RR, Keeney PM, Bennett JP. Impaired complex-I mitochondrial biogenesis in Parkinson disease frontal cortex. J Parkinsons Dis. 2012;2(1):67–76.
Grünewald A, Rygiel KA, Hepplewhite PD, Morris CM, Picard M, Turnbull DM. Mitochondrial DNA depletion in respiratory chain-deficient Parkinson disease neurons. Ann Neurol. 2016;79(3):366–78.
Chi J, Xie Q, Jia J, Liu X, Sun J, Deng Y, Yi L. Integrated analysis and identification of novel biomarkers in Parkinson’s disease. Front Aging Neurosci. 2018;18(10):178.
Shamir R, Klein C, Amar D, Vollstedt EJ, Bonin M, Usenovic M, Wong YC, Maver A, Poths S, Safer H, Corvol JC, Lesage S, Lavi O, Deuschl G, Kuhlenbaeumer G, Pawlack H, Ulitsky I, Kasten M, Riess O, Brice A, Peterlin B, Krainc D. Analysis of blood-based gene expression in idiopathic Parkinson disease. Neurology. 2017;89(16):1676–83.
Yalçınkaya N, Haytural H, Bilgiç B, Özdemir Ö, Hanağası H, Küçükali Cİ, Özbek Z, Akcan U, İdrisoğlu HA, Gürvit H, Tüzün E. Expression changes of genes associated with apoptosis and survival processes in Parkinson’s disease. Neurosci Lett. 2016;26(615):72–7.
• Henderson AR, Wang Q, Meechoovet B, Siniard AL, Naymik M, De Both M, Huentelman MJ, Caselli RJ, Driver-Dunckley E, Dunckley T. DNA methylation and expression profiles of whole blood in Parkinson’s disease. Front Genet. 2021;12:640266. This study performed a comparative analysis of the methylome and transcriptome in blood from PD patients, identifying patterns of altered disease-specific DNA methylation and associated gene expression.
Ahmed SS, Santosh W, Kumar S, Christlet HT. Metabolic profiling of Parkinson’s disease: evidence of biomarker from gene expression analysis and rapid neural network detection. J Biomed Sci. 2009;16(1):63.
Miki Y, Tanji K, Mori F, Kakita A, Takahashi H, Wakabayashi K. Alteration of mitochondrial protein PDHA1 in Lewy body disease and PARK14. Biochem Biophys Res Commun. 2017;489(4):439–44.
Wang J, Liu Y, Chen T. Identification of key genes and pathways in Parkinson’s disease through integrated analysis. Mol Med Rep. 2017;16(4):3769–76.
Simunovic F, Yi M, Wang Y, Macey L, Brown LT, Krichevsky AM, Andersen SL, Stephens RM, Benes FM, Sonntag KC. Gene expression profiling of substantia nigra dopamine neurons: further insights into Parkinson’s disease pathology. Brain. 2009;132(Pt 7):1795–809.
Plum S, Eggers B, Helling S, Stepath M, Theiss C, Leite REP, Molina M, Grinberg LT, Riederer P, Gerlach M, May C, Marcus K. Proteomic characterization of synaptosomes from human substantia nigra indicates altered mitochondrial translation in Parkinson’s disease. Cells. 2020;9(12):2580.
Bennett JP Jr, Keeney PM. Alzheimer’s and Parkinson’s brain tissues have reduced expression of genes for mtDNA OXPHOS Proteins, mitobiogenesis regulator PGC-1α protein and mtRNA stabilizing protein LRPPRC (LRP130). Mitochondrion. 2020;53:154–7.
Forés-Martos J, Boullosa C, Rodrigo-Domínguez D, Sánchez-Valle J, Suay-García B, Climent J, Falcó A, Valencia A, Puig-Butillé JA, Puig S, Tabarés-Seisdedos R. Transcriptomic and genetic associations between Alzheimer’s disease, Parkinson’s disease, and cancer. Cancers (Basel). 2021;13(12):2990.
Phung DM, Lee J, Hong S, Kim YE, Yoon J, Kim YJ. Meta-analysis of differentially expressed genes in the substantia nigra in Parkinson’s disease supports phenotype-specific transcriptome changes. Front Neurosci. 2020;18(14):596105.
Koks S, Pfaff AL, Bubb VJ, Quinn JP. Longitudinal intronic RNA-Seq analysis of Parkinson’s disease patients reveals disease-specific nascent transcription. Exp Biol Med (Maywood). 2022;247(11):945–57.
Jin J, Hulette C, Wang Y, Zhang T, Pan C, Wadhwa R, Zhang J. Proteomic identification of a stress protein, mortalin/mthsp70/GRP75: relevance to Parkinson disease. Mol Cell Proteomics. 2006;5(7):1193–204.
Hu D, Sun X, Liao X, Zhang X, Zarabi S, Schimmer A, Hong Y, Ford C, Luo Y, Qi X. Alpha-synuclein suppresses mitochondrial protease ClpP to trigger mitochondrial oxidative damage and neurotoxicity. Acta Neuropathol. 2019;137(6):939–60.
Barca E, Kleiner G, Tang G, Ziosi M, Tadesse S, Masliah E, Louis ED, Faust P, Kang UJ, Torres J, Cortes EP, Vonsattel JP, Kuo SH, Quinzii CM. Decreased coenzyme Q10 levels in multiple system atrophy cerebellum. J Neuropathol Exp Neurol. 2016;75(7):663–72.
•• Fernandes HJR, Patikas N, Foskolou S, Field SF, Park JE, Byrne ML, Bassett AR, Metzakopian E. Single-cell transcriptomics of Parkinson’s disease human in vitro models reveals dopamine neuron-specific stress responses. Cell Rep. 2020 Oct 13;33(2):108263. This study represents one of the largest single-cell transcriptomic study of human iPSC-derived dopaminergic neurons, focused on gene expression dynamics in response to cytotoxic and genetic stressors.
Walter J, Bolognin S, Antony PMA, Nickels SL, Poovathingal SK, Salamanca L, Magni S, Perfeito R, Hoel F, Qing X, Jarazo J, Arias-Fuenzalida J, Ignac T, Monzel AS, Gonzalez-Cano L, Pereira de Almeida L, Skupin A, Tronstad KJ, Schwamborn JC. Neural stem cells of Parkinson’s disease patients exhibit aberrant mitochondrial morphology and functionality. Stem Cell Reports. 2019;12(5):878–889.
Gautier CA, Erpapazoglou Z, Mouton-Liger F, Muriel MP, Cormier F, Bigou S, Duffaure S, Girard M, Foret B, Iannielli A, Broccoli V, Dalle C, Bohl D, Michel PP, Corvol JC, Brice A, Corti O. The endoplasmic reticulum-mitochondria interface is perturbed in PARK2 knockout mice and patients with PARK2 mutations. Hum Mol Genet. 2016;25(14):2972–84.
Bonzano S, Michelon F, Crisci I, Molineris I, Neri F, Oliviero S, Beckervordersandforth R, Lie Dieter C, Peretto P, Bovetti S, Studer M, De Marchis S. Nr2f1 controls mitochondrial dynamics in mouse adult-born hippocampal neurons. BioRxiv. 2022. https://doi.org/10.1101/2022.03.25.485113.
•• Walter J, Bolognin S, Poovathingal SK, Magni S, Gérard D, Antony PMA, Nickels SL, Salamanca L, Berger E, Smits LM, Grzyb K, Perfeito R, Hoel F, Qing X, Ohnmacht J, Bertacchi M, Jarazo J, Ignac T, Monzel AS, Gonzalez-Cano L, Krüger R, Sauter T, Studer M, de Almeida LP, Tronstad KJ, Sinkkonen L, Skupin A, Schwamborn JC. The Parkinson’s-disease-associated mutation LRRK2-G2019S alters dopaminergic differentiation dynamics via NR2F1. Cell Rep. 2021;37(3):109864. This study uses single-cell RNA-seq and high-throughput image analysis for describing a pathogenic mechanism involving the LRRK2-G2019S mutation, where the dynamics of dopaminergic differentiation are modified via NR2F1.
Pyle A, Brennan R, Kurzawa-Akanbi M, Yarnall A, Thouin A, Mollenhauer B, Burn D, Chinnery PF, Hudson G. Reduced cerebrospinal fluid mitochondrial DNA is a biomarker for early-stage Parkinson’s disease. Ann Neurol. 2015;78(6):1000–4.
Lowes H, Pyle A, Santibanez-Koref M, Hudson G. Circulating cell-free mitochondrial DNA levels in Parkinson’s disease are influenced by treatment. Mol Neurodegener. 2020;15(1):10.
Howlett EH, Jensen N, Belmonte F, Zafar F, Hu X, Kluss J, Schüle B, Kaufman BA, Greenamyre JT, Sanders LH. LRRK2 G2019S-induced mitochondrial DNA damage is LRRK2 kinase dependent and inhibition restores mtDNA integrity in Parkinson’s disease. Hum Mol Genet. 2017;26(22):4340–51.
Podlesniy P, Puigròs M, Serra N, Fernández-Santiago R, Ezquerra M, Tolosa E, Trullas R. Accumulation of mitochondrial 7S DNA in idiopathic and LRRK2 associated Parkinson’s disease. EBioMedicine. 2019;48:554–67.
Chuang YH, Paul KC, Bronstein JM, Bordelon Y, Horvath S, Ritz B. Parkinson’s disease is associated with DNA methylation levels in human blood and saliva. Genome Med. 2017;9(1):76.
Zeuner KE, Schäffer E, Hopfner F, Brüggemann N, Berg D. Progress of pharmacological approaches in Parkinson’s disease. Clin Pharmacol Ther. 2019;105(5):1106–20.
Shin EJ, Nam Y, Lee JW, Nguyen PT, Yoo JE, Tran TV, Jeong JH, Jang CG, Oh YJ, Youdim MBH, Lee PH, Nabeshima T, Kim HC. N-methyl, N-propynyl-2-phenylethylamine (MPPE), a selegiline analog, attenuates MPTP-induced dopaminergic toxicity with guaranteed behavioral safety: involvement of inhibitions of mitochondrial oxidative burdens and p53 gene-elicited pro-apoptotic change. Mol Neurobiol. 2016;53(9):6251–69.
Parkinson Study Group QE3 Investigators; Beal MF, Oakes D, Shoulson I, Henchcliffe C, Galpern WR, Haas R et al. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 2014;71(5):543–52.
Prasuhn J, Brüggemann N, Hessler N, Berg D, Gasser T, Brockmann K, Olbrich D, Ziegler A, König IR, Klein C, Kasten M. An omics-based strategy using coenzyme Q10 in patients with Parkinson’s disease: concept evaluation in a double-blind randomized placebo-controlled parallel group trial. Neurol Res Pract. 2019;23(1):31.
Lin MW, Lin CC, Chen YH, Yang HB, Hung SY. Celastrol inhibits dopaminergic neuronal death of Parkinson’s disease through activating mitophagy. Antioxidants (Basel). 2019;9(1):37.
Chandra G, Shenoi RA, Anand R, Rajamma U, Mohanakumar KP. Reinforcing mitochondrial functions in aging brain: an insight into Parkinson’s disease therapeutics. J Chem Neuroanat. 2019;95:29–42.
Ferreira AFF, Binda KH, Singulani MP, Pereira CPM, Ferrari GD, Alberici LC, Real CC, Britto LR. Physical exercise protects against mitochondria alterations in the 6-hidroxydopamine rat model of Parkinson’s disease. Behav Brain Res. 2020;1(387):112607.
Esteves AR, Gozes I, Cardoso SM. The rescue of microtubule-dependent traffic recovers mitochondrial function in Parkinson’s disease. Biochim Biophys Acta. 2014;1842(1):7–21.
Golpich M, Amini E, Mohamed Z, Azman Ali R, Mohamed Ibrahim N, Ahmadiani A. Mitochondrial Dysfunction and biogenesis in neurodegenerative diseases: pathogenesis and treatment. CNS Neurosci Ther. 2017;23(1):5–22.
Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN. Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet. 2003;33(2):177–82.
Billingsley KJ, Barbosa IA, Bandrés-Ciga S, Quinn JP, Bubb VJ, Deshpande C, Botia JA, Reynolds RH, Zhang D, Simpson MA, Blauwendraat C, Gan-Or Z, Gibbs JR, Nalls MA, Singleton A; International Parkinson’s Disease Genomics Consortium (IPDGC), Ryten M, Koks S. Mitochondria function associated genes contribute to Parkinson’s disease risk and later age at onset. NPJ Parkinsons Dis. 2019;5:8.
Prasuhn J, Davis RL, Kumar KR. Targeting mitochondrial impairment in Parkinson’s disease: challenges and opportunities. Front Cell Dev Biol. 2021;5(8):615461.
Funding
This work was partially supported by the Italian Ministry of Health (RRC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Andrea Legati and Daniele Ghezzi declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Legati, A., Ghezzi, D. Parkinson’s Disease, Parkinsonisms, and Mitochondria: the Role of Nuclear and Mitochondrial DNA. Curr Neurol Neurosci Rep 23, 131–147 (2023). https://doi.org/10.1007/s11910-023-01260-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-023-01260-8