Abstract
Purpose of Review
To elucidate the interconnection between sleep and stroke.
Recent Findings
Growing data support a bidirectional relationship between stroke and sleep. In particular, there is strong evidence that sleep-disordered breathing plays a pivotal role as risk factor and concur to worsening functional outcome. Conversely, for others sleep disorders (e.g., insomnia, restless legs syndrome, periodic limb movements of sleep, REM sleep behavior disorder), the evidence is weak. Moreover, sleep disturbances are highly prevalent also in chronic stroke and concur to worsening quality of life of patients.
Promising novel technologies will probably allow, in a near future, to guarantee a screening of commonest sleep disturbances in a larger proportion of patients with stroke.
Summary
Sleep assessment and management should enter in the routinary evaluation of stroke patients, of both acute and chronic phase. Future research should focus on the efficacy of specific sleep intervention as a therapeutic option for stroke patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep is a basic human need, essential for physical and mental health. Stroke is a leading cause of death and disability worldwide. A close relationship between sleep and stroke has been largely recognized [1••]. Several sleep disturbances have been studied as risk factors for stroke, including sleep-disordered breathing (SDB) [2], insomnia [3], restless legs syndrome (RLS) [4], periodic limb movements of sleep (PLMS) [4], REM sleep behavior disorder (RBD) [5], narcolepsy [6], circadian rhythm disorders [7•], and short and long sleep duration [8•]. Moreover, sleep is severely disrupted in patients with stroke [9] in both acute and chronic phase, and stroke patients experience sleep-dependent changes of physiological functions [10]. Sleep has a well-established role in synaptic plasticity [11]. Therefore, post-stroke sleep disruption may interfere with synaptic plasticity and with brain extracellular waste removal, that are essentials for stroke recovery [12]. As a matter of fact, in 2020, a task force of European experts in neurology, stroke, respiratory medicine, and sleep medicine proposed shared guidelines for the management of sleep disorders in patients with stroke [13••].
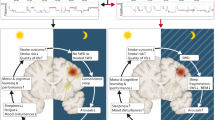
In the current paper, we will review how sleep and its disorders are strictly interconnected with stroke, playing a pivotal role as a stroke risk factor, appearing de novo as consequence of stroke, and modifying the course of the acute and chronic stroke phase (see Fig. 1).
Sleep Apnea and Stroke
Sleep apnea (SA) is the most extensively studied SDB in association with stroke [14]. SA is characterized by repetitive and intermittent cessation of airflow and encompasses two main phenotypes: obstructive SA (OSA), due to increased upper airways resistance, and central SA (CSA), due to lack of the respiratory drive.
SA, and particularly OSA, is one of the most common comorbidities in patients with stroke, acting as an independent risk factor [15], being high prevalent in the acute stroke phase [2], and playing a pivotal role in stroke recovery [16•] and stroke recurrence [17].
Sleep Apnea as Risk Factor for Stroke
OSA is a high prevalent in general population and is a potential modifiable risk factor for stroke. The hazard ratio of the risk of stroke in OSA population ranges from 1.97 [15] up to 4.63 [18]. OSA increases the risk of stroke through direct and indirect pathways. Among direct mechanisms, OSA leads to repetitive intermittent hypoxia, increased sympathetic activity, cerebral hemodynamic changes, hypercoagulability, endothelium dysfunction, and to an increased inflammatory response [19•, 20]. Moreover, OSA indirectly prompts stroke concurring to arterial hypertension and atrial fibrillation [21, 22].
The risk of stroke is directly related to OSA severity [23]; however, a definite phenotype of OSA, such as biomarkers predictive of cerebrovascular injury, is still missing. Recent evidence suggests that incidence of stroke is significantly higher in patients with OSA showing excessive daytime sleepiness (EDS) [24, 25]. Conversely, another recent research did not show any association between OSA symptoms and risk of cardiovascular diseases [26], but identified hypoxic burden, defined as the area under the desaturation curve associated with respiratory events, as a promising marker of cardiovascular risk in OSA population [26, 27]. From this point of view, new polygraphic metrics behind the number of apneas and hypopneas per hour of sleep (Apnea–Hypopnea Index, AHI) have been recently investigated (e.g., hypoxic burden [26, 27], hypoxia load [28], oximeter-derived pulse rate variability [29], and sleep breathing impairment index [30]) in order to identify markers of cerebrovascular risk in patients with OSA.
For what concerns CSA as risk factor for stroke, any convincing evidence is missing. Only one study on a large cohort of elderly patients showed that CSA and its severity were independent risk factors for stroke [31]. However, since CSA is frequently associated with atrial fibrillation and heart failure, it is possible that more than a specific risk factor, CSA represents a biomarker of other established risk factors for stroke.
Sleep Apnea in Stroke Patients: New Onset or Pre-existing Condition
Since SA is highly prevalent in patients with stroke, it is debated whether SA is a pre-existing condition or a consequence of stroke. In a recent meta-analysis performed by Seiler et al. [2] conducted on 86 studies for a total of 7096 patients, the prevalence of SA in patients with acute stroke or TIA ranged between 30% (with an AHI > 30/h, consistent with severe SDB) up to 71% (with an AHI > 5/h, consistent with mild SDB); only a slightly lower prevalence was observed in the chronic phase. However, most of the studies included in the meta-analysis evaluated the acute phase, while only few studies [32–37] evaluated the intra-individual evolution from acute to the chronic phase. Moreover, few studies [33–36, 38] evaluated the evolution of SDB in stroke by means of polysomnography (PSG), the standard diagnostic test for SDB in stroke patients [39], reporting an improvement, but not a resolution, from acute to sub-acute/chronic phase, of the SDB. These data were further confirmed by a meta-analysis conducted by Hasan et al. who evaluated the dynamic prevalence of sleep disorders after stroke or TIA [1••]. Recently, the SAS Care 1 study [35] evaluated longitudinally the progression of SDB in a large cohort patient with ischemic stroke or TIA by means of PSG: the authors observed a significant reduction of AHI (baseline: 21 events/h; at 3 months: 18 events/h) driven by a reduction of both the obstructive and central component. Nevertheless, the prevalence of SBD was similar at baseline (85.6%) and at 3 months (82.7%), and a reduction was observed exclusively for severe SBD (AHI > 30/h) and for CSA. Taken together, these data support the hypothesis that OSA may be a pre-existing condition aggravated by stroke, while CSA may appear de novo being a symptom of the acute phase.
Notably, the high prevalence of SDB in stroke is mainly determined by OSA, being the reported prevalence of CSA approximately 12% [2]. In a recent study conducted on a large population of stroke patients tested with a home sleep apnea test [40], the prevalence of CSA was even lower (1.4%). However, the prevalence of CSA in stroke patients is probably underestimated. In fact, CSA is diagnosed exclusively when more than 50% of total apneic events are scored as central. Furthermore, most of the studies evaluating the prevalence of SDB did not score central hypopnea [40]. Since the pathogenic pathways behind OSA and CSA are different, future studies should count separately the AHI of central and obstructive events and consider a mixed respiratory pattern when these two conditions coexist. In fact, studies that took into the account a mixed pattern, characterized by the coexistence of central and obstructive events, observed a high proportion of such pattern in both acute [41] and chronic phases [42•].
Several mechanisms can contribute to exacerbate or aggravate a pre-existing SDB in patients suffering of stroke. Though several studies failed to demonstrate a direct correlation among stroke topography and SDB [43], strokes involving the central respiratory pattern generator can induce respiratory instability and, in turn, promote both central and obstructive SDB [36, 42•]. In the acute stroke, several factors compromise the patency of the upper airways, such as a weakness or incoordination of the pharyngeal [44, 45], intercostal and diaphragmatic muscles [46], increased rostral fluid shift [47], and prolonged supine position [48]. Moreover, a reduced arousal response may concur to increase the length of apnea and the hypoxic burden. Taken together, these data suggest that stroke patients may present a peculiar SDB phenotype characterized by a multifactorial pathogenic mechanism [49], the coexistence of central and obstructive apnea, specific polygraphic features [50, 51], and different cardiovascular risk [52].
Sleep Apnea and Stroke Outcome
SDB negatively impacts on stroke outcome. In the BASIC [16•] and SAS Care 1 [35] studies, stroke patients with concomitant SDB showed poorer functional outcome at 3 months. Also, during acute phase, patients with SDB are at higher risk of early neurological deterioration [53]. It is supposed that sustained nocturnal hypoxia may play a detrimental role in extension of the ischemic penumbra [54], and that sleep fragmentation may interfere with synaptic plasticity [12]. Still, stroke patients with SDB exhibit a higher risk of stroke recurrence, and the recurrence risk is directly related to SDB severity [17]; the risk of recurrence appears to be linked to OSA rather than CSA [55]. Conversely, CSA, together with nocturnal hypoxia, may predict mortality [55]. Notably, most of studies evaluating the impact of OSA on the stroke outcome were conducted in pre-thrombectomy era. In fact, in last years, stroke outcome significantly improved due to the introduction of endovascular treatment [56]. A recent study conducted on a large stroke registry [57•] reported that stroke patients undergoing to thrombectomy and with a pre-existing diagnosis of OSA showed lower mortality and lower risk of intracranial hemorrhages compared to patients without OSA; the authors speculated that the repeated hypoxic/hypercapnic episodes that occur during sleep in OSA induce neuroprotective adaptations in the brain, increasing the tolerance to the hypoxia/ischemia [58, 59]. Although this study has several limitations, the main one being that OSA is probably largely under-recognized in the examined sample—it pinpoints that new evidence of the role of SDB and, eventually, its treatment are needed in the era of endovascular treatment.
Diagnosis of Sleep Apnea in Stroke Patients
Notably, in stroke patients’ signs and symptoms of OSA are not predictive [60]. Therefore, in the light of the high prevalence of SA in stroke patients, the search of sleep apnea should be part of routinary stroke care and, ideally, all stroke patients’ should undergo to an instrumental sleep assessment. Conversely, only a minority of stroke patients undergo to a formal sleep evaluation [61•]. Current guidelines of American Academy of Sleep Medicine recommend full-night PSG for screening and diagnosis of SDB in patients with stroke [39]. However, PSG is cost and time expensive, poorly available outside specialized sleep centers, and difficult to perform in acute setting. In the SLEAP SMART trial, home sleep apnea testing performed by means of respiratory polygraphy showed good feasibility and good diagnostic value compared to in-laboratory PSG [62•]. Therefore, in an acute setting, a sleep study with limited-channel devices is a reasonable alternative to PSG, keeping the latter study dedicated to ambiguous cases and to patients showing peculiar comorbidities (i.e., heart failure, chronic obstructive pulmonary diseases, and concomitant sleep disorders) [2, 14, 63].
Since performing a sleep study in all stroke patients is compelling, current research is focusing on identifying predictors of sleep apnea in this population applying promising artificial intelligent techniques [64•, 65, 66], new scores [67], or alternative recording techniques [68].
Treatment of Sleep Apnea in Stroke Patients
To date, studies evaluating the efficacy of SDB treatment in primary stroke prevention yielded conflicting results. Two recent large trials, the SAVE [69] and the ISAACC [70], failed to demonstrate the effectiveness of continuous positive airway pressure (CPAP) treatment for prevention of cardiovascular events, including stroke. Notably, in a subgroup analysis of SAVE trial, patients that reported a good adherence (> 4 h/night) showed a reduced risk of cerebrovascular events. Similarly, a recent meta-analysis including 13 studies (nine RCTs and four observational studies) reported an efficacy of CPAP treatment in reducing the risk of stroke in patients with good adherence and patients with moderate to severe OSA [71•]. This data was further confirmed by a retrospective cohort study conducted on 5757 Medicare beneficiaries aged ≥ 65 years that revealed that CPAP adherence was associated with a 2% reduction in risk of stroke for each month [72].
To date, studies that evaluated the effectiveness of positive airway pressure (PAP) treatment on neurological outcome and stroke recurrence in patients with stroke yielded conflicting results [73–76]. For a detailed review on studies evaluating the efficacy of SA treatment in patients with stroke, see Boulos et al. [77]. Nonetheless, available studies suggest weak evidence of efficacy of PAP treatment in terms of ameliorating functional outcome and reducing stroke recurrence. Moreover, such evidence comes from few trials, often underpowered, that included patients with mild SDB and with poor adherence to PAP treatment. In fact, treatment of SA in post-stroke patients is challenging: stroke patients usually show an altered state of consciousness, cognitive impairment, and presence of nasogastric tube that compromise the adherence of ventilatory treatment. Therefore, adherence to treatment is the major issue in this peculiar population [78]. Recent studies revealed how the implementation of specific adherence programs encompassing training strategy during hospitalization [79] and telemedicine monitoring after discharge [80•] can lead to an increase CPAP adherence in post-stroke patients.
Such uncertainty of efficacy of SA treatment in post-stroke patients is reflected by diverging recommendations coming from European guidelines [13••] that endorse to screen and treat SA in post-stroke patients and American Heart Association guidelines [81••] that does not propose this approach, suggesting to enroll patients in clinical trials. Ongoing trials [82, 83] evaluating the effect of early treatment of SDB with different nocturnal ventilatory supports in acute stroke on outcome will hopefully clarify the feasibility and efficacy of such treatment in acute stroke.
Alternative treatments to PAP in stroke patients have been poorly studied (e.g., trazodone [84], swallowing interventions [85], positional therapy [86]) and are currently not recommended in the management of stroke-related SDB [13••].
Sleep Duration, Insomnia, Hypersomnia, and Circadian Rhythms
Risk Factors
In the last few years, sleep duration outside the recommended sleep hours has been investigated as a possible risk factor of stroke by several studies, although providing inconsistent results [87–91, 92•, 93–95]. In a recent large population-based registry, involving over one million participants, both extremely short and long sleep duration were associated with higher odds of stroke [88]. Also, in the most recent meta-analysis, which included 20 prospective cohort studies, U-shaped relationships were observed between sleep duration and stroke incidence, and mortality [8•]. However, the evidence coming from this meta-analysis points towards a slightly higher risk for long sleep than short sleep. Indeed, for short sleep duration, the relative risk of stroke was 1.33 (95% CI: 1.19–1.49), while for long sleep it was 1.71 (95% CI: 1.50–1.95) [8•]. Such difference in stroke risk was even higher in a previous meta-analysis, which found a J-shaped association between stroke and sleep duration [96]. Interestingly, a large cohort study of 79,881 Swedish participants evaluated the effect on stroke risk of single-nucleotide polymorphisms with known association to different sleep traits [97]. Such analysis revealed no association of genetic liability to short or long sleep duration with overall stroke risk but suggested a possible association between short sleep duration and increased risk of large artery stroke [97].
It is worth noting that all the studies included in the meta-analysis [8•] evaluated sleep duration as self-reported by the subjects. Conversely, in the SAVE study [90], the authors performed an instrumental estimation of sleep duration using the oximetry recording time, showing that long sleep duration was significantly associated with stroke (HR 1.79, 95% CI 1.22–2.63).
Together with short sleep duration, insomnia symptoms have been linked with increased risk of cardiovascular and cerebrovascular events, as shown by a meta-analysis of 15 studies, reporting a pooled odds ratio for the different insomnia symptoms below 1.3 [3]. However, the risk of stroke remains uncertain, since the studies included in the meta-analysis assessing stroke as outcome did not find an association between stroke and insomnia or short sleep duration, nor did a later published meta-analysis [98].
Also, an increased risk of cardiovascular comorbidity is observed in patients with narcolepsy type 1 [6], a central hypersomnia characterized by orexin deficiency. Patients with narcolepsy show lack of nocturnal blood pressure dipping, and disrupted nighttime sleep, and other comorbidities (e.g., obesity, diabetes, and mood disorders) that may concur to raise the cardiovascular risk [54]; furthermore, drugs used to manage narcoleptic symptoms may concur to increase cardiovascular risk [99].
To date, the reasons why sleep duration may influence stroke risk remain unclear. Experimental data have linked sleep deprivation to increased cardiovascular risk through several intermediate pathophysiological mechanisms involving the autonomic nervous system, endothelial function, insulin and glucose regulation, inflammation, and coagulation [100•]. Moreover, short sleep has been linked to hypercholesterolemia and increased incidence of coronary artery calcification [100•, 101]. Other biological pathways connected to short sleep are decreased secretion of melatonin [102], increased ghrelin and reduced leptin levels [103], and therefore increased appetite. Conversely, a prolonged sleep was associated with increased levels of inflammatory markers [104].
Also, circadian misalignment has been linked to increased cardiovascular risk, including stroke [7•]. The possible influence of circadian rhythms on cardiovascular disorders was supported by the observation of a circadian rhythmicity in ischemic strokes, myocardial infarction, and sudden cardiac death, all having a peak of incidence in the morning hours [105]. The circadian system influences several cardiovascular risk factors, such as circulating catecholamine levels, blood pressure, heart rate, vagal modulation, platelet aggregability, and immune responses, thus having a possible impact on stroke risk [106]. Several circadian gene polymorphisms and haplotypes have been investigated as potential genetic risk factors of stroke [107]. Genes associated with a protective role against stroke were a single-nucleotide polymorphism of CLOCK gene [108] and PER1 and PER2 genes [109]. Genome-wide association studies demonstrated an association between genetic variants of melatonin receptors 2 and the risk of metabolic disorders, such as type 2 diabetes mellitus and insulin resistance, which may in turn increase the risk of stroke [107].
Overall, evidence coming from literature points towards a slight increase of stroke risk for both short and long sleepers, as well as a possible impact of circadian misalignments on stroke risk, severity, and outcome. While multiple possible pathogenetic mechanisms linking short sleep to stroke risk have been hypothesized, the literature on the association between long sleep and stroke is scarce. Future studies should focus on the possible pathogenetic effect of sleep duration on stroke risk and clarify the respective role of short and long sleep on stroke risk by means of prospective studies with objective measures of sleep duration. As concerns circadian rhythms, starting from the observation of a circadian rhythmicity in stroke occurrence, future research should test this hypothesis on a molecular level.
Stroke Recovery and Outcome
A growing evidence supports the knowledge that sleep disorders, pre-existent or appearing de novo, are frequent in stroke survivors and are associated with worse stroke outcomes and increased cardiocerebrovascular morbidity [110]. Notably, sleep is essential for synaptic plasticity by promoting an overall reduction in synaptic strength during slow-wave sleep and, in turn, synaptic plasticity is essential for stroke recovery. Therefore, it is presumed that poor sleep is associated to poorer stroke recovery [11].
A recent meta-analysis of PSG studies in acute ischemic stroke demonstrated that stroke patients have a poorer sleep than controls, in terms of sleep efficiency, total sleep time, and wake after sleep onset [111].
Among sleep disorders, insomnia is present in about one-third of stroke patients: in the studies assessing insomnia with validated diagnostic criteria, the pooled prevalence was 32.5% in the acute phase, and 34.8% in the subacute phase. When evaluating self-reported insomnia symptoms by means of questionnaires, the summary prevalence was 47.1% in the acute phase, and 50.4% in the subacute phase [1••]. However, it is worth to consider that insomnia is a multifactorial disorder, where a big contribution to sleep disruption is played by the hospital setting; importantly, sleep fragmentation in acute stroke is associated with an increased risk for stroke-associated delirium [112] that is, in turn, associated to poorer long-term outcome [113]. Nonetheless, studies in which insomnia was evaluated at different time points, up to 18 months from stroke onset, revealed a prevalence of insomnia symptoms near to 50% [114, 115]. Moreover, chronic post-stroke insomnia was associated with increased disability and mortality [115–117]. Even if only few studies investigated sleep complaints after the acute phase of stroke, thus limiting the generalizability of these results, the possible high risk of insomnia chronicization should be considered, and efforts should be made to limit iatrogenic sleep disruption. However, in the last years, growing evidence emerged on other factors contributing to post-stroke insomnia, further complicating the attempts to prevent this disorder. Indeed, post-stroke insomnia is often comorbid with post-stroke depression, anxiety, and fatigue with a bidirectional relationship [118•, 119]. A recent systematic review and meta-analysis on post-stroke fatigue found that depression, anxiety, and sleeping disturbances are associated with fatigue in stroke survivors, with sleeping disturbances nearly doubling the risk for post-stroke fatigue [120]. In a recent study assessing patients 1 month after stroke, poor sleep quality was independently associated with post-stroke anxiety [121]. A large, prospective study found a prevalence of post-stroke depression of 35% and of 25% at 3 and 12 months after stroke, respectively; in such cohort, sleep disturbances and fatigue were prevalent similarly to depression [122]. Interestingly, this study shows that at least 10% of patients without depression at 3 or 6 months will later develop depression at 12 months, thus suggesting the need for an active neuro-psychiatric follow-up of patients for at least 1 year after stroke.
On the other hand, hypersomnia may arise as consequence of stroke [123] affecting up to 5.6% of stroke survivors [124]. Hypersomnia moreover is a core feature of thalamic stroke [125•]. In a recent study conducted by Jaramillo et al., patients experiencing thalamic stroke showed a reduction of overnight slow wave slope changes suggesting an impaired thalamic-dependent synaptic renormalization, and therefore, an impaired recovery [125•]. Moreover, post-stroke hypersomnia in stroke patients is linked to poorer functional outcome and to an increase risk to go in a nursing home, suggesting an impaired sleep-dependent recovery of stroke patients [124].
The frequent co-existence of sleep disorders, depression, anxiety, and fatigue after stroke implies the need for interventions which could possibly target all these aspects. A 6-week therapy with modafinil, a wakefulness-promoting agent, was investigated for treatment of post-stroke fatigue persisting 3 months or more after stroke, showing a benefit on fatigue and quality of life [126]. Post-stroke depression is a clinical entity which is poorly responsive to pharmacological approaches [127, 128]. However, a recently published meta-analysis showed that SSRIs are effective in treating post-stroke depression and anxiety, and improving post-stroke recovery in terms of motor function, cognitive function, and dependence [129•]. Treatment of post-stroke insomnia is challenging as well: indeed, GABA agonists may have detrimental effects on stroke recovery; they have not been systematically evaluated in patients with post-stroke insomnia and therefore no recommendations can be made on their use [13••]. Apart from pharmacotherapy, other approaches have been investigated for post-stroke depression and insomnia, such as psychotherapy, bright light therapy, and acupuncture, with some evidence of a benefit on sleep parameters, daytime sleepiness, fatigue, mood, and quality of life [130–132]. In a recent randomized controlled trial, ischemic stroke patients with comorbid depression and insomnia were randomized to receive bright-light therapy and escitalopram or escitalopram alone. Compared to monotherapy, polytherapy significantly improved depressive symptoms and sleep complaints [133].
Sleep–wake cycle is impacted after stroke as well, as shown by studies involving actigraphy recordings and chronotype questionnaires [118•]. Moreover, circadian rhythm’s dysfunction is supported by the finding of reduced melatonin levels in patients with acute stroke [118•]. Therefore, the current pre-clinical research is oriented towards melatonin supplementation in experimental models of ischemic stroke. A recent study on mice found that daily melatonin administration during the subacute phase of stroke ameliorated stroke-induced sleep disturbances and resulted in reduction of infarct volume [134]. However, the efficacy of melatonin supplementation in human subjects with acute stroke has not been systematically evaluated, limiting the translationality of these results. Moreover, findings coming both from pre-clinical and clinical models of stroke suggest an impact of circadian rhythms also on the outcome of stroke. Indeed, a recent experimental study on mice suggested that stroke onset at different sleep–wake time points has an impact on stroke severity and outcome, which were worse for stroke occurring during sleep, compared to those occurring at wake [135]. In a multicenter study, including more than 17,000 patients, night-onset strokes, compared with day-onset strokes, were associated with worse presenting neurologic severity, more frequent early neurological deterioration, and worse functional outcome [136]. However, such association between sleep-onset and worse stroke outcome may be at least partially explained by a delayed recognition of stroke symptoms.
Overall, given the bidirectionality of relationship between sleep and mood disorders after stroke, treatment of post-stroke sleep and sleep–wake cycle disorders, depression, and fatigue should encounter a multi-component approach with target on sleep–wake cycle improvement, appropriate neuro-rehabilitation, and psychotherapy.
Restless Legs Syndrome, Periodic Limb Movement of Sleep, and Rem Sleep Behavior Disorder
RLS is a disturbance characterized by an unpleasant sensation in the legs and an irresistible urge to move them, occurring typically in the evening hours. RLS is often associated with PLMS, a condition with repetitive limb movements that occurring during sleep and may cause sleep disruption.
RBD is a REM-related disorder characterized by unpleasant dreams and vigorous motor behaviors in which the patients seem to be enacting their dreams. RBD is an early prodromal sign of the α-synucleinopathies.
Such disturbances have been investigated as risk factors for stroke and as consequence of stroke.
Risk Factors
In the last years, RLS and PLMS have been suggested as possible risk factors for stroke by different observational studies. Nevertheless, two systematic reviews and meta-analysis on RLS did not provide any evidence for an increased risk of stroke in patients with RLS [4, 137]. Conversely, a systematic review and meta-analysis on PLMS concluded that PLMS is associated with a mild increased risk for stroke (OR 1.267; 95% CI 1.040–1.543) [138]. However, the results of this meta-analysis should be taken cautiously since the five studies included did not control for other stroke-associated factors, such as presence of anti-thrombotic therapy, anti-hypertensive medications, strict diabetes mellitus management, smoking, and OSA. Therefore, further studies controlling for other stroke-related risk factors are needed to assess the true impact of PLMS on the risk of stroke. Also, long-term, prospective studies, controlling for potential confounders, are needed to rule out the hypothesized association between RLS and cardio-cerebrovascular morbidity.
RBD is characterized by early impairment of autonomic nervous system and of a non-dipping profile of blood pressure that may concur to increase the risk of cerebrovascular accidents [139]. However, to date, there is no convincing evidence of an increased risk of stroke in patients with such disorder; only a recent study suggests a higher risk of developing both hemorrhagic and ischemic stroke in patients with probable RBD, not confirmed by means of PSG [5].
Consequence of Stroke and Role on Stroke Recovery
In the last years, research on stroke-related RLS has focused on its incidence and on the identification of possible connections with the anatomy of stroke lesions. Data emerging from the most recent literature are not homogenous concerning stroke-related RLS incidence, which ranges between 2.3 and 15.1% [140•]. The most common sites of stroke lesions involved in stroke-related RLS have been identified in basal ganglia, corona radiata, thalamus, and brainstem, especially pons [140•]. In a recent prospective study, stroke lesions involving the body of caudate nucleus, the lenticulo-capsule, and corona radiata were significantly more frequent in the cohort of patients with stroke-related RLS compared with controls [141].
An element of uncertainty is whether the different clinical presentations of the disorder, which is more often unilateral, usually located in the paralyzed lower limb [142], may hide different pathogenetic mechanisms. Indeed, the pathogenesis of bilateral stroke-related RLS is yet to be determined, since the anatomical location of stroke can only partially explain the symptoms [140•]. A recent prospective study found bilateral symmetrical stroke-related RLS in 13/16 patients, even if all patients had unilateral stroke [143]. The lenticulostriate area was involved in eight patients, being either left-sided or right-sided, whereas seven patients had ventral brainstem stroke. Interestingly, a hyperdopaminergic tone in the putamen ipsilateral to the infarct was observed in all patients, except in one [143]. From a genetic point-of-view, direct evidence is rather scarce regarding the association between genes regulating dopaminergic neurotransmission, iron metabolism, and cardiovascular risk [107]. Some studies have argued that RLS is associated with a less favorable outcome of stroke [144, 145]; however, larger studies are needed to confirm these data [13••].
For what concerns PLMS, data on its prevalence after stroke are inconclusive [13••]. A large, prospective, polysomnographic study reported a similar frequency of PLMS in 169 stroke patients compared with 162 controls, both in the acute and subacute phase [146]. A meta-analysis, including 158 PLMS patients with stroke and 88 PLMS controls without stroke, revealed a significantly higher periodic limb movement index in patients with stroke, compared with controls [138]. However, studies investigating the pathophysiology of stroke-related PLMS are warranted to clarify the finding of a possible worse PLMS severity after stroke. Moreover, future studies with larger cohorts are needed to investigate its prevalence and its impact on the outcome of stroke.
Also, RBD may arise de novo as a consequence of brainstem stroke affecting nuclei involved in the regulation of REM sleep [147]. The pathogenic mechanisms are not clear; a recent study conducted on patients with brainstem stroke observed a preserved REM atonia of both phasic and tonic activities and only a reduction of the total time spent in REM [148].
To the present, data on the incidence of movement disorders of sleep and RBD after stroke, as well as their influence on further stroke risk and recovery, are inconclusive.
Conclusion
Despite substantial medical literature indicates a pivotal role of sleep and its disorders from pre-stroke up to chronic stroke phase, to date, poorness is known on pathogenic mechanisms and optimal management of sleep disorders in stroke population (see Table 1). From this point of view, rigorous and large trials are warranted in order to elucidate the role and the management of each specific sleep disorders in patients with stroke.
Even more important, sleep is poorly investigated by stroke physicians in the standard clinical practice outside research programs. Current knowledge supports that sleep assessment should promptly enter in the routinary stroke care.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hasan F, Gordon C, Wu D, Huang HC, Yuliana LT, Susatia B et al. Dynamic prevalence of sleep disorders following stroke or transient ischemic attack: systematic review and meta-analysis. Stroke. 2021;52(2):655–63. A systematic review and meta-analysis that evaluated the prevalence and evolution of main sleep disorders (sleep-disordered breathing, insomnia, restless legs syndrome, and periodic limb movements of sleep) from acute to chronic phase of stroke.
Seiler A, Camilo M, Korostovtseva L, Haynes AG, Brill AK, Horvath T, et al. Prevalence of sleep-disordered breathing after stroke and TIA: a meta-analysis. Neurology. 2019;92(7):e648–54.
He Q, Zhang P, Li G, Dai H, Shi J. The association between insomnia symptoms and risk of cardio-cerebral vascular events: a meta-analysis of prospective cohort studies. Eur J Prev Cardiol. 2017;24(10):1071–82.
Kendzerska T, Kamra M, Murray BJ, Boulos MI. Incident cardiovascular events and death in individuals with restless legs syndrome or periodic limb movements in sleep: a systematic review. Sleep. 2017;40(3):zsx013. https://doi.org/10.1093/sleep/zsx013.
Ma C, Pavlova M, Liu Y, Liu Y, Huangfu C, Wu S, et al. Probable REM sleep behavior disorder and risk of stroke: a prospective study. Neurology. 2017;88(19):1849–55.
Jennum PJ, Plazzi G, Silvani A, Surkin LA, Dauvilliers Y. Cardiovascular disorders in narcolepsy: review of associations and determinants. Sleep Med Rev. 2021;58:101440.
Lo EH, Albers GW, Dichgans M, Donnan G, Esposito E, Foster R et al. Circadian biology and stroke. Stroke. 2021;52(6):2180–90. A review that assessed molecular, cellular, and physiological pathways in circadian biology in relation to the clinical consequences in stroke.
Wang H, Sun J, Sun M, Liu N, Wang M. Relationship of sleep duration with the risk of stroke incidence and stroke mortality: an updated systematic review and dose-response meta-analysis of prospective cohort studies. Sleep Med. 2022;90:267–78. A meta-analysis on studies that evaluated the correlation between the risk of stroke and sleep duration.
Miano S, Fanfulla F, Nobili L, Heinzer R, Haba-Rubio J, Berger M, et al. SAS CARE 1: Sleep architecture changes in a cohort of patients with ischemic stroke/TIA. Sleep Med. 2022;98:106–13.
Brunetti V, Vollono C, Testani E, Pilato F, Della MG. Autonomic nervous system modifications during wakefulness and sleep in a cohort of patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28(6):1455–62.
de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science. 2017;355(6324):507–10.
Zunzunegui C, Gao B, Cam E, Hodor A, Bassetti CL. Sleep disturbance impairs stroke recovery in the rat. Sleep. 2011;34(9):1261–9.
Bassetti CLA, Randerath W, Vignatelli L, Ferini-Strambi L, Brill AK, Bonsignore MR et al. EAN/ERS/ESO/ESRS statement on the impact of sleep disorders on risk and outcome of stroke. Eur J Neurol. 2020;27(7):1117–36. A statement of four major European medical organizations (European Academy of Neurology, European Respiratory Society, European Stroke Organization, European Sleep Research Society) on current knowledge and management of sleep disorders in terms of stroke risk and outcome.
Baillieul S, Dekkers M, Brill AK, Schmidt MH, Detante O, Pepin JL, et al. Sleep apnoea and ischaemic stroke: current knowledge and future directions. Lancet Neurol. 2022;21(1):78–88.
Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–41.
Lisabeth LD, Sanchez BN, Lim D, Chervin RD, Case E, Morgenstern LB et al. Sleep-disordered breathing and poststroke outcomes. Ann Neurol. 2019;86(2):241–50. A large cohort study evaluating the impact of sleep-disordered breathing on functional and neurological outcome in patients with stroke.
Brown DL, Shafie-Khorassani F, Kim S, Chervin RD, Case E, Morgenstern LB, et al. Sleep-disordered breathing is associated with recurrent ischemic stroke. Stroke. 2019;50(3):571–6.
Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, Soler-Cataluna JJ, Almeida-Gonzalez C, De la Cruz MI, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186(9):909–16.
Tanayapong P, Kuna ST. Sleep disordered breathing as a cause and consequence of stroke: a review of pathophysiological and clinical relationships. Sleep Med Rev. 2021;59:10149. A review with an educational value evaluating the pathophysiological pathway of sleep-disordered breathing in patients with stroke.
Castello-Branco RC, Cerqueira-Silva T, Andrade AL, Goncalves BMM, Pereira CB, Felix IF, et al. Association between risk of obstructive sleep apnea and cerebrovascular reactivity in stroke patients. J Am Heart Assoc. 2020;9(6):e015313.
Pengo MF, Faini A, Grote L, Ludka O, Joppa P, Pataka A, et al. Impact of sleep apnea on cardioembolic risk in patients with atrial fibrillation: data from the ESADA cohort. Stroke. 2021;52(2):712–5.
Dalmar A, Singh M, Heis Z, Cumpian TL, Ceretto C, Mortada ME, et al. Risk of atrial fibrillation and stroke after bariatric surgery in patients with morbid obesity with or without obstructive sleep apnea. Stroke. 2021;52(7):2266–74.
Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O’Connor GT, Resnick HE, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–77.
Wu B, Tarraf W, Wallace DM, Stickel AM, Schneiderman N, Redline S, et al. Cardiovascular correlates of sleep apnea phenotypes: results from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). PLoS ONE. 2022;17(4):e0265151.
Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200(4):493–506.
Trzepizur W, Blanchard M, Ganem T, Balusson F, Feuilloy M, Girault JM, et al. Sleep apnea-specific hypoxic burden, symptom subtypes, and risk of cardiovascular events and all-cause mortality. Am J Respir Crit Care Med. 2022;205(1):108–17.
Blanchard M, Gerves-Pinquie C, Feuilloy M, Le Vaillant M, Trzepizur W, Meslier N, et al. Hypoxic burden and heart rate variability predict stroke incidence in sleep apnoea. Eur Respir J. 2021;57:2004022.
Muraja-Murro A, Kulkas A, Hiltunen M, Kupari S, Hukkanen T, Tiihonen P, et al. The severity of individual obstruction events is related to increased mortality rate in severe obstructive sleep apnea. J Sleep Res. 2013;22(6):663–9.
Sabil A, Gerves-Pinquie C, Blanchard M, Feuilloy M, Trzepizur W, Goupil F, et al. Overnight oximetry-derived pulse rate variability predicts stroke risk in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2021;204(1):106–9.
Cao W, Luo J, Huang R, Xiao Y. The association between sleep breathing impairment index and cardiovascular risk in male patients with obstructive sleep apnea. Nat Sci Sleep. 2022;14:53–60.
Munoz R, Duran-Cantolla J, Martinez-Vila E, Gallego J, Rubio R, Aizpuru F, et al. Central sleep apnea is associated with increased risk of ischemic stroke in the elderly. Acta Neurol Scand. 2012;126(3):183–8.
Parra O, Arboix A, Bechich S, Garcia-Eroles L, Montserrat JM, Lopez JA, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161(2 Pt 1):375–80.
Hui DS, Choy DK, Wong LK, Ko FW, Li TS, Woo J, et al. Prevalence of sleep-disordered breathing and continuous positive airway pressure compliance: results in Chinese patients with first-ever ischemic stroke. Chest. 2002;122(3):852–60.
Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37(4):967–72.
Ott SR, Fanfulla F, Miano S, Horvath T, Seiler A, Bernasconi C, et al. SAS Care 1: sleep-disordered breathing in cute stroke an transient ischaemic attack – prevalence, evolution and association with functional outcome at 3 months, a prospective observational polysomnography study. ERJ Open Res. 2020;6:00334-2019.
Pavsic K, Pretnar-Oblak J, Bajrovic FF, Dolenc-Groselj L. Prospective study of sleep-disordered breathing in 28 patients with acute unilateral lateral medullary infarction. Sleep Breath. 2020;24(4):1557–63.
Simonsen SA, Andersen AV, West AS, Wolfram F, Jennum P, Iversen HK. Sleep-disordered breathing and cerebral small vessel disease-acute and 6 months after ischemic stroke. Sleep Breath. 2022;26(3):1107–13.
Manconi M, Zavalko I, Cereda C, Pisarenco I, Ott S, Fulda S, et al. Longitudinal polysomnographic assessment from acute to subacute phase in infratentorial versus supratentorial stroke. Cerebrovasc Dis. 2014;37(2):85–93.
Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(3):479–504.
Schutz SG, Lisabeth LD, Hsu CW, Kim S, Chervin RD, Brown DL. Central sleep apnea is uncommon after stroke. Sleep Med. 2021;77:304–6.
Riglietti A, Fanfulla F, Pagani M, Lucini D, Malacarne M, Manconi M, et al. Obstructive and central sleep apnea in first ever ischemic stroke are associated with different time course and autonomic activation. Nat Sci Sleep. 2021;13:1167–78.
Baillieul S, Bailly S, Detante O, Alexandre S, Destors M, Clin R et al. Sleep-disordered breathing and ventilatory chemosensitivity in first ischaemic stroke patients: a prospective cohort study. Thorax. 2022;77:1006–14. A large cohort of study describing the peculiar ventilatory chemosensitivity characteristics of patients with acute stroke.
Fisse AL, Kemmling A, Teuber A, Wersching H, Young P, Dittrich R, et al. The association of lesion location and sleep related breathing disorder in patients with acute ischemic stroke. PLoS ONE. 2017;12(1):e0171243.
Estai M, Walsh J, Maddison K, Shepherd K, Hillman D, McArdle N, et al. Sleep-disordered breathing in patients with stroke-induced dysphagia. J Sleep Res. 2021;30(3):e13179.
Losurdo A, Brunetti V, Broccolini A, Caliandro P, Frisullo G, Morosetti R, et al. Dysphagia and obstructive sleep apnea in acute, first-ever, ischemic stroke. J Stroke Cerebrovasc Dis. 2018;27(3):539–46.
Alexiev F, Brill AK, Ott SR, Duss S, Schmidt M, Bassetti CL. Sleep-disordered breathing and stroke: chicken or egg? J Thorac Dis. 2018;10(Suppl 34):S4244–52.
Brown DL, Yadollahi A, He K, Xu Y, Piper B, Case E, et al. Overnight rostral fluid shifts exacerbate obstructive sleep apnea after stroke. Stroke. 2021;52(10):3176–83.
Brown DL, Lisabeth LD, Zupancic MJ, Concannon M, Martin C, Chervin RD. High prevalence of supine sleep in ischemic stroke patients. Stroke. 2008;39(9):2511–4.
Sacchetti ML, Della MG. Are stroke cases affected by sleep disordered breathings all the same? Med Hypotheses. 2014;83(2):217–23.
Leino A, Westeren-Punnonen S, Toyras J, Myllymaa S, Leppanen T, Yla-Herttuala S, et al. Acute stroke and TIA patients have specific polygraphic features of obstructive sleep apnea. Sleep Breath. 2020;24(4):1495–505.
Schutz SG, Lisabeth LD, Shafie-Khorassani F, Case E, Sanchez BN, Chervin RD, et al. Clinical phenotypes of obstructive sleep apnea after ischemic stroke: a cluster analysis. Sleep Med. 2019;60:178–81.
Chen CY, Chen CL. Recognizable clinical subtypes of obstructive sleep apnea after ischemic stroke: a cluster analysis. Nat Sci Sleep. 2021;13:283–90.
Yoon CW, Park HK, Bae EK, Rha JH. Sleep apnea and early neurological deterioration in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2020;29(2):104510.
Cananzi SG, White LA, Barzegar M, Boyer CJ, Chernyshev OY, Yun JW, et al. Obstructive sleep apnea intensifies stroke severity following middle cerebral artery occlusion. Sleep Med. 2020;67:278–85.
Huhtakangas JK, Saaresranta T, Vahanikkila H, Huhtakangas J. Nocturnal hypoxemia and central apneas increase mortality, but not recurrent ischemic events after ischemic stroke. Sleep Med. 2022;97:1–9.
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723–31.
Lapow JM, Dicpinigaitis AJ, Pammal RS, Coghill GA, Rechester O, Feldstein E, et al. Obstructive sleep apnea confers lower mortality risk in acute ischemic stroke patients treated with endovascular thrombectomy: National Inpatient Sample analysis 2010–2018. J Neurointerv Surg. 2021:neurintsurg-2021-018161. https://doi.org/10.1136/neurintsurg-2021-018161. The first study that evaluated the effect of sleep-disordered breathing on the outcome in terms of mortality in patients with acute ischemic stroke treated with mechanical thrombectomy.
Festic N, Alejos D, Bansal V, Mooney L, Fredrickson PA, Castillo PR, et al. Sleep apnea in patients hospitalized with acute ischemic stroke: underrecognition and associated clinical outcomes. J Clin Sleep Med. 2018;14(1):75–80.
Zhang L, Meng R, Shang S, Wu C, Wu D, Shang S, et al. Obstructive sleep apnea before ischemic stroke: clinical relevance to infarction volume and neurological recovery. J Stroke Cerebrovasc Dis. 2019;28(8):2132–9.
Brown DL, He K, Kim S, Hsu CW, Case E, Chervin RD, et al. Prediction of sleep-disordered breathing after stroke. Sleep Med. 2020;75:1–6.
Brown DL, Jiang X, Li C, Case E, Sozener CB, Chervin RD, et al. Sleep apnea screening is uncommon after stroke. Sleep Med. 2019;59:90–3. A study that showed as just a minority of patients with ischemic stroke are screened for sleep apnea.
Boulos MI, Kamra M, Colelli DR, Kirolos N, Gladstone DJ, Boyle K, et al. SLEAP SMART (sleep apnea screening using mobile ambulatory recorders after TIA/stroke): a randomized controlled trial. Stroke. 2022;53(3):710–8. A randomized control study evaluating the efficacy, feasibility and cost of home sleep apnea test compared to the polysomnography.
Huhtakangas JK, Huhtakangas J, Bloigu R, Saaresranta T. Unattended sleep study in screening for sleep apnea in the acute phase of ischemic stroke. Sleep Med. 2020;65:121–6.
Bernardini A, Brunello A, Gigli GL, Montanari A, Saccomanno N. AIOSA: An approach to the automatic identification of obstructive sleep apnea events based on deep learning. Artif Intell Med. 2021;118:102133. A study that applied artificial intelligence techniques in order to automatically identify obstructive sleep apnea relying on routinely recorded vital signs in stroke units.
Bernardini A, Brunello A, Gigli GL, Montanari A, Saccomanno N. OSASUD: A dataset of stroke unit recordings for the detection of obstructive sleep apnea syndrome. Sci Data. 2022;9(1):177.
Leino A, Nikkonen S, Kainulainen S, Korkalainen H, Toyras J, Myllymaa S, et al. Neural network analysis of nocturnal SpO2 signal enables easy screening of sleep apnea in patients with acute cerebrovascular disease. Sleep Med. 2021;79:71–8.
Siarnik P, Jurik M, Klobucnikova K, Kollar B, Pirosova M, Malik M, et al. Sleep apnea prediction in acute ischemic stroke (SLAPS score): a derivation study. Sleep Med. 2021;77:23–8.
Camilo MR, Machado LA, Castilho CM, Sander HH, Eckeli AL, Fernandes RF, et al. Diagnostic accuracy of positive airway pressure device for sleep apnea detection in acute stroke patients. Stroke. 2020;51(1):324–6.
McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–31.
Sanchez-de-la-Torre M, Sanchez-de-la-Torre A, Bertran S, Abad J, Duran-Cantolla J, Cabriada V, et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8(4):359–67.
Lin HJ, Yeh JH, Hsieh MT, Hsu CY. Continuous positive airway pressure with good adherence can reduce risk of stroke in patients with moderate to severe obstructive sleep apnea: an updated systematic review and meta-analysis. Sleep Med Rev. 2020;54:101354. A meta-analysis demonstrating the efficacy of continuous positive airway pressure for stroke prevention in patients with moderate to severe obstructive sleep apnea and good adherence.
Wickwire EM, Bailey MD, Somers VK, Srivastava MC, Scharf SM, Johnson AM, et al. CPAP adherence is associated with reduced risk for stroke among older adult Medicare beneficiaries with obstructive sleep apnea. J Clin Sleep Med. 2021;17(6):1249–55.
Barlinn K, Jakubicek S, Siepmann T, Chernyshev OY, Pallesen LP, Wienecke M, et al. Autotitrating bilevel positive airway pressure in large vessel steno-occlusive stroke patients with suspected sleep apnea: a multicenter randomized controlled study. Front Neurol. 2021;12:667494.
Bernasconi C, Ott SR, Fanfulla F, Miano S, Horvath T, Seiler A, et al. SAS CARE 2 - a randomized study of CPAP in patients with obstructive sleep disordered breathing following ischemic stroke or transient ischemic attack. Sleep Med X. 2020;2:100027.
Haba-Rubio J, Vujica J, Franc Y, Michel P, Heinzer R. Effect of CPAP Treatment of sleep apnea on clinical prognosis after ischemic stroke: an observational study. J Clin Sleep Med. 2019;15(6):839–47.
Kim H, Im S, Park JI, Kim Y, Sohn MK, Jee S. Improvement of cognitive function after continuous positive airway pressure treatment for subacute stroke patients with obstructive sleep apnea: a randomized controlled trial. Brain Sci. 2019;9(10):252.
Boulos MI, Dharmakulaseelan L, Brown DL, Swartz RH. Trials in sleep apnea and stroke: learning from the past to direct future approaches. Stroke. 2021;52(1):366–72.
Khot SP, Barnett HM, Davis AP, Byun E, McCann BS, Bombardier CH, et al. Novel and modifiable factors associated with adherence to continuous positive airway pressure therapy initiated during stroke rehabilitation: an exploratory analysis of a prospective cohort study. Sleep Med. 2022;97:43–6.
Khot S, Barnett H, Davis A, Siv J, Crane D, Kunze A, et al. Intensive continuous positive airway pressure adherence program during stroke rehabilitation. Stroke. 2019;50(7):1895–7.
Kotzian ST, Saletu MT, Schwarzinger A, Haider S, Spatt J, Kranz G, et al. Proactive telemedicine monitoring of sleep apnea treatment improves adherence in people with stroke- a randomized controlled trial (HOPES study). Sleep Med. 2019;64:48–55. A randomized controlled trial that demonstrated that telemedicine ameliorates the adherence of apnea treatment in post-stroke patients.
Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;144(3):e56-e67. A statement of American Heart Association on management of obstructive sleep apnea in patients with cardiovascular diseases.
Duss SB, Brill AK, Baillieul S, Horvath T, Zubler F, Flugel D, et al. Effect of early sleep apnoea treatment with adaptive servo-ventilation in acute stroke patients on cerebral lesion evolution and neurological outcomes: study protocol for a multicentre, randomized controlled, rater-blinded, clinical trial (eSATIS: early sleep apnoea treatment in stroke). Trials. 2021;22(1):83.
Brown DL, Durkalski V, Durmer JS, Broderick JP, Zahuranec DB, Levine DA, et al. Sleep for stroke management and recovery trial (Sleep SMART): rationale and methods. Int J Stroke. 2020;15(8):923–9.
Chen CY, Chen CL, Yu CC. Trazodone improves obstructive sleep apnea after ischemic stroke: a randomized, double-blind, placebo-controlled, crossover pilot study. J Neurol. 2021;268(8):2951–60.
Qian S, Zhang X, Wang T, Zhang L, Hu C, Jia R, et al. Effects of comprehensive swallowing intervention on obstructive sleep apnea and dysphagia after stroke: a randomized controlled trial. J Stroke Cerebrovasc Dis. 2022;31(8):106521.
Svatikova A, Chervin RD, Wing JJ, Sanchez BN, Migda EM, Brown DL. Positional therapy in ischemic stroke patients with obstructive sleep apnea. Sleep Med. 2011;12(3):262–6.
Li W, Taskin T, Gautam P, Gamber M, Sun W. Is there an association among sleep duration, nap, and stroke? Findings from the China Health and Retirement Longitudinal Study. Sleep Breath. 2021;25(1):315–23.
Lu C, Liao B, Nie J, Wang W, Wang Y. The association between sleep duration and chronic diseases: a population-based cross-sectional study. Sleep Med. 2020;73:217–22.
Lu H, Wu PF, Li RZ, Zhang W, Huang GX. Sleep duration and stroke: a Mendelian randomization study. Front Neurol. 2020;11:976.
Li J, Zheng D, Loffler KA, Wang X, McEvoy RD, Woodman RJ, et al. Sleep duration and risk of cardiovascular events: the SAVE study. Int J Stroke. 2020;15(8):858–65.
Krittanawong C, Kumar A, Wang Z, Jneid H, Baber U, Mehran R, et al. Sleep duration and cardiovascular health in a representative community population (from NHANES, 2005 to 2016). Am J Cardiol. 2020;127:149–55.
Wang YH, Wang J, Chen SH, Li JQ, Lu QD, Vitiello MV, et al. Association of longitudinal patterns of habitual sleep duration with risk of cardiovascular events and all-cause mortality. JAMA Netw Open. 2020;3(5):e205246. A cohort study on a large population demonstrating that short sleep as well as unstable sleep pattern are associated with higher risk of cardiovascular events.
Ji A, Lou H, Lou P, Xu C, Zhang P, Qiao C, et al. Interactive effect of sleep duration and sleep quality on risk of stroke: an 8-year follow-up study in China. Sci Rep. 2020;10(1):8690.
Zhou L, Yu K, Yang L, Wang H, Xiao Y, Qiu G, et al. Sleep duration, midday napping, and sleep quality and incident stroke: the Dongfeng-Tongji cohort. Neurology. 2020;94(4):e345–56.
Guo Q, Xie W, Peng R, Ma Y, Chong F, Wang Y, et al. A dose-response relationship between sleep duration and stroke according to nonhealth status in Central China: a population-based epidemiology survey. J Stroke Cerebrovasc Dis. 2019;28(7):1841–52.
Li W, Wang D, Cao S, Yin X, Gong Y, Gan Y, et al. Sleep duration and risk of stroke events and stroke mortality: a systematic review and meta-analysis of prospective cohort studies. Int J Cardiol. 2016;223:870–6.
Titova OE, Yuan S, Baron JA, Lindberg E, Michaelsson K, Larsson SC. Sleep-disordered breathing-related symptoms and risk of stroke: cohort study and Mendelian randomization analysis. J Neurol. 2022;269(5):2460–8.
Kwok CS, Kontopantelis E, Kuligowski G, Gray M, Muhyaldeen A, Gale CP, et al. Self-reported sleep duration and quality and cardiovascular disease and mortality: a dose-response meta-analysis. J Am Heart Assoc. 2018;7(15):e008552.
Mutti C, Brunetti V, Figorilli M, Liguori C, Pizza F, Proserpio P, et al. Clinical characteristics of a large cohort of patients with narcolepsy candidate for pitolisant: a cross-sectional study from the Italian PASS Wakix(R) Cohort. Neurol Sci. 2022;43(9):5563–74.
Tobaldini E, Fiorelli EM, Solbiati M, Costantino G, Nobili L, Montano N. Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nat Rev Cardiol. 2019;16(4):213–24. A review describing the effects of short sleep duration on cardiovascular health and diseases.
King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300(24):2859–66.
Dominguez-Rodriguez A, Abreu-Gonzalez P, Garcia-Gonzalez M, Ferrer-Hita J, Vargas M, Reiter RJ. Elevated levels of oxidized low-density lipoprotein and impaired nocturnal synthesis of melatonin in patients with myocardial infarction. Atherosclerosis. 2005;180(1):101–5.
Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62.
Grandner MA, Sands-Lincoln MR, Pak VM, Garland SN. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2013;5:93–107.
Zafar A, Dhruv P, Anwar A, Grigg-Damberger MM. Stroke: what’s sleep got to do with it? J Clin Neurophysiol. 2022;39(5):335–45.
Chellappa SL, Vujovic N, Williams JS, Scheer F. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab. 2019;30(10):767–79.
Korostovtseva L. Ischemic stroke and sleep: the linking genetic factors. Cardiol Ther. 2021;10(2):349–75.
Corella D, Asensio EM, Coltell O, Sorli JV, Estruch R, Martinez-Gonzalez MA, et al. CLOCK gene variation is associated with incidence of type-2 diabetes and cardiovascular diseases in type-2 diabetic subjects: dietary modulation in the PREDIMED randomized trial. Cardiovasc Diabetol. 2016;15:4.
Correa-Costa M, Gallo D, Csizmadia E, Gomperts E, Lieberum JL, Hauser CJ, et al. Carbon monoxide protects the kidney through the central circadian clock and CD39. Proc Natl Acad Sci U S A. 2018;115(10):E2302–10.
Duss SB, Seiler A, Schmidt MH, Pace M, Adamantidis A, Muri RM, et al. The role of sleep in recovery following ischemic stroke: a review of human and animal data. Neurobiol Sleep Circadian Rhythms. 2017;2:94–105.
Baglioni C, Nissen C, Schweinoch A, Riemann D, Spiegelhalder K, Berger M, et al. Polysomnographic characteristics of sleep in stroke: a systematic review and meta-analysis. PLoS ONE. 2016;11(3):e0148496.
Nakamizo T, Kanda T, Kudo Y, Sugawara E, Hashimoto E, Okazaki A, et al. Effects of uncomfortable care and histamine H2-antagonists on delirium in acute stroke: a propensity score analysis. J Neurol Sci. 2021;420:117251.
Rollo E, Brunetti V, Scala I, Callea A, Marotta J, Vollono C, et al. Impact of delirium on the outcome of stroke: a prospective, observational, cohort study. J Neurol. 2022. https://doi.org/10.1007/s00415-022-11309-2.
Palomaki H, Berg A, Meririnne E, Kaste M, Lonnqvist R, Lehtihalmes M, et al. Complaints of poststroke insomnia and its treatment with mianserin. Cerebrovasc Dis. 2003;15(1–2):56–62.
Li LJ, Yang Y, Guan BY, Chen Q, Wang AX, Wang YJ, et al. Insomnia is associated with increased mortality in patients with first-ever stroke: a 6-year follow-up in a Chinese cohort study. Stroke Vasc Neurol. 2018;3(4):197–202.
Glozier N, Moullaali TJ, Sivertsen B, Kim D, Mead G, Jan S, et al. The course and impact of poststroke insomnia in stroke survivors aged 18 to 65 years: results from the psychosocial outcomes in stroke (POISE) study. Cerebrovasc Dis Extra. 2017;7(1):9–20.
Fleming MK, Smejka T, Henderson Slater D, Chiu EG, Demeyere N, Johansen-Berg H. Self-reported and objective sleep measures in stroke survivors with incomplete motor recovery at the chronic stage. Neurorehabil Neural Repair. 2021;35(10):851–60.
Baylan S, Griffiths S, Grant N, Broomfield NM, Evans JJ, Gardani M. Incidence and prevalence of post-stroke insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2020;49:101222. A systematic review and meta-analysis on insomnia characteristics and changes in insomnia prevalence over time in post-stroke patients.
Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–68.
Zhang S, Cheng S, Zhang Z, Wang C, Wang A, Zhu W. Related risk factors associated with post-stroke fatigue: a systematic review and meta-analysis. Neurol Sci. 2021;42(4):1463–71.
Xiao M, Huang G, Feng L, Luan X, Wang Q, Ren W, et al. Impact of sleep quality on post-stroke anxiety in stroke patients. Brain Behav. 2020;10(12):e01716.
Dong L, Williams LS, Brown DL, Case E, Morgenstern LB, Lisabeth LD. Prevalence and course of depression during the first year after mild to moderate stroke. J Am Heart Assoc. 2021;10(13):e020494.
Falck RS, Best JR, Davis JC, Eng JJ, Middleton LE, Hall PA, et al. Sleep and cognitive function in chronic stroke: a comparative cross-sectional study. Sleep. 2019;42(5):zsz040. https://doi.org/10.1093/sleep/zsz040.
Harris AL, Elder J, Schiff ND, Victor JD, Goldfine AM. Post-stroke apathy and hypersomnia lead to worse outcomes from acute rehabilitation. Transl Stroke Res. 2014;5(2):292–300.
Jaramillo V, Jendoubi J, Maric A, Mensen A, Heyse NC, Eberhard-Moscicka AK, et al. Thalamic influence on slow wave slope renormalization during sleep. Ann Neurol. 2021;90(5):821–33. A study evaluating by means of high density electroencephalography and polysomnography patients with thalamic stroke showing a role of thalamus in the synpatic homeostasis.
Bivard A, Lillicrap T, Krishnamurthy V, Holliday E, Attia J, Pagram H, et al. MIDAS (modafinil in debilitating fatigue after stroke): a randomized, double-blind, placebo-controlled, cross-over trial. Stroke. 2017;48(5):1293–8.
Lundstrom E, Isaksson E, Greilert Norin N, Nasman P, Wester P, Martensson B, et al. Effects of fluoxetine on outcomes at 12 months after acute stroke: results from EFFECTS, a randomized controlled trial. Stroke. 2021;52(10):3082–7.
Collaboration FT. Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double-blind, randomised, controlled trial. Lancet. 2019;393(10168):265–74.
Kalbouneh HM, Toubasi AA, Albustanji FH, Obaid YY, Al-Harasis LM. Safety and efficacy of SSRIs in improving poststroke recovery: a systematic review and meta-analysis. J Am Heart Assoc. 2022;11(13):e025868. A meta-analysis of studies evaluated the role of SSRIs in post-stroke syndrome.
Herron K, Farquharson L, Wroe A, Sterr A. Development and evaluation of a cognitive behavioural intervention for chronic post-stroke insomnia. Behav Cogn Psychother. 2018;46(6):641–60.
Yang J. Acupuncture treatment for post-stroke insomnia: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2021;44:101396.
Kim WH, Joa KL, Kim CB, Lee HS, Kang SG, Jung HY, et al. The effect of bright light therapy on sleep and quality of life in patients with poststroke insomnia. Psychosom Med. 2022;84(1):123–30.
Xiao M, Feng L, Wang Q, Luan X, Chen S, He J. The therapeutic effects and safety of bright light therapy combined with escitalopram oxalate on insomnia in patients with poststroke depression. Int J Geriatr Psychiatry. 2021;36(1):182–9.
Hao SM, Zhong ZG, Qu WM, Huang ZL, Sun FY, Qiu MH. Melatonin supplementation in the subacute phase after ischemia alleviates postischemic sleep disturbances in rats. Brain Behav. 2021;11(10):e2366.
Kamat PK, Khan MB, Wood K, Siddiqui S, Rudic DR, Dhandapani K, et al. Preclinical evaluation of circadian rhythm in ischemic stroke outcomes. Cond Med. 2021;4(6):280–4.
Ryu WS, Hong KS, Jeong SW, Park JE, Kim BJ, Kim JT, et al. Association of ischemic stroke onset time with presenting severity, acute progression, and long-term outcome: a cohort study. PLoS Med. 2022;19(2):e1003910.
Katsanos AH, Kosmidou M, Konitsiotis S, Tsivgoulis G, Fiolaki A, Kyritsis AP, et al. Restless legs syndrome and cerebrovascular/cardiovascular events: systematic review and meta-analysis. Acta Neurol Scand. 2018;137(1):142–8.
Lin TC, Zeng BY, Chen YW, Wu MN, Chen TY, Lin PY, et al. Cerebrovascular accident risk in a population with periodic limb movements of sleep: a preliminary meta-analysis. Cerebrovasc Dis. 2018;46(1–2):1–9.
Terzaghi M, Pilati L, Ghiotto N, Arnaldi D, Versino M, Rustioni V, et al. Twenty-four-hour blood pressure profile in idiopathic REM sleep behavior disorder. Sleep. 2022;45(2):zsab239. https://doi.org/10.1093/sleep/zsab239.
Wang XX, Feng Y, Tan EK, Ondo WG, Wu YC. Stroke-related restless legs syndrome: epidemiology, clinical characteristics, and pathophysiology. Sleep Med. 2022;90:238–48. A review on epidemiology, clinical characteristics, pathophysiology, and impact on functional outcome of stroke-related RLS.
Wu X, Xu J, Lu B. Acute post-stroke restless legs syndrome: the body of caudate nucleus considerations. Sleep Med. 2020;70:66–70.
Gupta A, Shukla G, Sharma G, Roy A, Afsar M, Bhargava B. Restless legs syndrome/Willis-Ekbom disease among patients with resistant hypertension versus stroke patients-a prospective study. Sleep Breath. 2022;26(3):1245–51. https://doi.org/10.1007/s11325-021-02490-1.
Ruppert E, Hacquard A, Tatu L, Namer IJ, Wolff V, Kremer S, et al. Stroke-related restless legs syndrome: clinical and anatomo-functional characterization of an emerging entity. Eur J Neurol. 2022;29(4):1011–6.
Medeiros CA, de Bruin PF, Paiva TR, Coutinho WM, Ponte RP, de Bruin VM. Clinical outcome after acute ischaemic stroke: the influence of restless legs syndrome. Eur J Neurol. 2011;18(1):144–9.
Boulos MI, Wan A, Black SE, Lim AS, Swartz RH, Murray BJ. Restless legs syndrome after high-risk TIA and minor stroke: association with reduced quality of life. Sleep Med. 2017;37:135–40.
Manconi M, Fanfulla F, Ferri R, Miano S, Haba-Rubio J, Heinzer R, et al. Periodic limb movements during sleep in stroke/TIA: prevalence, course, and cardiovascular burden. Neurology. 2018;90(19):e1663–72.
Tang WK, Hermann DM, Chen YK, Liang HJ, Liu XX, Chu WC, et al. Brainstem infarcts predict REM sleep behavior disorder in acute ischemic stroke. BMC Neurol. 2014;14:88.
Tellenbach N, Schmidt MH, Alexiev F, Blondiaux E, Cavalloni F, Bassetti CL, et al. REM sleep and muscle atonia in brainstem stroke: a quantitative polysomnographic and lesion analysis study. J Sleep Res. 2022:e13640. https://doi.org/10.1111/jsr.13640.
Funding
Open access funding provided by Università Cattolica del Sacro Cuore within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization: Valerio Brunetti.
Writing—original draft preparation: Valerio Brunetti and Eleonora Rollo.
Writing—review and editing: Giacomo Della Marca.
Literature search: Valerio Brunetti and Eleonora Rollo.
Data analysis: Valerio Brunetti, Eleonora Rollo, Aldobrando Broccolini, Giovanni Frisullo, Irene Scala, and Giacomo Della Marca.
Critical revision for important intellectual content: Aldobrando Broccolini, Giovanni Frisullo, Irene Scala, and Giacomo Della Marca.
Study supervision: Valerio Brunetti.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interests/competing interests/funding to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stroke
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Brunetti, V., Rollo, E., Broccolini, A. et al. Sleep and Stroke: Opening Our Eyes to Current Knowledge of a Key Relationship. Curr Neurol Neurosci Rep 22, 767–779 (2022). https://doi.org/10.1007/s11910-022-01234-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-022-01234-2