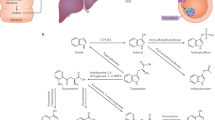
Abstract
Purpose of Review
We reviewed reasons for the high cardiovascular risk (CVD) of patients with chronic kidney disease (CKD), and explored alternatives to treatment of traditional risk factors to reduce CVD in CKD.
Recent Findings
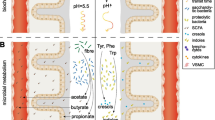
Besides traditional risk factors, patients with CKD are exposed to uremic toxins of two kinds: systemically derived toxins include asymmetric dimethylarginine (ADMA), total homocysteine (tHcy), thiocyanate, tumor necrosis factor alpha, and interleukin 6. Gut-derived uremic toxins (GDUT), products of the intestinal microbiome, include hippuric acid, indoxyl sulfate, p-cresyl sulfate, p-cresyl glucuronide, phenylacetylglutamine, and trimethylamine N-oxide (TMAO). Cyanocobalamin is toxic in patients with CKD. Approaches to reducing plasma levels of these uremic toxins would include diet to reduce GDUT, kidney transplantation, more intensive dialysis, and vitamin therapy to lower tHcy with methylcobalamin rather than cyanocobalamin.
Summary
The high CVD risk in CKD requires consideration of therapies beyond treatment of traditional risk factors.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wheeler DC. Cardiovascular disease in patients with chronic renal failure. Lancet. 1996;348(9043):1673–4.
Sozio SM, Armstrong PA, Coresh J, Jaar BG, Fink NE, Plantinga LC, et al. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: the choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis. 2009;54(3):468–77. https://doi.org/10.1053/j.ajkd.2009.01.261.
•• Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52. https://doi.org/10.1016/S0140-6736(13)60595-4. An important paper that quantifies cardiovascular risk by severity of CKD.
Bowman B, Abdel-Rahman EM. Cardiovascular outcomes in dialysis patients: one size does not fit all. Eur Heart J. 2019;40(11):899–901. https://doi.org/10.1093/eurheartj/ehy544.
• Ku E, McCulloch CE, Ahearn P, Grimes BA, Mitsnefes MM. Trends in cardiovascular mortality among a cohort of children and young adults starting dialysis in 1995 to 2015. JAMA Netw Open. 2020;3(9):e2016197. https://doi.org/10.1001/jamanetworkopen.2020.16197. Secular trends of CVD mortality in dialysis patients.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
• Ghoshal S, Freedman BI. Mechanisms of stroke in patients with chronic kidney disease. Am J Nephrol. 2019;50(4):229–39. https://doi.org/10.1159/000502446. A review of mechanisms of stroke in CKD.
Albakr RB, Bargman JM. A comparison of hemodialysis and peritoneal dialysis in patients with cardiovascular disease. Cardiol Clin. 2021;39(3):447–53. https://doi.org/10.1016/j.ccl.2021.04.013.
Loutradis C, Papadopoulos CE, Sarafidis P. Longer dialysis sessions improve cardiac systolic function by reducing myocardial stunning. J Card Fail. 2020;26(11):1026–7. https://doi.org/10.1016/j.cardfail.2020.06.001.
•• Sillesen H, Muntendam P, Adourian A, Entrekin R, Garcia M, Falk E et al. Carotid plaque burden as a measure of subclinical atherosclerosis: comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage Study. JACC Cardiovasc Imaging. 2012;5(7):681–9. S1936–878X(12)00397-X; https://doi.org/10.1016/j.jcmg.2012.03.013. An important study reporting that measurement of carotid plaque burden correlates highly with coronary calcium whereas IMT does not.
•• Baber U, Mehran R, Sartori S, Schoos MM, Sillesen H, Muntendam P et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015;65(11):1065–74. https://doi.org/10.1016/j.jacc.2015.01.017. An important paper reporting that carotid plaque burden is as predictive of CVD as coronary calcium whereas IMT is not.
•• Spence JD, Eliasziw M, DiCicco M, Hackam DG, Galil R, Lohmann T. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke. 2002;33(12):2916–22. The original description of the high CVD risk associated with high carotid plaque burden.
•• Spence JD, Hackam DG. Treating arteries instead of risk factors. a paradigm change in management of atherosclerosis. Stroke. 2010;41(6):1193–9. STROKEAHA.110.577973; https://doi.org/10.1161/STROKEAHA.110.577973. The original description of a process called “Treating Arteries” instead of merely treating risk factors to consensus targets.
•• Spence JD, Coates V, Li H, Tamayo A, Munoz C, Hackam DG et al. Effects of Intensive medical therapy on microemboli and cardiovascular risk in asymptomatic carotid stenosis. Arch Neurol. 2010;67(2):180–6. The original description of marked reduction of CVD risk by “Treating arteries.”
•• Spence JD, Solo K. Resistant Atherosclerosis: The Need for Monitoring of Plaque Burden. Stroke. 2017;48(6):1624–9. https://doi.org/10.1161/STROKEAHA.117.017392. The original description of “Resistant Atherosclerosis.”
Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–48. https://doi.org/10.1056/NEJMoa043545.
Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–407. https://doi.org/10.1056/NEJMoa0810177.
•• Lim YJ, Sidor NA, Tonial NC, Che A, Urquhart BL. Uremic toxins in the progression of chronic kidney disease and cardiovascular disease: mechanisms and therapeutic targets. Toxins (Basel). 2021;13(2). https://doi.org/10.3390/toxins13020142. A review of uremic toxins, mechanisms, and therapeutic targets.
• Rader DJ, Ischiropoulos H. ‘Multipurpose oxidase’ in atherogenesis. Nat Med. 2007;13(10):1146–7. An important review of thiocyanate and oxidation in atherosclerosis.
• Nygård O, Nordehaug JE, Refsum H, Ueland PM, Farstad M, Vollset SE. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337:230–6. Showed strong graded CVD risk with plasma total homocysteine.
•• Bogiatzi C, Gloor G, Allen-Vercoe E, Reid G, Wong RG, Urquhart BL et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis. 2018;273:91–7. https://doi.org/10.1016/j.atherosclerosis.2018.04.015. Plasma levels of the toxic metabolites of the intestinal microbiome are higher in patients with severe atherosclerosis despite low levels of traditional risk factors.
Poesen R, Claes K, Evenepoel P, de Loor H, Augustijns P, Kuypers D, et al. Microbiota-derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J Am Soc Nephrol. 2016;27(11):3479–87. https://doi.org/10.1681/ASN.2015121302.
• Nemet I, Saha PP, Gupta N, Zhu W, Romano KA, Skye SM et al. A cardiovascular disease-linked gut microbial metabolite acts via adrenergic receptors. Cell. 2020;180(5):862–77 e22. https://doi.org/10.1016/j.cell.2020.02.016. Phenylacetylglutamine affects platelet function by an adrenergic mechanism.
•• Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. Plasma TMAO increased 2.5-fold the 3-year CVD risk in patients referred for coronary angiography.
Senthong V, Wang Z, Fan Y, Wu Y, Hazen SL, Tang WH. Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. J Am Heart Assoc. 2016;5(10). https://doi.org/10.1161/JAHA.116.004237.
• Pignanelli M, Bogiatzi C, Gloor G, Allen-Vercoe E, Reid G, Urquhart BL et al. Moderate renal impairment and toxic metabolites produced by the intestinal microbiome: dietary implications. J Ren Nutr. 2019;29(1):55–64. https://doi.org/10.1053/j.jrn.2018.05.007. Even moderate renal impairment significantly increases plasma levels of the toxic metabolites of the intestinal microbiome.
•• Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55. CIRCRESAHA.116.305360 [pii]; https://doi.org/10.1161/CIRCRESAHA.116.305360. TMAO accelerates decline of renal function and increases mortality in CKD.
Spence JD, Urquhart BL, Bang H. Effect of renal impairment on atherosclerosis: only partially mediated by homocysteine. Nephrol Dial Transplant. 2016;31(6):937–44. https://doi.org/10.1093/ndt/gfv380.
Spence JD, Urquhart BL, Bang H. Effect of renal impairment on atherosclerosis: only partially mediated by homocysteine. Nephrol Dial Transplant. 2015. https://doi.org/10.1093/ndt/gfv380.
•• Koyama K, Yoshida A, Takeda A, Morozumi K, Fujinami T, Tanaka N. Abnormal cyanide metabolism in uraemic patients. Nephrol Dial Transplant. 1997;12(8):1622–8. Patients with severe CKD have high plasma cyanide/thiocyanate.
•• Koyama K, Ito A, Yamamoto J, Nishio T, Kajikuri J, Dohi Y et al. Randomized controlled trial of the effect of short-term coadministration of methylcobalamin and folate on serum ADMA concentration in patients receiving long-term hemodialysis. Am J Kidney Dis. 2010;55(6):1069–78. Methylcobalamin and folate significantly reduce tHcy and ADMA in dialysis patients.
Poesen R, Claes K, Evenepoel P, de Loor H, Augustijns P, Kuypers D, et al. Microbiota-derived phenylacetylglutamine associates with overall mortality and cardiovascular disease in patients with CKD. J Am Soc Nephrol. 2016. https://doi.org/10.1681/ASN.2015121302.
Poesen R, Evenepoel P, de Loor H, Kuypers D, Augustijns P, Meijers B. Metabolism, protein binding, and renal clearance of microbiota-derived p-cresol in patients with CKD. Clin J Am Soc Nephrol. 2016;11(7):1136–44. https://doi.org/10.2215/CJN.00160116.
Rossi M, Campbell K, Johnson D, Stanton T, Pascoe E, Hawley C, et al. Uraemic toxins and cardiovascular disease across the chronic kidney disease spectrum: an observational study. Nutr Metab Cardiovasc Dis. 2014;24(9):1035–42. https://doi.org/10.1016/j.numecd.2014.04.006.
Lin CJ, Pan CF, Chuang CK, Sun FJ, Wang DJ, Chen HH, et al. P-cresyl sulfate is a valuable predictor of clinical outcomes in pre-ESRD patients. Biomed Res Int. 2014;2014:526932. https://doi.org/10.1155/2014/526932.
Lin CJ, Wu V, Wu PC, Wu CJ. Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One. 2015;10(7):e0132589. https://doi.org/10.1371/journal.pone.0132589.
Lin CJ, Lin J, Pan CF, Chuang CK, Liu HL, Sun FJ, et al. Indoxyl sulfate, not P-cresyl sulfate, is associated with advanced glycation end products in patients on long-term hemodialysis. Kidney Blood Press Res. 2015;40(2):121–9. https://doi.org/10.1159/000368488.
Keys A. Mediterranean diet and public health: personal reflections. Am J Clin Nutr. 1995;61(6 Suppl):1321S-S1323.
•• Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–41. The Mediterranean diet is better for insulin resistance and diabetes than a low-fat or low-carbohydrate diet.
Spence JD. Reducing the risk of stroke in patients with impaired renal function: nutritional issues. J Stroke Cerebrovasc Dis. 2020;105376. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105376.
Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. nm.3145 https://doi.org/10.1038/nm.3145.
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. nature09922 https://doi.org/10.1038/nature09922.
•• Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40(7):583–94. https://doi.org/10.1093/eurheartj/ehy799. Switching from red meat to white meat or a meatless diet markedly reduces TMAO levels within a month.
• Spence JD, Srichaikul KK, Jenkins DJA. Cardiovascular harm from egg yolk and meat: more than just cholesterol and saturated fat. J Am Heart Assoc. 2021;10(7):e017066. https://doi.org/10.1161/JAHA.120.017066. Meat and egg yolk not only increase CVD risk because of cholesterol and saturated fat but also increase plasma levels of toxic intestinal metabolites.
Cailleux A, Subra JF, Riberi P, Tuchais E, Premel-Cabic A, Allain P. Cyanide and thiocyanate blood levels in patients with renal failure or respiratory disease. J Med. 1988;19(5–6):345–51.
Hasuike Y, Nakanishi T, Moriguchi R, Otaki Y, Nanami M, Hama Y, et al. Accumulation of cyanide and thiocyanate in haemodialysis patients. Nephrol Dial Transplant. 2004;19(6):1474–9.
•• House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, Fine A et al. Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA. 2010;303(16):1603–9. https://doi.org/10.1001/jama.2010.490. B vitamins containing 1000 mcg daily of cyanocobalamin doubled CVD risk in patients with diabetic nephropathy.
Spence JD, Eliasziw M, House AA. B-vitamin therapy for diabetic nephropathy: reply. JAMA. 2010;304(6):636–7.
•• Xu X, Qin X, Li Y, Sun D, Wang J, Liang M et al. Efficacy of folic acid therapy on the progression of chronic kidney disease: the renal substudy of the China Stroke Primary Prevention Trial. JAMA Intern Med. 2016;176(10):1443–50. https://doi.org/10.1001/jamainternmed.2016.4687. Folic acid significantly reduced the risk of stroke among >20,000 hypertensive patients over 4.5 years.
• Spence JD, Hankey GJ. Problem in the recent American Heart Association guideline on secondary stroke prevention: B vitamins to lower homocysteine do prevent stroke. Stroke. 2022:101161STROKEAHA122038640. https://doi.org/10.1161/STROKEAHA.122.038640. B vitamins do reduce the risk of stroke, but we should be using methylcobalamin, not cyanocobalamin.
EFSA Panel on Dietetic Products N, Allergies. Scientific opinion on dietary reference values for cobalamin (vitamin B12). EFSA Journal. 2015;13(7):4150. https://doi.org/10.2903/j.efsa.2015.4150.
Zhang Y-F, Ning G. Mecobalamin. Expert Opin Investig Drugs. 2008;17(6):953–64. https://doi.org/10.1517/13543784.17.6.953.
Herrington W, Haynes R, Staplin N, Emberson J, Baigent C, Landray M. Evidence for the prevention and treatment of stroke in dialysis patients. Semin Dial. 2015;28(1):35–47. https://doi.org/10.1111/sdi.12281.
Shih DM, Zhu W, Schugar RC, Meng Y, Jia X, Miikeda A, et al. Genetic deficiency of flavin-containing monooxygenase 3 ( Fmo3) protects against thrombosis but has only a minor effect on plasma lipid levels-brief report. Arterioscler Thromb Vasc Biol. 2019;39(6):1045–54. https://doi.org/10.1161/ATVBAHA.119.312592.
Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–95. https://doi.org/10.1016/j.cell.2015.11.055.
•• Pathak P, Helsley RN, Brown AL, Buffa JA, Choucair I, Nemet I et al. Small molecule inhibition of gut microbial choline trimethylamine lyase activity alters host cholesterol and bile acid metabolism. Am J Physiol Heart Circ Physiol. 2020;318(6):H1474-H86. https://doi.org/10.1152/ajpheart.00584.2019. An inhibitor of trimethylamine lyase reduces production of TMAO.
Kimber C, Zhang S, Johnson C, West RE, 3rd, Prokopienko AJ, Mahnken JD et al. Randomized, placebo-controlled trial of rifaximin therapy for lowering gut-derived cardiovascular toxins and inflammation in CKD. Kidney360. 2020;1(11):1206–16. https://doi.org/10.34067/kid.0003942020.
Din AU, Hassan A, Zhu Y, Yin T, Gregersen H, Wang G. Amelioration of TMAO through probiotics and its potential role in atherosclerosis. Appl Microbiol Biotechnol. 2019;103(23–24):9217–28. https://doi.org/10.1007/s00253-019-10142-4.
•• Petrof EO, Gloor GB, Vanner SJ, Weese SC, Carter D., Daigneaul MC, Brown EM et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013;1(3):1–12. An ecosystem therapeutic of cultured vitamins successfully treats Clostridium difficile infection.
Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27(1):305–13. https://doi.org/10.1681/ASN.2014111063.
Neirynck N, Glorieux G, Schepers E, Pletinck A, Dhondt A, Vanholder R. Review of protein-bound toxins, possibility for blood purification therapy. Blood Purif. 2013;35(Suppl 1):45–50. https://doi.org/10.1159/000346223.
Viaene L, Annaert P, de Loor H, Poesen R, Evenepoel P, Meijers B. Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm Drug Dispos. 2013;34(3):165–75. https://doi.org/10.1002/bdd.1834.
Itoh Y, Ezawa A, Kikuchi K, Tsuruta Y, Niwa T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal Bioanal Chem. 2012;403(7):1841–50. https://doi.org/10.1007/s00216-012-5929-3.
•• Nesrallah G, Suri R, Moist L, Kortas C, Lindsay RM. Volume control and blood pressure management in patients undergoing quotidian hemodialysis. Am J Kidney Dis. 2003;42(1 Suppl):13–7. https://doi.org/10.1016/s0272-6386(03)00532-8. Overnight daily hemodialysis nearly normalizes tHcy.
Du Q, Gao J, Lu R, Jin Y, Zou Y, Yu C, et al. Asymmetric dimethylarginine compartmental behavior during high-flux hemodialysis. Ren Fail. 2020;42(1):760–6. https://doi.org/10.1080/0886022X.2020.1797790.
Togawa T, Sengupta S, Chen H, Robinson K, Nonevski I, Majors AK, et al. Mechanisms for the formation of protein-bound homocysteine in human plasma. Biochem Biophys Res Commun. 2000;277(3):668–74. https://doi.org/10.1006/bbrc.2000.3723.
Urquhart BL, Freeman DJ, Spence JD, House AA. The effect of mesna on plasma total homocysteine concentration in hemodialysis patients. Am J Kidney Dis. 2007;49(1):109–17. https://doi.org/10.1053/j.ajkd.2006.10.002.
Pollay M, Stevens A, Davis C Jr. Determination of plasma-thiocyanate binding and the Donnan ratio under simulated physiological conditions. Anal Biochem. 1966;17(2):192–200. https://doi.org/10.1016/0003-2697(66)90197-7.
Hasuike Y, Nakanishi T, Moriguchi R, Otaki Y, Nanami M, Hama Y, et al. Accumulation of cyanide and thiocyanate in haemodialysis patients. Nephrol Dial Transplant. 2004;19(6):1474–9. https://doi.org/10.1093/ndt/gfh076.
Dreisbach AW, Hendrickson T, Beezhold D, Riesenberg LA, Sklar AH. Elevated levels of tumor necrosis factor alpha in postdialysis fatigue. Int J Artif Organs. 1998;21(2):83–6.
Devine E, Krieter DH, Ruth M, Jankovski J, Lemke HD. Binding affinity and capacity for the uremic toxin indoxyl sulfate. Toxins (Basel). 2014;6(2):416–29. https://doi.org/10.3390/toxins6020416.
Hyspler R, Ticha A, Safranek R, Moucka P, Nyvltova Z, Stochlova K, et al. Indoxyl sulfate elimination in renal replacement therapy: influence of citrate- versus acetate-buffering component during bicarbonate dialysis. Dis Markers. 2018;2018:3985861. https://doi.org/10.1155/2018/3985861.
Martinez AW, Recht NS, Hostetter TH, Meyer TW. Removal of P-cresol sulfate by hemodialysis. J Am Soc Nephrol. 2005;16(11):3430–6. https://doi.org/10.1681/ASN.2005030310.
Pelletier CC, Croyal M, Ene L, Aguesse A, Billon-Crossouard S, Krempf M et al. Elevation of trimethylamine-N-oxide in chronic kidney disease: contribution of decreased glomerular filtration rate. Toxins (Basel). 2019;11(11). https://doi.org/10.3390/toxins11110635.
Deltombe O, Van Biesen W, Glorieux G, Massy Z, Dhondt A, Eloot S. Exploring protein binding of uremic toxins in patients with different stages of chronic kidney disease and during hemodialysis. Toxins (Basel). 2015;7(10):3933–46. https://doi.org/10.3390/toxins7103933.
Lesaffer G, De Smet R, Lameire N, Dhondt A, Duym P, Vanholder R. Intradialytic removal of protein-bound uraemic toxins: role of solute characteristics and of dialyser membrane. Nephrol Dial Transplant. 2000;15(1):50–7. https://doi.org/10.1093/ndt/15.1.50.
Sirich TL, Fong K, Larive B, Beck GJ, Chertow GM, Levin NW, et al. Limited reduction in uremic solute concentrations with increased dialysis frequency and time in the Frequent Hemodialysis Network Daily Trial. Kidney Int. 2017;91(5):1186–92. https://doi.org/10.1016/j.kint.2016.11.002.
Velenosi TJ, Thomson BKA, Tonial NC, RaoPeters AAE, Mio MA, Lajoie GA, et al. Untargeted metabolomics reveals N, N, N-trimethyl-L-alanyl-L-proline betaine (TMAP) as a novel biomarker of kidney function. Sci Rep. 2019;9(1):6831. https://doi.org/10.1038/s41598-019-42992-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Neither author has a conflict of interest relating to this topic.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stroke
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spence, J.D., Urquhart, B.L. Cerebrovascular Disease, Cardiovascular Disease, and Chronic Kidney Disease: Interplays and Influences. Curr Neurol Neurosci Rep 22, 757–766 (2022). https://doi.org/10.1007/s11910-022-01230-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-022-01230-6