Abstract
Purpose of Review
To discuss the neurological complications and pathophysiology of organ damage following malaria infection.
Recent Findings
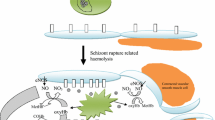
The principal advancement made in malaria research has been a better understanding of the pathogenesis of cerebral malaria (CM), the most dreaded neurological complication generally caused by Plasmodium falciparum infection. However, no definitive treatment has yet been evolved other than the use of antimalarial drugs and supportive care. The development of severe cerebral edema in CM results from two distinct pathophysiologic mechanisms. First, the development of “sticky” red blood cells (RBCs) leads to cytoadherence, where red blood cells (RBCs) get stuck to the endothelial walls and between themselves, resulting in clogging of the brain microvasculature with resultant hypoxemia and cerebral edema. In addition, the P. falciparum-infected erythrocyte membrane protein 1 (PfEMP1) molecules protrude from the raised knob structures on the RBCs walls and are in themselves made of a combination of human and parasite proteins in a tight complex. Antibodies to surfins, rifins, and stevors from the parasite are also located in the RBC membrane. On the human microvascular side, a range of molecules involved in host–parasite interactions, including CD36 and intracellular adhesion molecule 1, is activated during interaction with other molecules such as endothelial protein C receptor and thrombospondin. As a result, an inflammatory response occurs with the dysregulated release of cytokines (TNF, interleukins 1 and 10) which damage the blood–brain barrier (BBB), causing plasma leakage and brain edema. This second mechanism of CNS injury often involves multiple organs in adult patients in endemic areas but remains localized only to the central nervous system (CNS) among African children.
Summary
Neurological sequelae may follow both P. falciparum and P. vivax infections. The major brain pathology of CM is brain edema with diffuse brain swelling resulting from the combined effects of reduced perfusion and hypoxemia of cerebral neurons due to blockage of the microvasculature by parasitized RBCs as well as the neurotoxic effect of released cytokines from a hyper-acute immune host reaction. A plethora of additional neurological manifestations have been associated with malaria, including posterior reversible encephalopathy syndrome (PRES), reversible cerebral vasoconstriction syndrome (RCVS), malarial retinopathy, post-malarial neurological syndrome (PMNS), acute disseminated encephalomyelitis (ADEM), Guillain-Barré syndrome (GBS), and cerebellar ataxia. Lastly, the impact of the COVID-19 pandemic on worldwide malaria control programs and the possible threat from co-infections is briefly discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria is an infectious disease caused by parasites of the genus Plasmodium. The disease is transmitted to humans by the bite of an infected female Anopheles mosquito. Five species of Plasmodium exist, namely P. vivax, P. falciparum, P. ovale, P. malariae, and P. knowlesi which can infect humans. Plasmodium falciparum and Plasmodium vivax are the main species responsible for most infections in humans, with P. vivax being more prevalent in Southeast Asian countries and India [1,2,3, and]. Persons living in or traveling to areas of high endemicity (Central America, South America, sub-Saharan Africa, Indian subcontinent, Southeast Asia, Middle East, and Oceania) are at risk of contracting malaria. The global burden of malaria is on the rise of late. In 2017, an estimated 219 million people were affected by malaria, resulting in 435,000 deaths in the same year across the world. Globally, the overall mortality from malaria ranges from 0.3 to 2.2%, while the mortality increases to 11–30% in cases of the severe forms of the disease in tropical countries [4, 5].
Life Cycle of Malarial Parasite
The malarial parasite passes its life cycle in two different hosts. In humans, the parasite residing inside liver cells (extra-erythrocytic) and RBCs (intra-erythrocytic) reproduces asexually (schizogony). Hence, humans represent the intermediate host. In the female anopheles mosquito, for the initiation of the mosquito cycle, sexual forms (male and female gametocytes) are first developed inside the human host. These are then transferred to the insect host, where they develop further and are transformed into sporozoites [6•]. These sporozoites are infective to humans. In view of this sexual form of reproduction, the mosquitoes represent the definitive hosts of the malarial parasite. Plasmodium multiplies in the liver prior to invading erythrocytes initiating the symptomatic blood phase of the malaria infection. The sporozoites multiplying in the liver generate thousands of merozoites capable of erythrocyte invasion. The merozoites are then released from infected hepatocytes as merosomes, packets of hundreds of parasites surrounded by the host cell membrane. The merosomes survive the subsequent passage through the right heart undamaged and accumulate in the lungs. The merosomes eventually disintegrate inside pulmonary capillaries, thus liberating merozoites into the bloodstream. Merosome packaging protects hepatic merozoites from phagocytic attack by the sinusoidal Kupffer cells in the liver, and their release into the lung microvasculature enhances the chance of successful RBC invasion [6, 7]. The essential difference between the hepatic and erythrocytic phases of the development of the Plasmodium is that pigment granules (derived from hemoglobin) are not seen in the former, but are present in the latter. All Plasmodium species-infecting humans follow a similar life cycle pattern – the vector cycle and the human cycle, the latter having an intra-erythrocytic stage and an extra-erythrocytic stage in the liver. Some differences exist between P. falciparum and P. malariae infections. These parasites have a single liver schizont/sporozoite rupture even shortly after the sporozoite invasion. Conversely, P. vivax and P. ovale may “re-emerge” as quiescent forms of the schizonts/sporozoites in the liver (known as hypnozoites) may stay back for months to years in the liver from a single sporozoite exposure and continue to release merozoites into the circulation, causing recurrences of malarial symptoms [7]. Such relapses do not occur in P. falciparum and P. malariae infections for the reasons mentioned above.
Neurological Complications
The Plasmodium falciparum parasite is responsible for most of the neurological complications associated with malaria, but P. vivax-associated seizures in children and neurological complications like Guillain-Barré Syndrome (GBS) and cerebellar ataxia have also been reported in both adults and children [8, 9].
Nervous system involvement in malaria can manifest commonly as cerebral malaria or as late manifestations comprising of the post-malaria neurological syndromes (PMNS) after recovery from malaria, most commonly with complicated falciparum malaria [10••]. Other neurological manifestations include psychiatric manifestations, seizures, myelopathies, peripheral neuropathies, myopathies extrapyramidal syndromes, intracranial hemorrhage, and less commonly reversible cerebral vasoconstriction syndrome (RCVS) and posterior reversible encephalopathy syndrome (PRES). Neurological manifestations may also result as side effects of antimalarial medications.
Cerebral Malaria
A myriad of neurological complications may complicate P. falciparum infection, with CM causing the maximum number of deaths [1, 10,11,12]. Additionally, those patients who survive are often left with life-long sequelae, mostly neurological deficits, affecting activities of daily living and quality of life [13]. Clinical features of severe malaria due to P. falciparum infection with cerebral involvement vary between children and adults and also from region to region. Whereas mortality from pediatric CM is reportedly lower than mortality from CM in adults, pediatric CM is associated with a higher rate of seizures and post-CM neuro-cognitive deficits [14, 15]. The World Health Organization (WHO) has proposed the following definition of CM as “a clinical syndrome characterized by coma (inability to localize a painful stimulus) at least 1 h after termination of a seizure or correction of hypoglycemia, detection of asexual forms of P falciparum malaria parasites on peripheral blood smears, and exclusion of other causes of encephalopathy.” [16••]
Pathophysiology of Cerebral Malaria
CM is a multi-factorial syndrome characterized by a potentially reversible encephalopathy, which unfortunately still carries a mortality of 15–20% in spite of adequate antimalarial therapy and intensive care support, mostly due to the occurrence of multi-organ failure. The exact pathogenesis of fatal CM is still not very clear, although several probable pathogenetic mechanisms have been proposed. These include (a) reduction in tissue perfusion due to clogging of the microvasculature by parasite bearing RBCs, (b) excessive release of potentially harmful pro-inflammatory cytokines by hyperactivated host immune cells, and (c) associated coagulopathy induced as a result of the immune reaction. Two possible central mechanisms in the pathogenesis of CM have been proposed: vascular occlusion and inflammatory hypotheses [17, 18].
The unique characteristic of malaria caused by P. falciparum compared with the other species of malarial parasites is the sequestration of infected erythrocytes in the venules of various organs, particularly the brain. This feature is thought to cause many of the complications of malaria, especially the neurological features. Parasite-infected RBCs tend to clog the microvasculature by binding erythrocyte membrane protein 1 (PfEMP1) of the parasitized erythrocyte surface to surface proteins of the endothelial cells, namely intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM-1), and endothelial protein C receptor (EPCR). Microvascular clogging during P. falciparum infection may be made worse by the formation of rosettes and clumps resulting from the binding of non-infected RBCs by infected RBCs and aggregation of infected RBCs and platelets [17,18,19].
While the levels of both pro-inflammatory and anti-inflammatory cytokines have been found to be raised following P. falciparum infection, the exact roles played by these molecules in disease pathogenesis are unclear. Levels of pro-inflammatory tumor necrosis factor (TNF), interleukin (IL)-6, and anti-inflammatory IL–10 are increased in patients with CM as compared with those without malaria. Several polymorphisms in the TNF gene promoter regions are associated with an increased risk of CM, neurological sequelae, and death [20].
A study among Vietnamese adults demonstrated that levels of IL-6, IL-10, and TNF were raised significantly in patients with multi-organ severe disease, but not so in patients with CM alone without multi-organ involvement [21]. This may suggest that these cytokines are involved in the pathogenesis of severe malaria, but not coma per se.
An association between elevated serum levels of IL-1 receptor antagonist and severe malaria in children has been reported recently. Furthermore, plasma levels of inducible protein 10 (IP-10), the soluble TNF receptor TNF-R2, and soluble Fas proteins have been recently suggested as possible potential biomarkers of CM severity and mortality [22•]. By contrast, high levels of vascular endothelial growth factor were protective against death in CM. Post-mortem analysis of brains from patients with CM has suggested an increased local production of TNF, IL-1, and transforming growth factor in brain tissue. However, the production of or staining for these cytokines did not correlate with parasite sequestration. IL-10 is increased in patients with CM compared with those without malaria [23, 24].
Nitric oxide (NO) may act as a key effector for TNF. Inflammatory cytokines like TNF upregulate inducible NO synthetase in brain endothelial cells. This increases the production of NO, which diffuses into the brain tissue [25]. The increased NO level leads to a change in blood flow and reduction in glutamate uptake, leading to excite-toxicity. NO likely affects the level of consciousness rapidly and reversibly as this short-lived molecule can easily diffuse across the blood–brain barrier (BBB), resulting in neuronal dysfunction.
Given that parasites are largely confined to the intravascular space, one major question regarding the pathogenesis of CM is how these parasites cause neuronal dysfunction [26••]. The BBB seems to be impaired in patients with CM [27••] and in those with other neurological manifestations of malaria. Post-mortem analysis of brains of adults with CM has shown widespread vascular endothelial cell activation with disruption of cell junctional proteins (zonula occludens, occludin, and vinculin), particularly in vessels containing infected erythrocytes [24]. This process might be sufficient to allow metabolites to impair consciousness or precipitate seizures.
Even if these factors may explain the presenting features like coma or seizures, these do not seem to be adequate to explain the reversibility of the condition in most cases. Lack of proper therapy to deal with the cytoadherence of the parasitized RBCs and the resultant vasculopathy seems to be the principal cause of the high mortality seen in cases with CM [21, 28]. Imaging techniques have helped us to understand exactly what happens in the brain in CM patients ante mortem [29••]. Both head CT and brain MR imaging show features of brain swelling or diffuse cerebral edema. This edema may in part be vasogenic resulting from the disturbance in microcirculation and in part cytotoxic resulting from the release of inflammatory cytokines and other toxic chemicals released because of ischemia. Other mechanisms which may contribute are increased cerebral blood volume resulting from microvascular congestion generated by sequestered RBCs and decreased venous outflow and increased cerebral blood flow in response to fever, anemia, and seizures [30••]. It has been suggested by some workers that the distinction between vasogenic and cytotoxic edema may possibly be made with the use of diffusion-weighted sequences during MR imaging, and this may be helpful in developing more specific supportive therapies in the future [31••]. In addition to a generalized increase in brain volume, MR imaging may also demonstrate localized changes in the basal ganglia, pons, cerebral white matter, and splenium of the corpus callosum. Interestingly, while the increased brain volume accounts for the clinical manifestations of CM, a decrease in the elevated brain volume can be seen in imaging during the recovery phase of the CM [32••]. Additional roles of MR imaging to reveal the ongoing brain pathology during the acute phase of CM include diffusion tensor imaging to show axonal changes with tractography and damaged white matter with fractional anisotropy maps, MR spectroscopy to follow lactate in ischemic zones, susceptibility-weighted imaging to reveal hemozoin (a pigment derived from hemoglobin) deposition, and perfusion imaging to demonstrate the blocked arterioles by parasitized RBCs [29,30,31,32].
Autopsy studies have demonstrated sequestration of infected erythrocytes in all parts of the brain in patients with CM. Although the brain is often swollen, suggesting diffuse cerebral edema, features suggestive of frank herniation are uncommon in adults, but found to be more common in children. Extra-erythrocytic hemozoin is found inside cerebral vessels [33], suggesting rupture of sequestered infected erythrocytes. This might lead to an inflammatory process within and around brain capillaries. These findings are not seen consistently in adults and might reflect a difference between adults and children [34]. Brains of Asian adults dying of CM reveal this combination of vascular clogging, mononuclear cell migration, and enhanced vascular permeability [34, 35]. Additionally, pathogenic roles for heme activation of the blood coagulation cascade and platelet-induced clumping of P. falciparum-infected erythrocytes have been proposed in the pathogenesis of CM [36, 37]. However, the way the various pathological mechanisms discussed above are linked and how they are influenced by the host (genetic), parasitic, and environmental (possible co-infections) factors remains to be elucidated. Furthermore, the reason why circulating cytokines, coagulation factors, or parasitized RBCs, specifically target only the brains in African children, and the brains plus other organs, in Asian adults, still remains a mystery. The mere difference in pace of the disease between the two groups of patients, not giving adequate time for the involvement of other organs (before death from CNS disease), may not be the complete explanation.
Plasmodium vivax is known to cause relapsing malaria but rarely causes severe malaria with cerebral involvement. Only a little over fifty cases have so far been reported principally from India, Pakistan, and China [2, 3, 8, 9]. Serological and in some molecular genetic studies had been done to carefully exclude co-existent P. falciparum infection. The pathophysiology of multi-organ involvement in Plasmodium vivax infection may be similar to what had been discussed in relation to P. falciparum infection, but cytoadherence is not a characteristic feature with P. vivax infection.
Clinical Features
Presenting features of CM are those of diffuse encephalopathy as encountered in metabolic conditions. Occasionally, focal neurological signs may be present. Onset may be abrupt or may develop later in the progressive course of the worsening clinical condition of falciparum malaria with multi-organ involvement. In a typical case, there is a fever with severe headache and delirium which may rapidly progress to stupor, with hyperthermia (temperature >40°C). The stuporous state progresses to a coma with fluctuations in levels of consciousness. In some patients, the high fever is associated with irritability, restlessness, or psychotic behavior, suggesting cerebral involvement. Travel history and history of blood transfusions in the recent past often provide diagnostic clues [38••].
Some differences in clinical presentations between children (mostly African) and adults (mostly Asians) have already been highlighted. Coma usually develops rapidly in children with CM, often following a cluster of seizures. Coma develops gradually in adults, and seizures are less frequently encountered (around 20% compared to around 80% in children). In most instances, seizures are generalized tonic-clonic in nature. EEG may reveal a focal onset with secondary generalization. Rarely only electrographic seizures may be noted [39, 40]. With disease progression, brainstem dysfunction may be noted, especially in children with abnormal pupillary and corneal reflexes, dysconjugate/upward gaze, and irregular/stertorous breathing patterns. Decerebrate posturing is more common in children than in adults [38••]. While hypoglycemia needs exclusion in such cases, more commonly, such abnormal body posturing suggests raised intracranial pressure. Extrapyramidal signs may develop during convalescence when a strong pout reflex and a brisk jaw-closing reflex may also be elicited [38, 40]. In children with retinopathy, markedly increased brain volume, abnormal T2 signal intensity, and DWI abnormalities in the cortical, deep gray, and white matter structures had been noted [41••]. Focal abnormalities rarely respect arterial vascular territories [41••]. This increased brain volume suggesting raised intracranial pressure (ICP) has been more often noted among children who subsequently died. In survivors, the raised ICP had been a transient phenomenon [42].
Persistent residual neurological deficits are common among children who survive, and these include hemiplegia, tetraplegia, ataxia, seizure disorders, language deficits, altered behavior, and cognitive impairments [43]. Compared with controls, children surviving either retinopathy-negative or -positive CM are at similarly high risk for adverse neurologic outcomes like the occurrence of epilepsy or developmental disabilities [44••]. Cortical blindness is not uncommon but may improve over time [43].
Psychiatric Manifestations
Psychosis may develop in subjects with CM because of encephalopathy [45, 46]. Paranoid and manic syndromes may develop in the acute stage; depression often occurs as late sequelae. Disinhibitory behavior has been reported. Acute confusional state, agitation, delirium with hallucinations, transient amnesia, and schizophrenic features have also been described. Long-lasting personality disorders and even dementia might develop, albeit rather uncommonly [45,46,47]. On occasions, psychiatric problems may be the presenting feature in patients with acute uncomplicated malaria when associated with hyperpyrexia [45, 46]. It must, of course, be remembered that neuropsychiatric manifestations can also be caused by side effects of antimalarial drugs [48].
Neuro-cognitive Sequelae
Neurological sequelae of CM are more severe among children. The prevalence of neurological deficits varies between 6 and 29% at the time of hospital discharge [49,50,51] – Of these, some may be transient (like ataxia and psychoses) while others like paralytic sequelae and extrapyramidal features may be long lasting.
Residual neurological and/or psychiatric deficits are less common among adult patients. In India, 10% of adults had neurological sequelae on discharge, including psychosis, cerebeller ataxia, and extrapyramidal rigidity or hemiplegia [52••]. The depth and duration of coma and multiple seizures were independent risk factors for the development of significant residual neurological/psychiatric disorders [53].
African children with severe neurological sequelae following falciparum malaria (spastic quadriparesis and vegetative states) often die within a few months of discharge from hospitals. Epileptic seizures may ensue following CM [54, 55].
In one study, 10% of children recovering from CM developed at least one mental health issue within the next six to twelve months’ post-infection (median 21 months follow-up). The major ones included attention deficit hyperactivity disorder, conduct disorder, and oppositional defiant disorder [56••].
Cognitive impairment has been reported in a wide range of functions including memory, attention, executive functions, and language. Neuro-cognitive impairments can be associated with protracted seizures, deep and prolonged coma, hypoglycemia, and severe anemia [57,58,59].
In a study of 187 Ugandan children, 26% of children with CM and 13% with uncomplicated malaria had cognitive deficits in one or more areas at a 2-year follow-up examination, as compared with 8% of healthy children. The deficits in children with CM were primarily in the area of attention (18% CM vs. 3% controls) [60]. High levels of TNF production in the brain in CM may be responsible for residual neurological and cognitive morbidity.
Malarial Retinopathy
This had been described in African children with P. falciparum infection as well as in Bangladeshi and Indian adults [10, 15, 61, 62]. These retinal abnormalities, when properly evaluated, are 90% sensitive and 95% specific for detecting African children who have CM caused by Plasmodium falciparum [63••]. The degree of retinal changes bears some correlation with the severity of the disease process but cannot predict the ultimate prognosis. However, Malawi children with retinopathy-negative CM share a common clinical phenotype which seemed to have lower rates of mortality compared with those who have malarial retinopathy [44, 64]. It is possible that the clinical course, severity, or features of a patient infected with P. falciparum may vary depending on the presence or absence of a yet identified factor (alone or in combination) or perhaps co-infections, the latter being not uncommon in a tropical country. Malarial retinopathy has three components: retinal whitening, vessel changes, and retinal hemorrhages [10, 63]. Retinal whitening is similar to the patchy ischemic retinal changes seen in central vein occlusion and indicative of areas of hypoperfusion caused by the presence of infected erythrocytes clogging the retinal microvasculature [65]. Malarial retinopathy detection in an endemic region may have clinical diagnostic value: The presence of retinal whitening in a patient with altered sensorium hailing from an endemic area strongly suggests the possibility of CM with cerebral microvasculature sequestration [63••]. Proper diagnosis of retinal whitening, especially in the peripheral part, would need the use of indirect ophthalmoscopy [10••], of which physicians usually do not have much expertise. A trained ophthalmologist needs to be consulted. The recent availability of infra-red fundus photography is of much help. Disc changes and retinal hemorrhages can be detected more easily with direct ophthalmoscopy [15, 63].
Post-malaria Neurological Syndrome
Post-malaria neurological syndrome (PMNS) is an uncommon, monophasic disorder occurring within two months after recovering from Plasmodium falciparum malaria. Senanayake in 1987 and then Senanayake and Román in 1992 were the early investigators to report on this condition [66, 67]. Later, in 1994, the cases of seventy-four patients from Sri Lanka who developed a self-limiting delayed cerebellar ataxia (DCA) syndrome 3–41 days after fever onset were reported [68••]. However, in this study, it was difficult to establish a causal relationship with a post-malaria phenomenon as the onset of neurological signs occurred while almost half of the subjects continued to have peripheral smear parasitemia and some had not received any antimalarial treatment [66,67,68]. In 1996, Hong Mai and colleagues from Vietnam, in a large series of patients with falciparum malaria, reported on 22 patients (including 3 children) with subsequent PMNS [69••]. Clinical features included acute confusional state, psychosis, generalized seizures, prolonged confusional state following generalized convulsions, and new-onset tremors. All symptoms resolved spontaneously; in some cases, malarial treatment included oral mefloquine.
PMNS has now been defined by Yadava et al. (2019) as “the occurrence of de novo neurological signs following a symptom-free period after acute malaria (regardless of the Plasmodium species), associated with a negative blood smear and no retainable differential diagnosis.” [70]
It is important to remember that the demonstration of a symptom-free interval is a crucial criterion along with a negative peripheral blood smear. The incidence of PMNS in patients after falciparum malaria ranges from 0.7 to 1.8 per 1000, and it is 300 times more common in patients with severe rather than those with uncomplicated malaria [69••].
Schnorf and co-workers classified PMNS into three categories based on clinical severity: a mild form characterized by isolated cerebellar ataxia or postural tremors; a diffuse, relatively mild encephalopathic form associated with acute confusion or seizures; and a severe form with encephalopathy, aphasia, generalized myoclonus, postural tremors, and cerebellar ataxia [71].
The symptom-free period between remission of malarial fever and onset of PMNS symptoms is usually around 15 days [69••]. PMNS onset is often heralded by the recurrence of fever, confusion, seizures, psychosis, cerebellar ataxia, and paralysis [69, 70]. Less commonly, there may be involvement of cranial nerves, visual impairment, sphincteric disturbances, and headaches [70,71,72,73]. The cerebrospinal fluid (CSF) may be abnormal with high protein content (>1 g/L) and a lymphocytic pleocytosis [68, 69]. Brain MRI is generally normal following infection with P. falciparum and P. vivax infection (in contrast to post-malarial ADEM), but may occasionally demonstrate white matter signal alterations in some patients [70]. Clinical cerebellar syndromes may not always be accompanied by MRI signal changes. EEG may show diffuse slowing.
The Vietnam study suggested that mefloquine may be a risk factor for PMNS after severe malaria [69••]. However, only a small number of patients in other studies of PMNS had been treated with mefloquine. PMNS has also been observed in falciparum malaria patients who received treatment with other drugs including artemisin [74]. P. vivax infection-related PMNS patients were not treated with mefloquine. Mefloquine thus seemed not to have an important role in the pathogenesis of PMNS.
Delayed cerebellar ataxia (DCA) is a post-infectious syndrome following P. falciparum malaria and is characterized by gait ataxia without cerebral involvement [75]. PMNS and DCA are often viewed by some investigators as part of a single neurological spectrum [75, 76]. However, PMNS presenting as the brainstem and/or spinal cord syndrome has not yet been described. Cerebellar signs have been observed in cases of severe malaria responding to standard antimalarial treatment, with the absence of parasites. This would support a possible immune-mediated mechanism. Overall, 30% of the studied PMNS cases presented with cerebellar involvement with or without other neurological signs and symptoms [75]. PMNS thus should include DCA as they share the same prognosis and outcome [68,69,70, 76].
Some authors classify PMNS as a variant of ADEM [77, 78] rather than a distinct nosological entity due to their similarity. While both PMNS and ADEM are often acute monophasic illnesses [73, 77, 78], and both respond to corticosteroids, there indeed are important differences between these two conditions. Firstly, PMNS appears to be more common in adults, while ADEM occurs more frequently in children. Secondly, many patients with PMNS have normal MRI, while patients with ADEM almost invariably have abnormal imaging studies. Thirdly, all patients with PMNS appear to recover completely, while patients with ADEM, despite carrying a favorable prognosis, may have long-term neurological sequelae.
Several pathogenetic mechanisms have been postulated for the development of ADEM following malarial infection [79]. These include an abnormal immune reaction to some cerebral antigens after a neurotropic infection, initiated by molecular mimicry between some protein of the infective agent and the cerebral myelin, and a T cell-activated cerebral aggression. However, the relationship between infection with P. falciparum and P. vivax and the development of ADEM-like features is, in fact, an indirect one.
The exact pathogenesis of PMNS is unknown. It has been suggested [69, 80] that blockage of the brain microvasculature by parasitized RBCs might result in cerebral hypoxemia causing neurological and psychiatric dysfunction similar to what had been postulated for CM. Hsieh et al. [81] described brain single-photon emission computed tomography (SPECT) findings with markedly decreased radioactivity, a feature which would support this hypothesis. However, this mechanism seemed unlikely as features of PMNS develop only after a clear period of clinical remission when obstruction of the microvasculature by parasite-loaded RBCs would unlikely to occur. Another postulated hypothesis is immune-mediated-based, supported by a positive response to corticosteroid therapy in some patients and delay of onset of PMNS symptoms after resolution of malaria symptoms [70]. Further evidence in favor of the immunological hypothesis is the increased levels of inflammatory cytokines like TNF-alpha, IL 2, and IL 6 in serum and CSF, described in some cases of delayed post-malaria cerebellar syndrome [75].
There also exist shared clinical features between PMNS and auto-immune encephalitis (AIE). Much attention has been devoted toward neurological disorders caused by neuronal auto-antibodies, commonly referred to as AIE [82]. Such disorders commonly occur in the setting of malignancy or post-viral infections [82, 83]. No neuronal auto-antibodies have yet been found to be related to malaria [83]. But there are striking similarities between PMNS and AIE in the sense that both conditions tend to develop after a symptom-free interval following the offending infection, both conditions have normal MRI studies in the majority of cases, and lastly, improvements occur in both with corticosteroids, often with full resolution. Such common features would argue in favor of a post-infectious immunologically mediated cerebral disorder. Sahuguet and colleagues reported a case of AIE with anti-voltage-gated-potassium-channel antibodies in the setting of PMNS [84••]. This observation would raise the need for evaluation of an encephalitic autoantibody profile in patients who otherwise would have been diagnosed as simply PMNS. Perhaps, PMNS with normal MRI findings should be investigated in the line of an AIE as therapeutic strategies may be different.
In cases of ADEM, MRI is a key point for diagnosis as it is almost always abnormal, and a normal MRI is often cited as against the diagnosis of ADEM [85]. Although the exact mechanisms underlying PMNS are poorly understood, delayed onset, negativity of blood smears, association with fever, and sometimes elevated CRP levels all suggest an inflammatory process. As mentioned earlier, some studies of DCA have reported increased levels of blood and CSF pro-inflammatory cytokines [86]. The brain microvasculature could be the site of this immune reaction since parasites and pigments are known to be sequestered there due to cytoadherence. Cytoadherence is less frequent in cases of P. vivax infection which could explain why most PMNS cases follow P. falciparum infections rather than P. vivax [87,88,89,90].
If corticosteroids are ineffective, the diagnosis of PMNS should be reconsidered, and thereafter, the role of intravenous immunoglobulin or plasma exchange needs to be entertained.
Other Neurological Manifestations
Guillain-Barré syndrome (GBS) following malaria is rare. A review of 12 cases of GBS following malaria included eight patients who had preceding falciparum malaria and four had P. vivax infections [8•]. Four patients with falciparum malaria had severe paralysis with respiratory failure, and three of these patients died. Other forms of neuropathies described in association with malaria include mononeuritis like facial palsy, trigeminal neuralgia, optic neuritis, ulnar, circumflex, and lateral popliteal nerve involvement. The pathogenesis of these peripheral nerve disorders is not precisely known. Possibilities include parasitized red blood cell corpuscles obstructing the vasa nervorum, liberation of neurotoxins from the parasite, and/or metabolic or nutritional disturbances [91,92,93].
There have also been isolated case reports of RCVS associated with CM. Yamamoto et al. reported one of the first such cases in an adult patient of CM where brain MRI and MR angiography demonstrated narrowing and dilatation in multiple cerebral arteries [94•]. With the demonstration of cerebral arterial vasoconstriction during the acute phase of CM, the possibility of primary angiitis of the CNS (PACNS) [95] may be raised. However, histopathological evidence of cerebral vessel infiltration by inflammatory cells has not yet been observed in autopsy studies of patients dying of CM [96, 97].
Mohanty et al. reported that in about 41% of patients with non-fatal CM, brain MRI showed findings of PRES or PRES-like features [21]. Such changes improved within 48–72 h [21]. Although the pathogenesis of CM may be associated with microcirculatory dysfunction, damage to the BBB, and the effects of cytokines, vasoconstriction of large cerebral arteries may also occur. P. falciparum-parasitized RBCs, adhering to endothelial cells causing endothelial dysfunction, likely play a key role. Newton et al. reported that 15 of 50 Kenyan children with CM had increased cerebral blood flow velocities suggestive of narrowed cerebral blood vessel diameters [98••]. Malarial infection can also result in various hematological and hemorrhagic complications associated with thrombocytopenia [99]. Ocular and intracranial hemorrhagic complications are rare [100,101,102,103,104]. Although falciparum malaria is known to be associated with both intracerebral and subarachnoid hemorrhages, there have also been reports of intracranial hemorrhagic complications associated with P. vivax infection [105].
Cerebellar syndrome
Cerebellar involvement is a major neurological manifestation of malaria [106, 107]. The Purkinje cells are commonly damaged, possibly due to hyperpyrexia. Cerebellar involvement may dominate the clinical manifestations of CM and generally resolve along with other cerebral manifestations. Presentation is characterized by gait and truncal ataxia due to midline cerebellar involvement. A clear gap of several days of apyrexia generally precedes the onset of cerebellar symptoms [108, 109]. Opsoclonus-myoclonus is rare and may respond to clonazepam; co-activation of some neurotropic viruses may be the causative mechanism [110,111,112].
Neurotoxicity of Antimalarial Drugs
Chloroquine is usually very well tolerated, but it may rarely produce transient neuropsychiatric disturbances or cerebellar dysfunction. Precipitation of acute intermittent porphyria (AIP) has also been reported with its use [113]. Prolonged use of chloroquine may also result in vacuolar myopathy. Cinchonism, characterized by nausea, tinnitus, and high-frequency hearing loss, may be associated with quinine and quinidine. Mefloquine, a new antimalarial drug, may be associated with serious but self-limiting neuropsychiatric reactions. Confusion has been reported in 0.5 to 1.0 percent of Europeans and Africans, but only 0.1% of Southeast Asian patients [114]. Furthermore, a case of “central anticholinergic syndrome” associated with mefloquine use has been described [115••]. In patients with epilepsy, mefloquine should be avoided as it has potent epileptogenic effects. Similarly, van Hensbroek et al., in a comparative study of artemether and quinine in children with CM, observed an increased incidence of convulsions in the artemether-treated group [115••]. Ataxia and slurring of speech have been described after artesunate treatment for falciparum malaria [116].
Malaria and COVID-19 Infection: Threat of a Syndemic?
The devastating effects of the COVID-19 pandemic on the health care systems of most countries in the world, rich or poor, have been felt in one way or another in the past two–three years. The pandemic’s effects on the occurrences of malaria and the consequent alterations in the morbidity and mortality have been a subject of much interest and serious public health concerns due to the fact that malaria happens to be endemic mostly in the low- or middle-income countries in Asia, Africa, and South America. These countries have not escaped the ravages caused by the COVID-19 pandemic and, being resource deficient, have experienced a tremendous burden on their health care systems as well as on their overall economy. Interestingly, the WHO noted an important difference between the incidence and mortality from COVID-19 disease between North America, Western Europe, and South Asia on the one hand and most African countries on the other hand, especially among the malaria-endemic countries as “nine scenarios for potential disruptions in access to core malaria control tools during the pandemic in 41 countries, and the resulting increases that may be seen in cases and deaths. Under the worst-case scenario, in which all insecticide-treated net campaigns are suspended and there is a 75% reduction in access to effective antimalarial medicines, the estimated tally of malaria deaths in sub-Saharan Africa in 2020 would reach 769 000, twice the number of deaths reported in the region in 2018. This would represent a return to malaria mortality levels last seen in the year 2000.” [https://www.who.int/publications-detail/thepotential-impact-of-health-service-disruptions-on-the-bur]
Travel restrictions, lockdowns, and other COVID-appropriate behaviors had their impact on health care services, causing significant delays in diagnosis and treatment for other diseases, including malaria. A similar situation happened in African countries during the Ebola epidemic, with a rise in cases of malaria and increased mortality [117]. On the brighter front, of course, travel restrictions and social distancing are likely to curtail the spread of vector-borne diseases like malaria or dengue.
Another possibility that has been raised is of SARS-CoV-2 virus (or the resultant immune reaction) interacting with parasitic infections and altering the rate of severe outcomes, particularly among younger populations that have been relatively less affected by COVID-19 in 2020 [118].
Returning to the darker sides, the chances of misdiagnosis and co-infections are likely to be factors for concern. The initial clinical features of both malaria and SARS-CoV-2 infections are similar, and hence with the dearth of proper laboratory-based diagnostic facilities, the chances of misdiagnosis for one condition for the other would remain high with delays in delivering appropriate therapeutic measures [119••]. This would certainly affect both morbidity and mortality from either condition.
Syndemics or synergistic epidemics occur when two or more concurrent epidemics have a deleterious interaction [120]. For example, (a) malaria plays a role in Epstein–Barr virus (EBV) infection, leading to Burkitt’s lymphoma by contributing to B-cell proliferation and increased viral loads [121]. (b) HIV-infected individuals experience a higher frequency of severe malaria and increased HIV viral load following co-infection with Plasmodium falciparum and other parasites [122]. Co-infections may also result in altered immune dynamics. Malaria can induce a cytokine storm and a pro-coagulant state similar to that seen in severe SARS-CoV-2 infections. A co-infection could also possibly result in substantially worse outcomes than singular infections with either pathogen and could alter the age pattern of severe SARS-CoV-2 infection in younger age groups. The balance between pro- and anti-inflammatory responses to either of these two diseases (malaria and SARS-CoV-2 infection) determines the ultimate fate of the infection in individual patients [123, 124].
Two further immunogenic mechanisms have been postulated in relation to the interaction between SARS-CoV-2 infection and malaria. By now, it is well established that SARS-CoV-2 (except perhaps some very new variants like Omicron) uses the angiotensin-converting enzyme 2 (ACE2) receptor to enter the host cells, and such receptors are highly expressed in the heart and type II alveolar cells of the lungs. In addition to the membrane-bound form, there are soluble forms in the plasma and urine as well. The ACE2 receptors can produce Ang-(1–7) from angiotensin II (ANG II), the former having a protective activity on lung parenchymal cells [125]. Downregulation of the ACE2 receptor would result in the accumulation of ANG II which is the substrate for ACE2 in the alveolar cells. Accumulated ANG II would increase neutrophilic aggregation and enhance vascular permeability leading to pulmonary edema and ARDS [126]. Conversely, it had been demonstrated that ANG II decreases the build-up of sporozoites in mosquitoes’ salivary glands by directly disturbing the parasite membrane [127••]. Thus, ANG II appears to have a dual role – harmful for the host but beneficial for the host by way of its role in the vector.
Various types of interferons are produced by lymphocytes as an immune response to infection by malaria parasites. Interferons can have both in vitro and in vivo efficacy against the corona viruses causing SARS, MERS, and COVID-19 [128,129,130]. Malaria-affected patients develop antibodies (IgG types) against Plasmodium antigens. These IgG antibodies target Glycosyl-phosphatidyl-inositol (GPI) molecules, which bind some membrane proteins of Plasmodium species. SARS-CoV-2 has various glycoproteins (GPs), namely, membrane GPs, spike GPs, and GPs that have acetyl esterase and hemagglutination features. The anti-GPI antibodies in a malaria-affected patient could identify and bind to different GPs on the virus, inactivating them and thus may provide protection against virus infection or result in a milder form of the disease [131].
Unfortunately, none of the aforementioned hypotheses have been adequately tested in a real-world clinical setting, and hence, at this time, these should not evoke any false sense of security in the minds of the scientific community neither the people living in malaria-endemic areas.
Malaria per se can induce immunosuppression/immunomodulation in some co-infections, significantly inhibiting immune responses to the other infection, as had been seen in Salmonella infection, and also this can be protective against the severity of some respiratory viruses [132,133,134]. It is possible that similar phenomena could occur during Plasmodium–SARS-CoV-2 co-infection; on the one hand, malaria-induced immunosuppression might lead to a clinically milder form of COVID-19 but at the same time, potentially increasing or sustaining viral loads, hamper virus control, thereby increasing the potential for viral transmission. Predicting outcomes in cases of syndemics thus become quite complex.
In malaria-endemic countries, especially in Africa, age-related vulnerability to malaria and COVID-19 cases is different. Younger children are more vulnerable to malaria, especially falciparum infections, with a higher risk for severe malaria, including the cerebral form. For SARS-CoV-2 infections, children are less likely to develop severe disease, whereas older adults are more likely to be affected, with a higher risk of severe disease and death. This may be related to differences in age-related immune status as well as residual immunity provided by past malarial infections. Severe respiratory distress may also occur in SARS-CoV-2 infection. This combined respiratory compromise, thus, often would lead to a worsened outcome in cases of co-infections.
The occurrence of coagulopathy is a major pathogenetic mechanism underlying a number of complications in both SARS-CoV-2 and malaria infections. Clinically, this hypercoagulable state in COVID-19 patients presents with a high occurrence rate of venous thrombosis, arterial thrombosis, and also DIC caused by consumption coagulopathy [135, 136] with associated thrombocytopenia. Thrombocytopenia develops in 60–80% of falciparum malaria cases. A pro-coagulant state may also develop in severe falciparum infections due to activation of the coagulation cascade, mediated by TNF-alpha and IL-6 [137••]. In addition to micro-thrombotic complications, thrombosis of large vessels, including cerebral venous thrombosis, and pulmonary embolism may also occur [138, 139]. All of these factors would be additive in assessing the outcome of co-infections in a syndemic situation.
Finally, outcomes from both severe malarial infection and also SARS-CoV-2 depend upon the nutritional status of children, and malnutrition is related to poor immune status. Malnutrition or undernutrition is a major health problem in parts of the developing world which are also endemic to malaria infections [123••]. The effect of a syndemic in such regions would thus probably be devastating.
Rapidly developing surveillance platforms to monitor signals of SARS-CoV-2 co-infection with malaria will be critical. One early indication of a potential interaction would be a shift in the age pattern of severe COVID-19 with increased occurrence in children. Unfortunately, our knowledge of epidemiology and clinical course of SARS-CoV-2 infections in countries with substantial burdens of malaria and other vector-borne diseases is still rather limited, as community transmission, on the whole, started later in these countries and surveillance had been poor because of resource deficiency. The immune status of the population in countries with low socioeconomic status and being breeding grounds for vectors transmitting multiple diseases is bound to be variable, and hence, the outcomes of co-infections would remain variable as well.
India has witnessed a significant decline in the numbers of malaria cases in recent times through the Malaria Elimination efforts which had been in vogue since 2015 and subsequently enhanced through the National Framework for Malaria Elimination in 2016 and the National Strategic Plan for Malaria Elimination (2017–22). Between 2000 and 2019, there had been a 71.8% drop in malaria infections and a 73.9% decline in mortality [140••]. As happened elsewhere in the World, the existing health care system in India became extremely stretched out with the onslaught of the pandemic. All available resources were directed toward containing the COVID-19 infection. No doubt, routine malaria monitoring was also hampered in the past couple of years or so, with frontline workers being shifted to pandemic-related activities, including vaccination. As a result, there had been an under-reporting of malaria cases, with a lesser number of tests being performed. Hence, a correct estimate of the number of cases of malaria while the pandemic had been at its peaks (2020 and 2021) could not be made. Notwithstanding the funding issues and lack of qualified clinicians in village/slum areas, India has been able to contain the virus’s growing trends over time, as this undoubtedly seemed to be of top most priority [141]. As expected, the country faced difficulties in the epidemiological control of infectious diseases, and the danger of a malaria epidemic to escalate in the near future is indeed a real one. The need of the hour would be the re-implementation of methods to combat malarial infection, which would include chemoprophylaxis as well as steps to stop the spread of parasites from vector to host by removing breeding sites of mosquitoes and educating people about how to prevent mosquito bites.
Concluding Remarks
Even in the 21st century, malaria continues to be a major infectious disease in tropical and sub-tropical countries. Cerebral malaria, a complex and potentially reversible encephalopathy, is a severe complication of P. falciparum infection, which may lead to multi-organ failure and death. The mortality remains high even with the use of highly effective antimalarial drugs and intensive care management.
It is heartening that the World Health Organization (WHO) has recommended the widespread use of the RTS, S/AS01 (RTS,S) malaria vaccine among children in sub-Saharan Africa and in other regions with moderate-to-high P. falciparum malaria transmission. This recommendation has been based on results from ongoing pilot programs in Ghana, Kenya, and Malawi that have reached more than 900,000 children since 2019.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO. Malaria Key Facts. Geneva, World Health Organization, 2019. https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed April 2022. Fact sheet from WHO on Malaria: diagnosis and management.
Kochar DK, Das A, Kochar SK, Saxena V, Sirohi P, Garg S, et al. Severe Plasmodium vivax malaria: a report on serial cases from Bikaner in northwestern India. Am J Trop Med Hyg. 2009;80:194–8.
Battle KE, Lucas TCD, Nguyen M, Howes RE, Nandi AK, Twohig KA, et al. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000-17: a spatial and temporal modelling study. Lancet. 2019;394:332–43. A major global epidemiological study report on Plasmodium vivax infection.
World Health Organization. World Malaria Report 2018. Geneva: WHO; 2018. WHO report on global Plasmodium infection
White NJN, Pukrittayakamee S, Hien TTT, Faiz MA, Mokuolu OAO, Dondorp AAM. Malaria. Lancet. 2014;383:723–35.
Soulard V, Henriette Bosson-Vanga H, Lorthiois A, Roucher C, et al. Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nature. Communications. 2015;6:7690. https://doi.org/10.1038/ncomms8690. Article describes the full life cycles of Plasmodium falciparum and Plasmodium ovale covering both the erythrocytic and extra-erythrocytic stages.
Baer K, Klotz C, Kappe SHI, Schnieder T, Frevert U. Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature. PLoSPathog. 2007;3(11):e171. https://doi.org/10.1371/journal.ppat.003017.
• Chakravarty A, Ghosh B, Bhattacharyya R, Sengupta S, Mukherjee S. Acute inflammatory demyelinating polyneuropathy following Plasmodium vivax malaria. Neurol India. 2004;52:130–1. Article describes a small series of cases of Guillain Barre syndrome following P.vivax malaria, which is uncommon.
Kochar DK, Saxena V, Singh N, et al. Plasmodium vivax malaria. Emerg Inf Dis. 2005;11(1). www.cdc.gov/eid. Accessed April 2022
Chakravarty A. The malarial maladies. In: Chakravarty A, editors. Neurology and internal medicine. A case based study. New Delhi London: Jaypee Brothers Medical Publication; 2021. p. 33–39. A case-based interactive discussion on neurological complications in Malaria.
Gething PW, Casey DC, Weiss DJ, Bisanzio D, Bhatt S, Cameron E, et al. Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N Engl J Med. 2016;375:2435–45 A major epidemiological study.
Weiss DJ, Lucas TCD, Nguyen M, Nandi AK, Bisanzio D, Battle KE, et al. Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum, 2000–17: a spatial and temporal modelling study. Lancet. 2019;394:322–31 A major epidemiological study.
Severe malaria (no authors listed). Trop Med Int Health. 2014;19(Suppl 1):7–131. https://doi.org/10.1111/tmi.12313_2
Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119:e360–6.
Birbeck GL, Beare N, Lewallen S, Glover SJ, Molyneux ME, Kaplan PW, et al. Identification of malaria retinopathy improves the specificity of the clinical diagnosis of cerebral malaria: findings from a prospective cohort study. Am J Trop Med Hyg. 2010;82:231–4 A major study on the recognition of malarial retinopathy and its value in the diagnosis of severe malaria and prognostication.
WHO. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(suppl 1):1–90. WHO recommendations on the diagnosis and management of severe falciparum malaria.
Storm J, Craig AG. Pathogenesis of cerebral malaria – inflammation and cytoadherence. Front Cell Infect Microbiol. 2014;4:100. https://doi.org/10.3389/fcimb.2014.00100This article provides an in-depth discussion on the pathogenesis of cerebral malaria.
Milner DA Jr. Malaria pathogenesis. Cold Spring Harb Perspect Med. 2018;8:a025569 This article provides an in-depth discussion on the pathogenesis of multi-organ involvement in malaria, in addition to observations on the life cycle of the Plasmodium species.
Ponsford MJ, Medana IM, Prapansilp P, Hien TT, Lee SJ, Dondorp AM, et al. Sequestration and microvascular congestion are associated with coma in human cerebral malaria. J Infect Dis. 2012;205:663–71. https://doi.org/10.1093/infdis/jir812This article provides an in-depth discussion on the pathogenesis of cerebral malaria.
Lennartz F, Adams Y, Bengtsson A, Olsen RW, Turner L, Ndam NT, et al. Structure-guided identification of a family of dual receptor-binding PfEMP1 that is associated with cerebral malaria. Cell HostMicrobe. 2017;21:403–14. https://doi.org/10.1016/j.chom.2017.02.009.
Gimenez F, de Lagerie S, Barraud, Fernandez C, Pino P, Mazier D. Tumor necrosis factor α in the pathogenesis of cerebral malaria. Cell Mol Life Sci. 2003;60:1623–35. Article describes the immunopathogenesis of cerebral malaria with special reference to the role of TNF.
Day NP, et al. The prognostic and pathophysiologic role of pro- and anti-inflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–97. Article describes the inflammatory response to severe P falciparum in the brain and its regulation by pro- and anti-inflammatory cytokines.
John CC, Park GS, Sam-Agudu N, Opoka RO, Boivin MJ. Elevated serum levels of IL-1ra in children with Plasmodium falciparum malaria are associated with increased severity of disease. Cytokine. 2008;41:204–8.
Jain V, Armah HB, Tongren JE, Ned RM, et al. Plasma IL-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malar J. 2008;7:83.
Clark IA, Rockett KA, Cowden WB. Possible central role of nitric oxide in conditions clinically similar to cerebral malaria. Lancet. 1992;340:894–6.
Brown H, Hien TT, Day N, Mai NT, Chuong LV, Chau TT, et al. Evidence of blood–brain barrier dysfunction in human cerebral malaria. Neuropathol Appl Neurobiol. 1999;25:331–40 Highlight on the mechanism of plasma leakage in cerebral malaria-causing brain edema.
Gitau EN, Newton CR. Blood–brain barrier in falciparum malaria. Tropical Med Int Health. 2005;10:285–92 Highlight on the mechanism of plasma leakage in cerebral malaria-causing brain edema.
Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2009;11:e16. Published 2009 May 26. https://doi.org/10.1017/S1462399409001082.
Mohanty S, Benjamin LA, Majhi M, et al. Magnetic resonance imaging of cerebral malaria patients reveals distinct pathogenetic processes in different parts of the brain. mSphere. 2017;2:e00193–17 Detail observations on the development of brain edema in cerebral malaria in life. References 31–33 also highlight the “in life” changes in the brain microvasculature contributing to the pathology of cerebral malaria.
Wassmer SC, Taylor TE, Rathod PK, Mishra SK, Mohanty S, Arevalo-Herrera M, Duraisingh MT, Smith JD. Investigating the pathogenesis of severe malaria: a multidisciplinary and cross-geographical approach. Am J Trop Med Hyg. 2015;93(3 Suppl):42–56. https://doi.org/10.4269/ajtmh.14-0841. This review highlights progress in understanding severe malaria pathophysiology and summarizes key areas of pathogenesis research within the International Centers of Excellence for Malaria Research program.
Potchen MJ, Kampondeni SD, Seydel KB, Haacke EM, Sinyangwe SS, Mwenechanya M, et al. 1.5 tesla magnetic resonance imaging to investigate potential etiologies of brain swelling in pediatric cerebral malaria. Am J Trop Med Hyg. 2018;98:497–504. Article highlights the role of high field MRI to indicate that vascular congestion associated with parasite sequestration, local inflammation from microhemorrhages and autoregulatory dysfunction likely contribute to brain swelling in cerebral malaria.
Mohanty S, Taylor TE, Kamponden S, et al. Magnetic resonance imaging during life: the key to unlock cerebral malaria pathogenesis? Malar J. 2014;13(276). In this article the emergence of neuroimaging as a revolutionary tool in identifying critical structural and functional modifications of the brain during cerebral malaria is discussed and highly promising areas of clinical research using MRI are highlighted.
Grau GE, et al. Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J Infect Dis. 2003;187:461–6. The article describes that platelet accumulation occurs in themicrovasculature of brains of patients with cerebral malaria and may play a role in the pathogenesis of the disease.
Pongponratn E, et al. An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. Am J Trop Med Hyg. 2003;69:345–59.
Patnaik JK, et al. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg. 1994;51:642–7.
Francischetti IM. Does activation of the blood coagulation cascade have a role in malaria pathogenesis? Trends Parasitol. 2008;24:258–63 Discussion on the pro-coagulant state occurring in severe malaria.
Wassmer SC, et al. Platelet-induced clumping of Plasmodium falciparum-infected erythrocytes from Malawian patients with cerebral malaria—possible modulation in vivo by thrombocytopenia. J Infect Dis. 2008;197:72–8.
Newton CR, Warrell DA. Neurological manifestations of falciparum malaria. Ann Neurol. 1998;43:695–702 An early exhaustive review of the neurological manifestations of falciparum malaria.
Crawley J, Smith S, Kirkham F, Muthinji P, Waruiru C, Marsh K. Seizures and status epileticus in childhood cerebral malaria. QJM. 1996;89:591–7.
Crawley J, Smith S, Muthinji P, Marsh K, Kirkham F. Electroencephalographic and clinical features of cerebral malaria. Arch Dis Child. 2001;84:247–53.
Potchen MJ, Kampondeni SD, Seydel KB, Birbeck GL, Hammond CA, Bradley WG, et al. Acute brain MRI findings in 120 Malawian children with cerebral malaria: new insights into an ancient disease. Am J Neuroradiol. 2012;33:1740–6 A detailed account of MRI changes in cerebral malaria in a large series of affected children.
Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, et al. Brain swelling and death in children with cerebral malaria. N Engl J Med. 2015;372:1126–37.
Idro R, Carter JA, Fegan G, Neville BG, Newton CR. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child. 2006;91:142–8.
Postels DG, Taylor TE, Molyneux M, Mannor K, Kaplan PW, Seydel KB, et al. Neurologic outcomes in retinopathy-negative cerebral malaria survivors. Neurology. 2012;79:1268–72 Highlights the importance of detecting malarial retinopathy in prognostication.
Blocker WW, Kastl AJ, Daroff RB. The psychiatric manifestations of cerebral malaria. Am J Psychiatry. 1968;125:192–6.
Arun Prakash MV, Stein G. Malaria presenting as a typical depression. Br J Psychiatry. 1990;156:594–5.
Kochar DK, Shudhakaran, Kumawat B. Neurosychiatric manifestations in malaria. J Assoc Physicians India. 1998;46:886–90.
Weinke T, Trautmann M, Held T, et al. Neuropsychiatric side effects after the use of mefloquine. Am J Trop Med Hyg. 1991;45:86–91.
Newton CR, Krishna S. Severe falciparum malaria in children: current understanding of pathophysiology and supportive treatment. Pharmacol Ther. 1998;79:1–53.
Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet. 1990;336:1039–43.
van Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual neurologic sequelae after childhood cerebral malaria. J Pediatr. 1997;131:125–9.
Bajiya HN, Kochar DK. Incidence and outcome of neurological sequelae in survivors of cerebral malaria. J Assoc Physicians India. 1996;44:679–81. This hospital based study described the various neurological and psychiatric features noted in surviving patients with cerebral malaria.
Jain V, Nagpal AC, Joe PK, Shukla M, Singh MP, et al. Burden of cerebral malaria in central India (2004–2007). Am J Trop Med Hyg. 2008;79:636–42.
Carter JA, Mung’ala-Odera V, Neville BG, et al. Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry. 2005;76:476–81. This study from Kenya showed that after severe malaria, some children have neurocognitive impairments that are evident as long as nine years later. Impairments may become more evident as children progress and face more complex cognitive and linguistic demands, socially and educationally.
Carter JA, Neville BG, White S, et al. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45:978–81.
Idro R, Kakooza-Mwesige A, Asea B, Ssebyala K, Bangirana P, Opoka RO, et al. Cerebral malaria is associated with long-term mental health disorders: a cross sectional survey of a long-term cohort. Malar J. 2016;15:184 A reminder for clinicians to enquire about the past history of cerebral malaria in children with mental health problems hailing from endemic zones.
Boivin MJ. Effects of early cerebral malaria on cognitive ability in Senegalese children. J Dev Behav Pediatr. 2002;23:353–64.
Dugbartey AT, Spellacy FJ, Dugbartey MT. Somatosensory discrimination deficits following pediatric cerebral malaria. Am J Trop Med Hyg. 1998;59:393–6.
Carter JA, Ross AJ, Neville BG, et al. Developmental impairments following severe falciparum malaria in children. Tropical Med Int Health. 2005;10:3–10.
John CC, Bangirana P, Byarugaba J, Opoka RO, et al. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122:e92–9.
Singh J, Verma R, Tiwari A, et al. Retinopathy as a prognostic marker in cerebral malaria. Indian Pediatr. 2016;53:315. The article highlighted that malarial retinopathy is significantly associated with mortality in children with cerebral malaria and hence can be used for prognostication.
Sayeed AA, Maude RJ, Hasan MU, et al. Malarial retinopathy in Bangladeshi adults. Am J Trop Med Hyg. 2011;84(1):141–7. Assessment of malarial retinopathy in adult malaria using ophthalmoscopy by non-ophthalmologists has clinical and prognostic significance. Severity of retinopathy increased with severity of disease, and renal failure, acidosis, and moderate/severe retinopathy were independent predictors of mortality.
Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75(5):790–7. This article reviews current knowledge of malarial retinopathy, including its use as a diagnostic test in the comatose child, and its value as a tool for research into the pathogenesis of cerebral malaria.
Postels DG, Birbeck GL. Children with retinopathy-negative cerebral malaria: a pathophysiologic puzzle. Pediatr Infect Dis J. 2011;30(11):953–6. https://doi.org/10.1097/INF.0b013e3182271c69.
Beare NA, Harding SP, Taylor TE, Lewallen S, Molyneux ME. Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J Infect Dis. 2009;199(2):263–71.
Senanayake N, Roman GC. Neurological complications of malaria. S Asian J Trop Med Public Health. 1992;23:672–80 Early recognition of post-malarial neurological syndromes including delayed cerebeller ataxia. Further discussion on the subject is provided in References 68–71 and References 77 and 79, highlighting nosological issues.
Senanayake N. Delayed cerebellar ataxia: a new complication of falciparum malaria? BMJ. 1987;294:1253–4. The first report on the occurrence of delayed cerebeller ataxia following P falciparum malaria.
Senanayake N, de Silva HJ. Delayed cerebellar ataxia complicating falciparum malaria: a clinical study of 74 patients. J Neurol. 1994;241:456–9. A detailed report on the development of delayed cerebeller ataxia complicating falciparum malaria. The condition has a good prognosis, with spontaneous and complete recovery within 3 months.
Hong Mai NT, Day NP, Chuong LV, Waller D, Phu NH, Bethell DB, et al. Postmalarial neurological syndrome. Lancet. 1996;348:917–21. The article describes the clinical aspects of Post Malarial Neurological Syndrome(PMNS). At the time of PMNS diagnosis all patients were aparasitaemic. The syndrome was self-limiting, median duration 60 h (range 24-240). PMNS was associated with the use of oral mefloquine. The authors opined that mefloquine is not the only risk factor for PMNS but it is a strong one. Where an effective alternative drug is available, mefloquine should not be used after treatment of severe malaria.
Yadava SK, Laleker A, Fazili T. Post-malaria neurological syndrome: a rare neurological complication of malaria. Infection. 2019;47:183–93.
Schnorf H, Diserens K, Schnyder H, et al. Corticosteroid responsive postmalaria encephalopathy characterized by motor aphasia, myoclonus, and postural tremor. Arch Neurol. 1998;55:417–20.
Kochar DK, Sirohi P, Kochar SK, Bindal D, Kochar A, Jhajharia A, et al. Post-malaria neurological syndrome – a case of bilateral facial palsy after Plasmodium vivax malaria. J Vector Borne Dis. 2007;44:227–9.
Kasundra GM, Bhargava AN, Bhushan B, Shubhakaran K, Sood I. Post- Plasmodium vivax malaria cerebellar ataxia and optic neuritis: a new form of delayed cerebellar ataxia or cerebellar variant of acute disseminated encephalomyelitis? J Pediatr Neurosci. 2015;10:58.
Odawara T, Matsumura T, Maeda T, Washizaki K, Iwamoto A, Fujii T. A case of post-malarial neurological syndrome (PMNS) after treatment of falciparum malaria with artesunate and mefloquine. Trop Med Health. 2009;37(3):125–8.
van derWal G, Verhagen WIM, Dofferhoff ASM. Neurological complications following Plasmodium falciparum infection. Neth J Med. 2005;63(5):180–3.
Tamzali Y, Demeret S, Haddad E, Guillot H, Caumes E, Jauréguiberry S. Post-malaria neurological syndrome: four cases, review of the literature and clarification of the nosological framework. Malar J. 2018;17(1):387. In this article the authors commented that PMNS is a rare entity encompassing various neurological signs after severe or non-severe malaria appearing after a symptom-free period. PMNS occurred following treatment of malaria with a wide range of anti-malarials.. MRI patterns underline a possible link with acute disseminated encephalomyelitis (ADEM) or auto-immune encephalitis.
Koibuchi T, Nakamura T, Miura T, Endo T, Nakamura H, Takahashi T, et al. Acute disseminated encephalomyelitis following Plasmodium vivax malaria. J Infect Chemother. 2003;9:254–6.
Mohsen AH, McKendrick MW, Schmid ML, Green ST. Post-malarial neurological syndrome: a case of acute disseminated encephalomyelitis? J NeurolNeurosurg. Psychiatry. 2000;68:388–90. In this communication the authors reported the first case of PMNS showing spontaneous and complete resolution of not only the clinical but also the MRI abnormalities. There seemed to be no identifiable clinical or radiological features that can distinguish PMNS from ADEM. It was felt that Plasmodium falciparum malaria should therefore be added to the list of infections able to precipitate ADEM.
Steiner I, Kennedy PGE. Acute disseminated encephalomyelitis: current knowledge and open questions. J Neuro-Oncol. 2015;21:473–9.
Carreira J, Casella MI, Ascenção BB, Luis NP, Gonçalves AC, Brito AP, Sá JE, Parreira M, Lopes D, Poças J. Acute disseminated encephalomyelitis, a rare post-malaria neurological complication: case report and review of the literature. Travel Med Infect Dis. 2019;28:81–5. There are four neurological complications that can occur after malaria treatment at a time when the patient is aparasitaemic: delayed cerebellar ataxia, acute inflammatory demyelinating polyneuropathy, post-malaria neurological syndrome and acute disseminated encephalomyelitis (ADEM). In the present series diagnosis of post-malaria ADEM was made based on the acute onset of the neurological symptoms, characteristic findings in magnetic resonance imaging of the brain and prompt response to steroid therapy.
Hsieh CF, Shih PY, Lin RT. Postmalaria neurological syndrome: a case report. Kaohsiung J Med Sci. 2006;22:630–5.
Dalmau J. NMDA receptor encephalitis and other antibody-mediated disorders of the synapse: The 2016 Cotzias Lecture. Neurology. 2016;87:2471–82.
Mailles A, Stahl J-P, Bloch KC. Update and new insights in encephalitis. Clin Microbiol Infect. 2017;23:607–13.
Sahuguet J, Poulet A, Bou Ali H, Parola P, Kaphan E. Post-malaria neurologicsyndrome—autoimmune encephalitis with anti-voltage-gatedpotassium-channel antibodies. Ann Intern Med. 2017;167:70. Herein the authors described a patient with PMNS and many features of autoimmune encephalitis who tested positive for VGKC antibodies.
Menge T, Hemmer B, Nessler S, Wiendl H, Neuhaus O, Hartung H-P, et al. Acute disseminated encephalomyelitis: an update. Arch Neurol. 2005;62:1673–80.
de Silva HJ, Hoang P, Dalton H, de Silva NR, Jewell DP, Peiris JB. Immune activation during cerebellar dysfunction following Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:129–31.
Silamut K, Phu NH, Whitty C, Turner GD, Louwrier K, Mai NT, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol. 1999;155:395–410.
Anstey NM, Douglas NM, Poespoprodjo JR, Price RN. Plasmodium vivax: clinical spectrum, risk factors and pathogenesis. Adv Parasitol. 2012;80:151–201 A detailed review of P. vivax infection-related problems and their mechanisms.
Carvalho BO, Lopes SCP, Nogueira PA, Orlandi PP, Bargieri DY, Blanco YC, et al. On the cyto-adhesion of Plasmodium vivax—infected erythrocytes. J Infect Dis. 2010;202:638–47.
Markley JD, Edmond MB. Post-malaria neurological syndrome: a case report and review of the literature. J Travel Med. 2009;16:424–30.
Arya TVS, Prasad RN. Falciparum malaria presenting as Guillain-Barre syndrome. BMJ. 1986;292:1430.
Connor DH, Manz HJ. Parasitic infections of the peripheral nervous system. In: Dyck PJ, Thomas PK, editors. Peripheral neuropathy, vol. 2. Philadelphia: WB Saunders; 1993. p. 1338–90.
Drago SD, Sa ND, Golapalli U, et al. Guillian Barre syndrome in a case of falciparum malaria. J Assoc Physicians India. 1997;45:161.
Yamamoto K, Kato Y, Shinohara K, Kutsuna S, Takeshita N, Hayakawa K, Iwagami M, Kano S, Watanabe S, Ohmagari N. Case report: reversible cerebral vasoconstriction syndrome in cerebral malaria. Am J Trop Med Hyg. 2018;98(2):505–7. Herein the authors described a of cerebral malaria in a young subject, in whom the MRA findings indicated the involvement of reversible cerebral vasoconstriction syndrome.
Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible cerebral vasoconstriction syndrome, Part 1: epidemiology, pathogenesis, and clinical course. AJNR Am J Neuroradiol. 2015;36:1392–9.
Hajj-Ali RA, Calabrese LH. Diagnosis and classification of central nervous system vasculitis. J Autoimmun. 2014;48–49:149–52.
Milner DA Jr, Whitten RO, Kamiza S, Carr R, Liomba G, Dzamalala C, Seydel KB, Molyneux ME, Taylor TE. The systemic pathology of cerebral malaria in African children. Front Cell Infect Microbiol. 2014;4:104. Pediatric cerebral malaria carries a high mortality rate in sub-Saharan Africa. In this report the authors described detailed histopathological findings found at autopsy in the brain in such cases. Sequestration of parasites in the brain demonstrated two patterns: (a) the "classic" appearance (i.e., ring hemorrhages, dense sequestration, and extra-erythrocytic pigment) which was associated with evidence of systemic activation of coagulation and (b) the "sequestration only" appearance associated with shorter duration of illness and higher total burden of parasites in all organs including the spleen and gastrointestinal tract.
Newton CR, Marsh K, Peshu N, Kirkham FJ. Perturbations of cerebral hemodynamics in Kenyans with cerebral malaria. Pediatr Neurol. 1996;15:41–9 Highlights early observations on alterations in cerebral hemodynamics which later led to the recognition of RCVS and PRES in patients with severe malaria.
Moxon CA, Heyderman RS, Wassmer SC. Dysregulation of coagulation in cerebral malaria. Mol BiochemParasitol. 2009;166(2):99–108.
White VA, Lewallen S, Beare N, Kayira K, Carr RA, Taylor TE. Correlation of retinal haemorrhages with brain haemorrhages in children dying of cerebral malaria in Malawi. Trans R Soc Trop Med Hyg. 2001;95:618–21.
Murugavel K, Saravanapavananthan S, Anpalahan A, James RF. Subarachnoid haemorrhage in Plasmodium falciparum malaria. Postgrad Med J. 1989;65:236–7.
Mathur SL, Hakim A, Lodha R, Chowdhry P, Jain R. Subarachnoid haemorrhage in falciparum malaria: an unreported presentation. J Assoc Physicians India. 1992;40:348.
Saraswat DK. Case of cerebral malaria presenting as subarachnoid haemorrhage. J Assoc Physicians India. 1994;42:756.
Gall C, Spuler A, Fraunberger P. Subarachnoid hemorrhage in a patient with cerebral malaria. N Engl J Med. 1999;341:611–3. Subarachnoid hemorrhage due to plasmodium infection is very rare, but its incidence may be underestimated.
Pittella JE. Commentary. J Neurosci Rural Pract. 2014;5(3):321–2.
Chitkara AJ, Anand NK, Saini L, et al. Indian Pediatr. 1984;21:908–19.
Kalita J, Dhanuka AK, Misra UK. Cerebeller ataxia in patients with cerebral malaria. Neurol India. 1996;44:227–8. Cerebeller ataxia associated with acute malaria occurs InIndian and that the features are similar to those described from other parts of the world. This neurological complication of falciparum malaria has a good prognosis, resolving completely in virtually all cases.
Kochar DK, Kumawat BL, Kochar SK, et al. Delayed carebellar ataxia – a complication of Plasmodium falciparum malaria. J Assoc Physicians India. 1996;44:686–8. Cerebellar ataxia is an unusual post malarial complication. The authors encountered 10 such patients during the malaria epidemic in Rajasthan, India between 1992-1994. All the patients improved within one month without any residual deficit.
Kochar DK, Kumawat BL. Cerebeller ataxia in patients of falciparum malaria. Neurol India. 1997;45:118–9.
Lev D, Yahalom G, Rabinowicz S, Leshem E, Schwartz E. Opsoclonus-myoclonus as a presentation of post-malaria neurological syndrome. J Travel Med. 2020;27(4):taaa051. https://doi.org/10.1093/jtm/taaa051. Opsoclonus-myoclonus ataxia (OMA) syndrome is rare in children, mostly caused by neuroblastoma. OMA may occur as a very rare complication of falciparum malaria.
Bose K, Saha S, Islam R, et al. Opsoclonus myoclonus ataxia syndrome due to falciparum malaria in two Indian children. Indian J Ophthalmol. 2016;64(11):852–4. The Opsoclonus-myoclonus syndrome rarely developing in children with falciparum malaria responds well to high dose corticosteroids and intravenous immunoglobulins.
Puri AS, Rawal KK, Gupta R, et al. Precipitation of acute intermittent porphyria by chloroquine. Indian Pediatr. 1996;33:241–3.
Phillips-Howard P, ter Kuile F. CNS adverse events associated with antimalarial agents, fact or ficition ? Drug Saf. 1995;12:370–83.
Speich R, Haller A. Central anticholinergic syndrome with the antimalarial drug mefloquine. N Engl J Med. 1994;331:57–8.
van Hensbroek MB, Onyiorah E, Jaffar S, et al. A trial of aretemether or quinine in children with cerebral malaria. N Engl J Med. 1996;335:69–75. This trial carried out in Gambia showed that Artemether is as effective as quinine in the treatment of cerebral malaria in children.
Muller LG, Panosian CB. Ataxia and slurred speech after artisunate treatment for falciparum malaria. N Engl J Med. 1997;336:1328.
Plucinski MM, Guilavogui T, Sidikiba S, Diakité N, Diakité S, et al. Effect of the ebola-virus-disease epidemic on malaria case management in Guinea, 2014: a crosssectional survey of health facilities. Lancet Infect Dis. 2015;15:1017–23.
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–95.
Hussein MIH, Albashir AAD, Elawad OAMA, Homeida A. Malaria and COVID-19: unmasking their ties. Malar J. 2020;19:457–67 Critical evaluation of possible areas of interactions between the malarial parasite and the SARS-CoV-2 virus infection.
Singer M. Introduction to syndemics: a critical systems approach to public and community health. San Francisco: John Wiley & Sons; 2009.
Mulama DH, Bailey JA, Foley J, Chelimo K, Ouma C, Jura WGZO, Otieno J, Vulule J, Moormann AM. Sickle cell trait is not associated with endemic Burkitt lymphoma: an ethnicity and malaria endemicity-matched case-control study suggests factors controlling EBV may serve as a predictive biomarker for this pediatric cancer. Int J Cancer. 2014;134:645–53.
Kwenti TE. Malaria and HIV coinfection in sub-Saharan Africa: prevalence, impact, and treatment strategies. Res Rep Trop Med. 2018;9:123–36.
Gutman JR, Lucchi NW, Cantey PT, Steinhardt LC, et al. Malaria and parasitic neglected tropical diseases: potential syndemics with COVID-19? Am J Trop Med Hyg. 2020;103(2):572–7. Both malaria and parasitic Neglected Tropical Diseases (NTD) can alter immunologic responses to other infectious agents. Malaria can induce a cytokine storm and pro-coagulant state similar to that seen in severe COVID-19. Consequently, co-infections with malaria parasites and SARS-CoV-2 could result in substantially worse outcomes than mono-infections with either pathogen, and could shift the age pattern of severe COVID-19 to younger age-groups. Enhancing surveillance platforms could provide signals that indicate whether malaria, NTDs, and COVID-19 have caused syndemics (synergistic epidemics).
Akanmori BD, Kurtzhals JA, Goka BQ, Adabayeri V, Ofori MF, Nkrumah FK, Behr C, Hviid L. Distinct patterns of cytokine regulation in discrete clinical forms of Plasmodium falciparum malaria. Eur Cytokine Netw. 2000;11:113–8.
Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51.
Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care. 2017;21:305.
Silva LS, Silva-Filho JL, Caruso-Neves C, Pinheiro AAS. New concepts in malaria pathogenesis: the role of the renin-angiotensin system. Front Cell Infect Microbiol. 2016;5:103 This article draws attention to a mechanism that might be of much interest during possible co-infection with P. falciparum and the COVID-19 virus.
King T, Lamb T. Interferon-γ: the Jekyll and Hyde of malaria. PLoSPathog. 2015;11:e1005118.
Strayer D, Dickey R, Carter W. Sensitivity of SARS/MERS CoV to interferons and other drugs based on achievable serum concentrations in humans. Infect Disord Drug Targets. 2014;14:37–43.
Fauci AS, Lane HC, Redfeld RR. Covid-19—navigating the uncharted. N Engl J Med. 2020;382:1268–9.
Gomes LR, Martins YC, Ferreira-Da-Cruz MF, Daniel-Ribeiro CT. Autoimmunity, phospholipid-reacting antibodies and malaria immunity. Lupus. 2014;23:1295–8.
Lokken KL, Stull-Lane AR, Poels K, Tsolis RM. Malaria parasite-mediated alteration of macrophage function and increased iron availability predispose to disseminated non-typhoidal Salmonella infection. Infect Immun. 1918;86:e00301–18.
Mooney JP, Butler BP, Lokken KL, Xavier MN, Chau JY, Schaltenberg N, et al. The mucosal inflammatory response to non-typhoidal Salmonella in the intestine is blunted by IL-10 during concurrent malaria parasite infection. Mucosal Immunol. 2014;7:1302–11.
Thompson MG, Breiman RF, Hamel MJ, Desai M, et al. Influenza and malaria coinfection among young children in western Kenya, 2009–2011. J Infect Dis. 2012;206:1674–84.
Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers, Damp J, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020. https://doi.org/10.1016/j.thromres.2020.04.013. The incidence of thrombotic complications in ICU patients with COVID-19 infections is remarkably high. The authors reinforce the recommendation to strictly apply pharmacological thrombosis prophylaxis in all COVID-19 patients admitted to the ICU, and are strongly in favor of increasing the prophylaxis towards high-prophylactic doses, even in the absence of randomized evidence.
Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. N Engl J Med. 2020;382:e60.
Angchaisuksiri P. Coagulopathy in malaria. Thromb Res. 2014;133:5–9. Blood coagulation activation is frequently found in patients with malaria. Clinically apparent bleeding or disseminated intravascular coagulation (DIC) isassociated with very severe disease and a high mortality. Alterations in levels of several coagulation factors have been noted in plasmodium falciparum infections as compared to infections with Plasmodium vivax. It has been demonstrated that severe P. falciparum infection is associated with acute endothelial cell (EC) activation, abnormal circulating ultralarge vWF multimers, and a significant reduction in plasma ADAMTS13 function. These changes may result in intravascular platelet aggregation, thrombocytopenia, and microvascular disease.
Krishnan A, Karnad DR, Limaye U, Siddharth W. Cerebral venous and dural sinus thrombosis in severe falciparum malaria. J Infect. 2004;48:86–90. The diagnosis of cerebral venous sinus thrombosis should be suspected in patients with severe malaria who develop focal neurological deficits along with alteration in levels of consciousness. The diagnoses should be confirmed by appropriate imaging; judicious use of local thrombolytic therapy may help improve outcome.
Musoke C, Ssendikadiwa C, Babua C, Schwartz JI. Severe falciparum malaria associated with massive pulmonary embolism. Ann Afr Med. 2014;13:4. Microthrombotic complications are the best described in association with Plasmodium falciparum infections. Howevera number of cases of thrombosis involving larger vessels have also been reported..Herein, the authors described the case of a woman with malaria associated with massive pulmonary embolism.
Mohan A, Wara UU, Amjadh SW, et al. Malaria amidst COVID-19 in India: challenges, efforts, and recommendations. Clin Epidemiol Global Health. 2021;12:100867 An article of much public health importance in the Indian subcontinent and other developing countries with high case loads of both malaria and COVID19.
Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Coronavirus pandemic (COVID-19). Our World in Data. 2020;7:345. https://doi.org/10.1038/s41597-020-00688-8
Author information
Authors and Affiliations
Contributions
Both the authors had contributed equally to the preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Sweety Trivedi and Ambar Chakravarty each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology of Systemic Diseases
Rights and permissions
About this article
Cite this article
Trivedi, S., Chakravarty, A. Neurological Complications of Malaria. Curr Neurol Neurosci Rep 22, 499–513 (2022). https://doi.org/10.1007/s11910-022-01214-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-022-01214-6