Abstract
Purpose of the review
Neurocysticercosis (NCC) has been well recognized as a leading cause of epilepsy. More recently, studies of other parasitic diseases such as cerebral malaria (CM) and onchocerciasis are yielding novel insights into the pathogenesis of parasite-associated epilepsy. We compare the clinical and electrophysiological findings in epilepsy associated with these highly prevalent parasites and discuss the mechanisms involved in epileptogenesis.
Recent Findings
Electrophysiological and imaging biomarkers continue to emerge, and individuals who are at-risk of developing parasite-associated epilepsies are being identified with greater reliability. While both Taenia solium and Plasmodium falciparum directly affect the brain parenchyma, Onchocerca volvulus is not known to invade the central nervous system. Thus, the causal association between O. volvulus and epilepsy remains controversial.
Summary
Both NCC and CM have a well-defined acute phase when the parasites directly or indirectly invade the brain parenchyma and lead to local inflammatory changes. This is followed by a chronic phase marked by recurrent seizures. However, these stages of epileptogenic process have not been identified in the case of O. volvulus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epilepsy is one of the most common neurological disorders globally, affecting approximately 50 million people worldwide [1]. The causes of epilepsy are varied and multifactorial, including genetic and acquired etiologies, such as stroke, brain injury, and neurotoxicity [2]. Of the acquired etiologies, central nervous system (CNS) infections are an important acquired cause of seizures and epilepsy, especially in the low- and middle-income countries. The worldwide pool incidence rate of epilepsy is 61.4 per 100,000-person years (95% CI 50.7–74.4), with a higher incidence rate in low-/middle-income (LMI) countries than in high-income (HI) countries [3]. While there are numerous factors contributing to this disparity including socioeconomic status [4], this may also be due to the higher rates of CNS infections and traumatic brain injury in low-/middle-income countries [5, 6].
Of the infectious etiologies of acquired epilepsy, bacteria, viruses, fungi, and parasites are well-known causes of seizures worldwide. Epilepsy has been long recognized as sequelae of meningitis and encephalitis [7], with a higher incidence in LMI countries [8] than in HI countries. Per Baraff et al., the mean probability of unprovoked seizures after bacterial meningitis was 4.2% [9] in a meta-analysis of 45 studies. The three most common causes of meningitis are meningococcus, pneumococcus, and Haemophilus influenzae B (HiB). However, due to factors such as vaccination and access to healthcare, the distribution and proportion of meningitis caused by these bacteria are different between LMI and HI countries. The causative viruses are far more numerous varying widely in geographic distribution, and the most well-known include Herpes simplex virus, Japanese encephalitis, West Nile virus, human herpes virus 6, and human immunodeficiency virus (HIV) [10]. It is believed that the inflammatory response to the viral and bacterial pathogens plays a key role in epileptogenesis [11,12,13]. Fortunately, many of the viral and bacterial infections of the brain now can be prevented with vaccines and likely reduce the overall burden of infectious epilepsy through primary prevention.
In contrast to most bacterial and viral infection of brain, parasitic infection usually affects the host chronically [14]. This warrants parasitic causes of epilepsy to be considered with special importance. While highly diverse, parasites can be classified into single-celled organism protozoa and multicellular helminths (worms). There is a significant disparity in the incidence of parasitic diseases between LMI and HI countries, and indeed, most burden of helminth infections worldwide occur in LMI countries [15], where 80% of people with epilepsy reside. In sub-Saharan Africa, exposure to parasitic infection has been associated with an increased prevalence of epilepsy and, importantly, co-infection with multiple parasites has been found to increase the risk of epilepsy [16].
Most parasites known to infect the brain have been implicated in causing seizures. Neurocysticercosis (NCC) has been most well recognized as a leading cause of epilepsy, and recent studies of other parasitic diseases including cerebral malaria (CM) and onchocerciasis are yielding novel insight into the pathogenesis of parasite-associated epilepsy. Understanding the mechanism(s) by which parasitic infections may cause epilepsy will help elucidate the pathophysiology involved in acquired etiologies of epilepsy as well. In this review, we evaluate and compare the clinical and electrophysiological findings in epilepsy associated with common and highly prevalent parasites of interest and discuss pathomechanism involved in epileptogenesis.
Parasite and Epilepsies
Neurocysticercosis
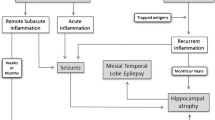
One of the most frequently studied and recognized parasitic causes of epilepsy is neurocysticercosis (NCC), an infection of the CNS by the larvae of Taenia solium. Although infection with NCC does not always lead to seizures, patients who are symptomatic constitute a significant proportion of persons with epilepsy in endemic regions of Latin America and Asia [17, 18]. Given the high seroprevalence of exposure to multiple parasites infections in low-resource regions, establishing a causal relationship between parasite and epilepsy has been controversial [19]. However, treatment of persons with viable parenchymal cysts with anti-helminthic albendazole showed decreased risk of seizure recurrence, supporting the role of NCC in the epileptogenic process [20, 21]. From a public health perspective, epilepsy associated with NCC is especially important given the preventable nature of the disease is exacerbated by the lack of resources (i.e., availability of diagnostic tools, physicians, and both surgical and pharmacological treatment options) [22].
Consensus about the process by which NCC causes seizures involves two complex and intertwined mechanisms of epileptogenesis: structural change to the CNS parenchyma [10, 17] caused by the larval cyst and an inflammatory reaction to infection [11]. The parasite likely disrupts the blood–brain barrier and enables influx of inflammatory cells into the parenchyma, consistent with the finding that patients with NCC have higher matrix metalloproteinases (a molecule involved in the breakdown of the blood–brain barrier) compared to healthy individuals [23]. The parasite itself undergoes unique distinguishable changes within the brain: a viable cyst, which does not illicit much inflammatory response, followed by a degeneration of the cyst with surrounding brain edema and, subsequent formation of a granuloma that sometimes leads to calcified brain lesions [24]. Each stage of infection might illicit variable inflammatory responses and determine the overall course of NCC-associated epilepsy [25].
There is evidence from electroencephalogram (EEG) and imaging studies to suggest that the NCC-related structural lesions in the brain parenchyma and recurrent inflammation may also lead to hippocampal sclerosis and subsequent mesial temporal lobe epilepsy [26, 27]. However, it is important to note that while dual pathology of NCC-related lesions and hippocampal sclerosis frequently exists, a causal relationship between the two is yet to established [26]. Typical seizure semiology observed in NCC is focal. EEG abnormalities with NCC are commonly focal as well, manifesting as slowing and epileptiform discharges. While there could be a clinico-electrographic correlation with the anatomical location of the cyst-associated lesions [28, 29], the electrophysiological abnormalities are not always concordant with the site of the NCC lesions [30,31,32]. Follow-up studies of people with NCC found that seizures occur both acutely and in the chronic phase of the infection and about fifty percent of individuals have recurrence of seizures (defined as occurrence of any seizures after one week of index seizure). The persistence of brain lesions on imaging is particularly associated with increased the risk of seizure recurrence [33]. Moreover, younger individuals and persons with a higher burden of cysts are more likely to experience seizures [34]. Unlike the risk of clinical seizures, the electrographic abnormalities do not depend on the burden of the lesion [35, 36]. Interestingly, persons with NCC-associated epilepsy who have electrographic abnormalities on EEG are also more likely to have hippocampal atrophy [37•]. These electroclinical findings reinforce the hypothesis that epileptogenesis in NCC could be mediated through hippocampal sclerosis [38].
Malaria
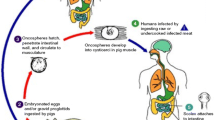
Similar to neurocysticercosis, malaria is another common parasitic disease endemic to many low- and middle-income countries in sub-Saharan Africa, several regions of south and southeast Asia, and South America. Caused primarily by five species of Plasmodium (P. vivax, P. ovale, P. malariae, P. knowlesi, and P. falciparum), malaria is a mosquito-borne parasitic disease that can manifest in various organ systems, with cerebral malaria (CM) as one of the most common causes of seizures in Southeast Asia and Africa [39, 40]. Among the five species of Plasmodium known to cause disease in human, P. falciparum has the greatest propensity to cause the most malignant form of malaria, i.e., cerebral malaria [39]. The life cycle of this parasite and the pathophysiology of malarial disease has been well established in the literature [41], though the exact mechanism of epileptogenesis is still under investigation.
Plasmodium does not invade the brain directly, as it remains inside erythrocytes. Parasite-infected red blood cells sequestered in the intravascular space subsequently cause perivascular damage, which is believed to be the primary step in ictogenesis [42, 43]. This is followed by loss of perfusion to the parenchyma leading to localized ischemia of the brain tissue. This structural change to neurovasculature and its subsequent effect on the brain parenchyma is believed to induce an inflammatory reaction [44], mirroring the mechanisms of epileptogenesis hypothesized in neurocysticercosis. In addition, there are other mechanisms of seizure generation in malaria, though these are often sequelae of non-neurological systemic effects of malaria such as hypoglycemia, severe metabolic acidosis, and shock [45].
Cerebral malaria, a complicated form of malaria, is clinically defined as unarousable coma following the correction of hypoglycemia, detection of Plasmodium parasite in the peripheral blood, and absence of any other causes of encephalopathy [46]. Clinically, seizures are commonly seen during the acute phase of both cerebral and uncomplicated form of malaria [47]. About 10% of survivors with CM develop epilepsy within two years [47]. High fever and acute seizures during CM increase the risk of subsequent malaria associated epilepsy [47]. The semiology of seizures associated with CM is typically focal with or without secondary generalization [10, 48, 49]. Status epilepticus also occurs frequently and may contribute to epileptogenesis [50].
In the attempt to further understand CM-associated epilepsy, EEG has been a powerful tool in investigation, treatment, and prognosis [51, 52]. EEG findings during hospitalization predicted morbidity and mortality among of children with malaria [52]. Acutely, electrographic features found on EEG review include focal epileptiform discharges, most commonly in the parieto-temporal regions [50]. A prospective follow-up of 70 children with CM found that while there is no statistically significant difference in the EEG on visual inspection between persons with and without epilepsy, quantitative spectral analysis showed a higher gamma-delta ratio in children with epilepsy who survive CM [53]. This suggests that the presence of gamma activity might serve as a potential biomarker in epileptogenesis in CM, similar to other epilepsies [54].
More than 80% of individuals with CM-associated epilepsy continue to have seizures despite treatment with available epileptic drugs [47]. As EEG and imaging biomarkers continue to emerge and at-risk individuals are identified with greater reliability, strategies to prevent CM-associated epilepsy must be prioritized.
Onchocerciasis
More recently, epilepsy due to exposure to the arthropod-borne nematode Onchocerca volvulus has gained attention [55]. Unlike T. solium and Plasmodium which invade the brain parenchyma, Onchocerca volvulus is not known to directly enter the CNS [56]. Thus, the causal association between Onchocerca volvulus and epilepsy has been questioned. However, several epidemiological studies have found an a strong association between the parasite O. volvulus and epilepsy [57, 58] and a dose–response relationship was reported between exposure to O. volvulus and the risk of developing epilepsy [59, 60••]. Several researchers have proposed an immune-mediated hypothesis in the pathogenesis of epilepsy associated with O. volvulus [61, 62]. This hypothesis is supported by the recent discovery of antibodies against leiomodin-1 and DJ-1 in nodding syndrome, an endemic form of epilepsy strongly associated with O. volvulus infection [63]. The theorized pathogenesis of epilepsy in such cases involves parasitic infection resulting in the induction of both the innate and adaptive immune responses. This in turn promotes neuroinflammation, as inflammatory molecules cross the blood–brain barrier. Ultimately, this neuroinflammatory response is believed to result in disruption of neural networks and, subsequent, epileptogenesis [62]. There is already precedent for an immune-mediated disease process associated with O. volvulus, as in the cases of “river blindness” [64] where immune response to the parasite can cause sclerosing keratitis, chorioretinitis, or optic neuritis. In the case of other recently discovered immune-mediated epilepsies such as NMDAR and anti-LGI1, treatment of the underlying autoimmune disorder often results in improvement of seizures, suggesting that the seizures are driven by the inflammatory process [65,66,67]. It remains to be seen if anti-inflammatory treatments could alter the course of O. volvulus-associated epilepsies.
Multiple seizure types and epilepsy subtypes have been associated with O. volvulus, including endemic forms of epilepsy syndromes such as nodding syndrome and Nakalanga syndrome [62, 68,69,70]. Generalized tonic–clonic seizures are commonly reported semiology associated with O. volvulus infection [71]. Subtypes of O. volvulus-associated epilepsy include nodding syndrome, an epileptic encephalopathy characterized by atonic seizures with repetitive dorso-ventral head drop, from which the name of the syndrome is derived [72]. The Nakalanga phenotype associated with O. volvulus is defined by growth retardation, physical deformities (including severe kyphosis), endocrine dysfunction and generalized tonic–clonic seizures [69]. Electrographic findings of O. volvulus-associated epilepsies are also varied, reflecting the clinical heterogeneity. Interictal electroencephalography often revealed bifronto-temporal spike and slow wave epileptiform discharges [68]. Moreover, other findings include frequent generalized slowing of the background activity and, in some focal epileptiform activities [73]. In nodding syndrome, the EEG is very different from what is typically encountered in other forms of epilepsy associated with O. volvulus. Interictal EEG is dominated by generalized slow spike-and-wave discharges, as expected with many epileptic encephalopathies [72]. Further details of epileptiform abnormalities associated with nodding syndrome may be found in another comprehensive review [72].
It is unclear whether the clinical heterogeneity of epilepsies associated with O. volvulus parasite is due to a misattribution of the cause and a limitation in knowledge regarding other etiological factors. Further studies are needed to evaluate if the parasite O. volvulus causes heterogenous forms of epilepsies with variable electroclinical features. Imaging and electroclinical studies with detailed phenotyping of the varied epilepsies associated with O. volvulus remain scarce.
Comparing the natural history of the aforementioned parasitic diseases, a stark distinction between O. volvulus and the other two parasites, T. solium and P. falciparum, becomes apparent. In both NCC and CM, there is an acute phase of new-onset seizures as the parasitic invasion of the brain parenchyma leads to local inflammatory changes, which is then followed by a chronic phase of recurrent seizures. In epilepsies associated with O. volvulus parasitic infection, however, the latency of developing the disease remains unknown. Further complicating the matter is the lack of any imaging or neuropathological evidence of parasitic invasion of the brain parenchyma. In addition, chronic inflammatory changes involving macrophage clusters have been noted in a minority of cases [74]. An important point to emphasize is that a causal relationship between O. volvulus and the development of epilepsy has not yet been definitively established and further research is needed.
Host Genetics in Parasitic Infection and Epilepsy
Parasites have co-evolved with humans and provided selective pressure on host genetics [75]. Thus, when evaluating the effect of parasites on epileptogenesis, the human–host factors also need to be understood. Several studies have found evidence of host-genetic susceptibility that leads to an increased risk of neuronal injury due to the parasitic infection [76,77,78,79,80, 81•, 82, 83•]. However, the exact mechanism by which host genetics interact with exposure to a parasitic infection and lead to epilepsy remains unknown. Several possibilities could be considered: a) host-genetic predisposition increases the risk of a parasitic infection; b) host genetics affect the immune-response to a parasite leading to epileptogenesis; and c) chronic parasitic infections change the inflammatory milieu and thus lower the seizure threshold in individuals who are genetically susceptible to developing seizures, acting as a “second hit” in the epileptogenic process.
Persons with CM-associated epilepsy commonly have first-degree relatives with epilepsy in their family [76, 77]. A multi-site study involving four African countries found that polymorphism in genes involved in the inflammatory pathways such as IL-10 are associated with acute seizures [78]. The study also found that the association of the genetic polymorphism differed based on the seizure phenotype and the community where the participants were enrolled. Furthermore, host-genetic variants affecting erythrocyte function and tight-junction proteins in the endothelial cells increase the susceptibility to severe malaria infection [79]. Future studies are necessary to evaluate how these varied biological pathways and susceptibility factors ultimately lead to epileptogenesis.
In people with neurocysticercosis, host genetics is also known to play an important role in epileptogenesis. Polymorphisms in Toll-like receptor- 4, which plays an important role in the immune response against NCC within the central nervous system, have been associated with seizure recurrence due to NCC [80, 81•]. Specific genetic variants are more likely to trigger neuroinflammation and subsequent provocation of seizures. One study from Mexico found an association between developing severe NCC and polymorphisms in TRAF-1 (a member of the TNF receptor family) and C5 (a complement component that acts as anaphylatoxins and chemotactic factor). HLA genotyping among families affected with NCC has revealed inherited susceptibility of epilepsy associated with certain HLA haplotypes [82].
Similarly, in O. volvulus-associated epilepsy including nodding syndrome, HLA haplotypes have been found to be associated with both protection and susceptibility to the disorder [83•]. Authors have argued that the presence of HLA variants may explain the heterogeneity of the O. volvulus-associated epilepsies and why under similar infectious exposure there is such variation in the phenotype, including unaffected family members [83•]. These studies highlight the importance of further research into the role of host genetics in infectious etiologies of acquired epilepsy. Clarifying these pathways may allow for the development of novel anti-epileptogenic treatment mechanisms and achieve primary prevention of epilepsy.
Conclusion
Infectious etiologies of epilepsy, particularly, parasitic diseases, play a significant role in the burden of neurological disorders worldwide. While primary parasitic infections are preventable, they are hindered by access to resources along with the disparate geographic distribution of the infectious agents. In addition to primary prevention of parasitic infection, future research efforts should focus on understanding how these infectious agents cause neuronal injury that ultimately leads to epilepsy. Electrophysiological and imaging biomarkers continue to emerge, and individuals who are at-risk of developing parasite-associated epilepsies are being identified with greater reliability.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Epilepsy. https://www.who.int/news-room/fact-sheets/detail/epilepsy. Accessed 12 Apr 2021
Thomas RH, Berkovic SF. The hidden genetics of epilepsy - A clinically important new paradigm. Nat Rev Neurol. 2014;10:283–92.
Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, Pringsheim T, Lorenzetti DL, Jetté N. Prevalence and incidence of epilepsy. Neurology. 2017;88:296–303.
Beghi E, Hesdorffer D. Prevalence of epilepsy - An unknown quantity. Epilepsia. 2014;55:963–7.
Beghi E. The Epidemiology of Epilepsy. Neuroepidemiology. 2020;54:185–91.
Feigin VL, Nichols E, Alam T, et al. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–80.
Marks DA, Kim J, Spencer DD, Spencer SS. Characteristics of intractable seizures following meningitis and encephalitis. Neurology. 1992;42:1513–8.
Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: A systematic review and meta-analysis. Lancet Infect Dis. 2010;10:317–28.
Baraff LJ, Lee SI, Schriger DL. Outcomes of bacterial meningitis in children: A meta-analysis. Pediatr Infect Dis J. 1993;12:389–94.
Singhi P. Infectious causes of seizures and epilepsy in the developing world. Dev Med Child Neurol. 2011;53:600–9.
Vezzani A, Fujinami RS, White HS, Preux PM, Blümcke I, Sander JW, Löscher W. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131:211–34.
Marchi N, Granata T, Janigro D. Inflammatory pathways of seizure disorders. Trends Neurosci. 2014;37:55–65.
Kwon YS, Pineda E, Auvin S, Shin D, Mazarati A, Sankar R. Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J Neuroinflammation. https://doi.org/10.1186/1742-2094-10-30
Sacks DL. Vaccines against tropical parasitic diseases: A persisting answer to a persisting problem. Nat Immunol. 2014;15:403–5.
Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: The great neglected tropical diseases. J Clin Invest. 2008;118:1311–21.
Kamuyu G, Bottomley C, Mageto J, et al. Exposure to Multiple Parasites Is Associated with the Prevalence of Active Convulsive Epilepsy in Sub-Saharan Africa. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0002908
Garcia HH. Neurocysticercosis. Neurol Clin. 2018;36:851–64.
Jallon P. Epilepsy in developing countries. In: Epilepsia. Epilepsia, pp 1143–1151
Wagner RG, Newton CR. Do helminths cause epilepsy? Parasite Immunol. 2009;31:697–705.
Garcia HH, Pretell EJ, Gilman RH, Martinez SM, Moulton LH, Del Brutto OH, Herrera G, Evans CAW, Gonzalez AE. A Trial of Antiparasitic Treatment to Reduce the Rate of Seizures Due to Cerebral Cysticercosis. N Engl J Med. 2004;350:249–58.
Monk EJ, Abba K, Ranganathan LN. Anthelmintics for people with neurocysticercosis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD000215.pub5
Reddy DS, Volkmer R. Neurocysticercosis as an infectious acquired epilepsy worldwide. Seizure. 2017;52:176–81.
Verma A, Prasad KN, Nyati KK, Singh SK, Singh AK, Paliwal VK, Gupta RK. Association of MMP-2 and MMP-9 with clinical outcome of neurocysticercosis. Parasitology. 2011;138:1423–8.
Nash TE, Del Brutto OH, Butman JA, et al. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–8.
Fujita M, Mahanty S, Zoghbi SS, De Ferraris Araneta M, Hong J, Pike VW, Innis RB, Nash TE. PET Reveals Inflammation around Calcified Taenia solium Granulomas with Perilesional Edema. PLoS One. https://doi.org/10.1371/journal.pone.0074052
Del Brutto OH, Engel J, Eliashiv DS, García HH. Update on Cysticercosis Epileptogenesis: the Role of the Hippocampus. Curr Neurol Neurosci Rep. 2016;16:1–7.
Rathore C, Thomas B, Kesavadas C, Radhakrishnan K. Calcified neurocysticercosis lesions and hippocampal sclerosis: Potential dual pathology? Epilepsia. https://doi.org/10.1111/j.1528-1167.2011.03386.x
Duque KR, Burneo JG. Clinical presentation of neurocysticercosis-related epilepsy. Epilepsy Behav. 2017;76:151–7.
Nash TE, Bustos JA, Garcia HH. Disease Centered Around Calcified Taenia solium Granuloma. Trends Parasitol. 2017;33:65–73.
Velasco TR, Zanello PA, Dalmagro CL, et al. Calcified cysticercotic lesions and intractable epilepsy: A cross sectional study of 512 patients. J Neurol Neurosurg Psychiatry. 2006;77:485–8.
del Brutto OHD, Santibañez R, Noboa CA, Aguirre R, Díaz E, Alarcón TA. Epilepsy due to neurocysticercosis: Analysis of 203 patients. Neurology. 1992;42:389–92.
Issa NP, Sedler MJ, Del Brutto VJ, Darsan E, Milla L, Montes J, Zambrano M, Del Brutto OH. EEG patterns in patients with calcified neurocysticercosis with or without hippocampal atrophy. J Clin Neurophysiol. 2018;35:232–8.
Carpio A, Hauser WA. Prognosis for seizure recurrence in patients with newly diagnosed neurocysticercosis. Neurology. 2002;59:1730–4.
Kelvin EA, Carpio A, Bagiella E, Leslie D, Leon P, Andrews H, Hauser WA. Seizure in people with newly diagnosed active or transitional neurocysticercosis. Seizure. 2011;20:119–25.
Chayasirisobhon S, Menoni R, Chayasirisobhon W, Locke GE. Correlation of Electroencephalography and the Active and Inactive Forms of Neurocysticercosis. Clin EEG Neurosci. 1999;30:9–11.
Murthy JMK, Sreenivas RV. Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion. Seizure. 1998;7:153–7.
• Del Brutto OH, Mera RM, Wu S, Recalde BY, Issa NP. Epilepsy, interictal EEG abnormalities and hippocampal atrophy in patients with calcified neurocysticercosis: a population study in an endemic milieu. Epileptic Disord. 2021;23:357–365. The burden of infection, and subsequent recurrent inflammation around calcified cysts, contributes to hippocampal atrophy development in neurocysticercosis.
Nash TE, Mahanty S, Loeb JA, et al. Neurocysticercosis: A natural human model of epileptogenesis. Epilepsia. 2015;56:177–83.
Ngoungou EB, Preux PM. Cerebral malaria and epilepsy. Epilepsia. 2008;49:19–24.
White NJ. Plasmodium knowlesi: The fifth human malaria parasite. Clin Infect Dis. 2008;46:172–3.
Garcia LS. Malaria. Clin Lab Med. 2010;30:93–129.
White NJ, Ho M. The Pathophysiology of Malaria. Adv Parasitol. 1992;31:83–173.
Newton CRJC, Hien TT, White N. Cerebral malaria. J Neurol Neurosurg Psychiatry. 2000;69:433–41.
Löscher W, Gernert M, Heinemann U. Cell and gene therapies in epilepsy - promising avenues or blind alleys? Trends Neurosci. 2008;31:62–73.
Idro R, Marsh K, John CC, Newton CRJ. Cerebral malaria: Mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res. 2010;68:267–74.
Idro R, Jenkins NE, Newton CRJ. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–40.
Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, Taylor TE. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: A prospective cohort study. Lancet Neurol. 2010;9:1173–81.
Ogutu BR, Newton C. Management of seizures in malaria. 2004;71–75.
Carter JA, Neville BGR, White S, Ross AJ, Otieno G, Mturi N, Musumba C, Newton CRJC. Increased prevalence of epilepsy associated with severe falciparum malaria in children. Epilepsia. 2004;45:978–81.
Crawley J, Smith S, Kirkham F, Muthinji P, Waruiru C, Marsh K. Seizures and status epilepticus in childhood cerebral malaria. QJM - Mon J Assoc Physicians. 1996;89:591–7.
Crawley J, Smith S, Muthinji P, Marsh K, Kirkham F. Electroencephalographic and clinical features of cerebral malaria. Arch Dis Child. 2001;84:247–53.
Postels DG, Wu X, Li C, et al. Admission EEG findings in diverse paediatric cerebral malaria populations predict outcomes. Malar J. https://doi.org/10.1186/s12936-018-2355-9
Patel AA, Jannati A, Dhamne SC, Sapuwa M, Kalanga E, Mazumdar M, Birbeck GL, Rotenberg A. EEG markers predictive of epilepsy risk in pediatric cerebral malaria – A feasibility study. Epilepsy Behav. https://doi.org/10.1016/j.yebeh.2020.107536
Nariai H, Beal J, Galanopoulou AS, Mowrey WB, Bickel S, Sogawa Y, Jehle R, Shinnar S, Moshé SL. Scalp EEG Ictal gamma and beta activity during infantile spasms: Evidence of focality. Epilepsia. 2017;58:882–92.
Colebunders R, Njamnshi AK, Menon S, Newton CR, Hotterbeekx A, Preux PM, Hopkins A, Vaillant M, Fodjo JNS. Onchocerca volvulus and epilepsy: A comprehensive review using the bradford hill criteria for causation. PLoS Negl Trop Dis. 2021;15:1–24.
Hotterbeekx A, Raimon S, Abd-Elfarag G, et al. Onchocerca volvulus is not detected in the cerebrospinal fluid of persons with onchocerciasis-associated epilepsy. Int J Infect Dis. 2020;91:119–23.
Mmbando BP, Suykerbuyk P, Mnacho M, Kakorozya A, Matuja W, Hendy A, Greter H, Makunde WH, Colebunders R. High prevalence of epilepsy in two rural onchocerciasis endemic villages in the Mahenge area, Tanzania, after 20 years of community directed treatment with ivermectin. Infect Dis Poverty. https://doi.org/10.1186/s40249-018-0450-3
Kaiser C, Pion SDS, Boussinesq M. Case-control Studies on the Relationship between Onchocerciasis and Epilepsy: Systematic Review and Meta-analysis. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0002147
Chesnais CB, Bizet C, Campillo JT, Njamnshi WY, Bopda J, Nwane P, Pion SD, Njamnshi AK, Boussinesq M. A Second Population-Based Cohort Study in Cameroon Confirms the Temporal Relationship Between Onchocerciasis and Epilepsy. Open Forum Infect Dis. https://doi.org/10.1093/ofid/ofaa206
•• Chesnais CB, Nana-Djeunga HC, Njamnshi AK, Lenou-Nanga CG, Boullé C, Bissek ACZK, Kamgno J, Colebunders R, Boussinesq M. The temporal relationship between onchocerciasis and epilepsy: a population-based cohort study. Lancet Infect Dis. 2018;18:1278–1286. O. volvulus infection in childhood was associated with the risk of either seizures or epilepsy in an onchocerciasis focus in Cameroon.
Levite M, Zelig D, Friedman A, et al. Dual-Targeted Autoimmune Sword in Fatal Epilepsy: Patient’s glutamate receptor AMPA GluR3B peptide autoimmune antibodies bind, induce Reactive Oxygen Species (ROS) in, and kill both human neural cells and T cells. J Autoimmun. https://doi.org/10.1016/j.jaut.2020.102462
Johnson TP, Sejvar J, Nutman TB, Nath A. The Pathogenesis of Nodding Syndrome. Annu Rev Pathol Mech Dis. 2020;15:395–417.
Johnson TP, Tyagi R, Lee PR, et al. Nodding syndrome may be an autoimmune reaction to the parasitic worm Onchocerca volvulus. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaf6953
Pearlman E, Hall LR. Immune mechanisms in Onchocerca volvulus-mediated corneal disease (river blindness). In: Parasite Immunol. Parasite Immunol, pp 625–631
Dutra LA, Abrantes F, Toso FF, Pedroso JL, Barsottini OGP, Hoftberger R. Autoimmune encephalitis: A review of diagnosis and treatment. Arq Neuropsiquiatr. 2018;76:41–9.
Hermetter C, Fazekas F, Hochmeister S. Systematic review: Syndromes, early diagnosis, and treatment in autoimmune encephalitis. Front Neurol. https://doi.org/10.3389/fneur.2018.00706
Höftberger R. Neuroimmunology: An expanding frontier in autoimmunity. Front Immunol. https://doi.org/10.3389/fimmu.2015.00206
Morin A, Guillaume M, Ngarka L, et al. Epilepsy in the Sanaga‐Mbam valley, an onchocerciasis‐endemic region in Cameroon: electro‐clinical and neuropsychological findings. Epilepsia Open. https://doi.org/10.1002/epi4.12510
Föger K, Gora-Stahlberg G, Sejvar J, Ovuga E, Jilek-Aall L, Schmutzhard E, Kaiser C, Winkler AS. Nakalanga Syndrome: Clinical Characteristics, Potential Causes, and Its Relationship with Recently Described Nodding Syndrome. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0005201
Sejvar JJ, Kakooza AM, Foltz JL, et al. Clinical, neurological, and electrophysiological features of nodding syndrome in Kitgum, Uganda: An observational case series. Lancet Neurol. 2013;12:166–74.
Siewe Fodjo JN, Mandro M, Mukendi D, et al. Onchocerciasis-associated epilepsy in the Democratic Republic of Congo: Clinical description and relationship with microfilarial density. PLoS Negl Trop Dis. https://doi.org/10.1371/journal.pntd.0007300
Spencer PS, Mazumder R, Palmer VS, Pollanen MS. Nodding syndrome phenotypes. Rev Neurol (Paris). 2019;175:679–85.
Ogwang R, Ningwa A, Akun P, et al. Epilepsy in Onchocerca volvulus Sero-Positive Patients From Northern Uganda—Clinical, EEG and Brain Imaging Features. Front Neurol. 2021;12:687281.
Hotterbeekx A, Lammens M, Idro R, et al. Neuroinflammation and not tauopathy is a predominant pathological signature of nodding syndrome. J Neuropathol Exp Neurol. 2019;78:1049–58.
Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, Clerici M, Bresolin N, Sironi M. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206:1395–408.
Thierry A, Falilatou A, Covalic B, Elodie D, Mendinatou A, Didier A, Alphonse N, Joseph A. Epilepsy and Malaria in Children Aged 1 to 15 Years in Parakou in 2018: Case-Control Study. Child Neurol Open. 2020;7:2329048X2095411.
Versteeg AC, Carter JA, Dzombo J, Neville BG, Newton CRJC. Seizure disorders among relatives of Kenyan children with severe falciparum malaria. Trop Med Int Heal. 2003;8:12–6.
Kariuki SM, Rockett K, Clark TG, Reyburn H, Agbenyega T, Taylor TE, Birbeck GL, Williams TN, Newton CRJC. The genetic risk of acute seizures in African children with falciparum malaria. Epilepsia. 2013;54:990–1001.
Timmann C, Thye T, Vens M, et al. Genome-wide association study indicates two novel resistance loci for severe malaria. Nature. 2012;489:443–6.
Lachuriya G, Garg RK, Jain A, Malhotra HS, Singh AK, Jain B, Kumar N, Verma R, Sharma PK. Toll-like Receptor-4 Polymorphisms and Serum Matrix Metalloproteinase-9 in Newly Diagnosed Patients with Calcified Neurocysticercosis and Seizures. Medicine. 2016; 17-pe3288.
• Villegas M, Sciutto E, Rosetti M, Fleury A, Fragoso G. Association of TRAF1/C5 locus polymorphisms with epilepsy and clinical traits in Mexican patients with neurocysticercosis. Infect Immun. 2019; 87(12):e00347–19. Polymorphism in C5 and TRAF1 play a role in the risk of developing severe neurocysticercosis in the Mexican population.
Jain S, Padma MV, Kanga U, Mehra NK, Puri A, Maheshwari MC. Family studies and human leukocyte antigen class II typing in Indian probands with seizures in association with single small enhancing computed tomography lesions. Epilepsia. 1999;40:232–8.
• Benedek G, El Latif MA, Miller K, et al. Protection or susceptibility to devastating childhood epilepsy: Nodding syndrome associates with immunogenetic fingerprints in the hla binding groove. PLoS Negl Trop Dis. 2020;14:1–15. The authors found that immunogenetic fingerprints in HLA peptide-binding grooves probably are associated with protection and/or susceptibility to nodding syndrome.
Waruiru CM, Newton CRJC, Forster D, New L, Winstanley P, Mwangi I, Marsh V, Winstanley M, Snow RW, Marsh K. Epileptic seizures and malaria in Kenyan children. Trans R Soc Trop Med Hyg. 1996;90:152–5.
Funding
This study was supported by Department of Neurology, David Geffen School of Medicine, University of California, Los Angeles.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Rajarshi Mazumder and John K Lee declare that they do not have any conflict of interest.
Human and Animal Ethics and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Epilepsy
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mazumder, R., Lee, J.K. Epileptogenesis in Common Parasitic Infections. Curr Neurol Neurosci Rep 22, 285–291 (2022). https://doi.org/10.1007/s11910-022-01187-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-022-01187-6