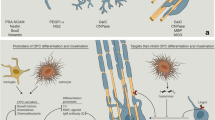
Abstract
Purpose of Review
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system (CNS). Inflammatory attacks in MS lead to both demyelination and axonal damage. However, due to incomplete remyelination most MS lesions remain chronically demyelinated. In parallel, there is axonal degeneration in the CNS of MS patients, contributing to progressive disability. There are currently no approved therapies that adequately restore myelin or protect axons from degeneration. In this review, we will discuss the pathophysiology of axonal loss and chronic demyelination in MS and how understanding this pathophysiology is leading to the development of new MS therapeutics.
Recent Findings
Ongoing research into the function of oligodendrocytes and myelin has revealed the importance of their relationship with neuronal health. Demyelination in MS leads to a number of pathophysiologic changes contributing to axonal generation. Among these are mitochondrial dysfunction, persistent neuroinflammation, and the effects of reactive oxygen and nitrogen species. With this information, we review currently approved and investigational therapies designed to restore lost or damaged myelin and protect against neuronal degeneration.
Summary
The development of therapies to restore lost myelin and protect neurons is a promising avenue of investigation for the benefit of patients with MS.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wallin MT, Culpepper WJ, Nichols E, Bhutta ZA, Gebrehiwot TT, Hay SI, et al. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology. 2019;18(3):269–85. https://doi.org/10.1016/s1474-4422(18)30443-5.
Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029–e40. https://doi.org/10.1212/WNL.0000000000007035.
Feigin VL, Abajobir AA, Abate KH, Abd-Allah F, Abdulle AM, Abera SF, et al. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Neurology. 2017;16(11):877–97. https://doi.org/10.1016/s1474-4422(17)30299-5.
• Paz-Zulueta M, Parás-Bravo P, Cantarero-Prieto D, Blázquez-Fernández C, Oterino-Durán A. A literature review of cost-of-illness studies on the economic burden of multiple sclerosis. Multiple Sclerosis and Related Disorders. 2020;43:102162. https://doi.org/10.1016/j.msard.2020.102162This review details the evidence demonstrating the well-known large economic burden of multiple sclerosis, highlighting the continued need for improved therapies and outcomes.
Rommer PS, Milo R, Han MH, Satyanarayan S, Sellner J, Hauer L, et al. Immunological aspects of approved MS therapeutics. Front Immunol. 2019;10:1564. https://doi.org/10.3389/fimmu.2019.01564.
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. New England Journal of Medicine. 2017;376(3):209–20. https://doi.org/10.1056/nejmoa1606468.
Patti F, Visconti A, Capacchione A, Roy S, Trojano M. Long-term effectiveness in patients previously treated with cladribine tablets: a real-world analysis of the Italian multiple sclerosis registry (CLARINET-MS). Therapeutic Advances in Neurological Disorders. 2020;13:175628642092268. https://doi.org/10.1177/1756286420922685.
Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. The Lancet. 2018;391(10127):1263–73. https://doi.org/10.1016/s0140-6736(18)30475-6.
Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. The Lancet. 2017;390(10111):2481–9. https://doi.org/10.1016/s0140-6736(17)32346-2.
Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17(1):R29–35. https://doi.org/10.1016/j.cub.2006.11.042.
Ford MC, Alexandrova O, Cossell L, Stange-Marten A, Sinclair J, Kopp-Scheinpflug C, et al. Tuning of Ranvier node and internode properties in myelinated axons to adjust action potential timing. Nat Commun. 2015;6:8073. https://doi.org/10.1038/ncomms9073.
Koles ZJ, Rasminsky M. A computer simulation of conduction in demyelinated nerve fibres. J Physiol. 1972;227(2):351–64.
Felts PA, Baker TA, Smith KJ. Conduction in segmentally demyelinated mammalian central axons. J Neurosci. 1997;17(19):7267–77.
Hamada MS, Kole MH. Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability. J Neurosci. 2015;35(18):7272–86. https://doi.org/10.1523/JNEUROSCI.4747-14.2015.
Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurology. 2009;8(3):280–91.
Saab AS, Tzvetanova ID, Nave KA. The role of myelin and oligodendrocytes in axonal energy metabolism. Curr Opin Neurobiol. 2013;23(6):1065–72. https://doi.org/10.1016/j.conb.2013.09.008.
Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci. 2012;32(1):356–71. https://doi.org/10.1523/JNEUROSCI.3430-11.2012.
Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11(4):275–83. https://doi.org/10.1038/nrn2797.
Tallantyre EC, Bo L, Al-Rawashdeh O, Owens T, Polman CH, Lowe JS, et al. Clinico-pathological evidence that axonal loss underlies disability in progressive multiple sclerosis. Multiple Sclerosis Journal. 2010;16(4):406–11. https://doi.org/10.1177/1352458510364992.
Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487(7408):443–8. https://doi.org/10.1038/nature11314.
Philips T, Mironova YA, Jouroukhin Y, Chew J, Vidensky S, Farah MH, et al. MCT1 Deletion in oligodendrocyte lineage cells causes late-onset hypomyelination and axonal degeneration. Cell Reports. 2021;34(2):108610. https://doi.org/10.1016/j.celrep.2020.108610.
Micu I, Plemel JR, Lachance C, Proft J, Jansen AJ, Cummins K, et al. The molecular physiology of the axo-myelinic synapse. Exp Neurol. 2016;276:41–50. https://doi.org/10.1016/j.expneurol.2015.10.006.
Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron. 2016;91(1):119–32. https://doi.org/10.1016/j.neuron.2016.05.016.
Schirmer L, Mobius W, Zhao C, Cruz-Herranz A, Ben Haim L, Cordano C, et al. Oligodendrocyte-encoded Kir4.1 function is required for axonal integrity. Elife. 2018;7. 10.7554/eLife.36428.
Witte ME, Schumacher A-M, Mahler CF, Bewersdorf JP, Lehmitz J, Scheiter A, et al. Calcium influx through plasma-membrane nanoruptures drives axon degeneration in a model of multiple sclerosis. Neuron. 2019;101(4):615–24.e5. https://doi.org/10.1016/j.neuron.2018.12.023.
Wilkins A, Majed H, Layfield R, Compston A, Chandran S. Oligodendrocytes promote neuronal survival and axonal length by distinct intracellular mechanisms: a novel role for oligodendrocyte-derived glial cell line-derived neurotrophic factor. Journal of Neuroscience. 2003;23(12):4967–74.
• Mukherjee C, Kling T, Russo B, Miebach K, Kess E, Schifferer M, et al. Oligodendrocytes provide antioxidant defense function for neurons by secreting ferritin heavy chain. Cell Metab. 2020;32(2):259–72 e10. https://doi.org/10.1016/j.cmet.2020.05.019This study details a function outside of myelination whereby oligodendrocytes serve to preserve axon integrity by preventing oxidative damage.
Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte–neuron communication. PLoS Biol. 2013;11(7):e1001604. https://doi.org/10.1371/journal.pbio.1001604.
Zawadzka M, Rivers LE, Fancy SP, Zhao C, Tripathi R, Jamen F, et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6(6):578–90. https://doi.org/10.1016/j.stem.2010.04.002.
•• Bacmeister CM, Barr HJ, McClain CR, Thornton MA, Nettles D, Welle CG, et al. Motor learning promotes remyelination via new and surviving oligodendrocytes. Nat Neurosci. 2020;23(7):819-31. 10.1038/s41593-020-0637-3. This study demonstrates that motor learning can stimulate remyelination and that it occurs via both the differentiation of new oligodendrocytes and existing mature oligodendrocytes. .
Duncan ID, Radcliff AB, Heidari M, Kidd G, August BK, Wierenga LA. The adult oligodendrocyte can participate in remyelination. Proc Natl Acad Sci U S A. 2018;115(50):E11807–E16. https://doi.org/10.1073/pnas.1808064115.
•• Yeung MSY, Djelloul M, Steiner E, Bernard S, Salehpour M, Possnert G, et al. Dynamics of oligodendrocyte generation in multiple sclerosis. Nature. 2019;566(7745):538–42. https://doi.org/10.1038/s41586-018-0842-3This study provides evidence that preexisting mature oligodendrocytes contribute to remyelination in the human MS brain.
Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16(6):668–76. https://doi.org/10.1038/nn.3390.
Frischer JM, Weigand SD, Guo Y, Kale N, Parisi JE, Pirko I, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Annals of neurology. 2015;78(5):710–21. https://doi.org/10.1002/ana.24497.
Goldschmidt T, Antel J, Konig FB, Bruck W, Kuhlmann T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72(22):1914–21. https://doi.org/10.1212/WNL.0b013e3181a8260a.
Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–72. https://doi.org/10.1093/Brain/Awl217.
Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346(3):165–73. https://doi.org/10.1056/NEJMoa010994.
Kuhlmann T, Miron V, Cuo Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–58. https://doi.org/10.1093/brain/awn096.
Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–55. https://doi.org/10.1038/nrn2480.
Neumann B, Segel M, Chalut KJ, Franklin RJ. Remyelination and ageing: reversing the ravages of time. Multiple sclerosis. 2019;25(14):1835–41. https://doi.org/10.1177/1352458519884006.
Charles P, Reynolds R, Seilhean D, Rougon G, Aigrot MS, Niezgoda A, et al. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain. 2002;125(Pt 9):1972–9.
Stoffels JM, de Jonge JC, Stancic M, Nomden A, van Strien ME, Ma D, et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain. 2013;136(Pt 1):116–31. https://doi.org/10.1093/brain/aws313.
Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11(9):966–72. https://doi.org/10.1038/nm1279.
John GR, Shankar SL, Shafit-Zagardo B, Massimi A, Lee SC, Raine CS, et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nat Med. 2002;8(10):1115–21. https://doi.org/10.1038/nm781.
Foote AK, Blakemore WF. Inflammation stimulates remyelination in areas of chronic demyelination. Brain. 2005;128(Pt 3):528–39. https://doi.org/10.1093/brain/awh417.
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–8. https://doi.org/10.1038/nn.3469.
Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125(Pt 2):338–49.
Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, et al. Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions. Am J Pathol. 2000;157(1):267–76. https://doi.org/10.1016/S0002-9440(10)64537-3.
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. New England Journal of Medicine. 1998;338(5):278–85. https://doi.org/10.1056/nejm199801293380502.
Lovas G, Szilagyi N, Majtenyi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000;123(Pt 2):308–17. https://doi.org/10.1093/brain/123.2.308.
Bjartmar C, Kidd G, Mork S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48(6):893–901.
Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280(5369):1610–3.
Edgar JM, McLaughlin M, Werner HB, McCulloch MC, Barrie JA, Brown A, et al. Early ultrastructural defects of axons and axon-glia junctions in mice lacking expression of Cnp1. Glia. 2009;57(16):1815–24. https://doi.org/10.1002/glia.20893.
Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, et al. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nature Genetics. 2003;33(3):366–74. https://doi.org/10.1038/ng1095.
Oluich L-J, Stratton JAS, Lulu Xing Y, Ng SW, Cate HS, Sah P, et al. Targeted ablation of oligodendrocytes induces axonal pathology independent of overt demyelination. Journal of Neuroscience. 2012;32(24):8317–30. https://doi.org/10.1523/jneurosci.1053-12.2012.
Mei F, Lehmann-Horn K, Shen YA, Rankin KA, Stebbins KJ, Lorrain DS, et al. Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery. Elife. 2016;5. https://doi.org/10.7554/eLife.18246.
Bodini B, Veronese M, Garcia-Lorenzo D, Battaglini M, Poirion E, Chardain A, et al. Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann Neurol. 2016. https://doi.org/10.1002/ana.24620.
Bramow S, Frischer JM, Lassmann H, Koch-Henriksen N, Lucchinetti CF, Sorensen PS, et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 2010;133(10):2983–98. https://doi.org/10.1093/brain/awq250.
Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nature Reviews Neuroscience. 2006;7(12):932–41. https://doi.org/10.1038/nrn2023.
Haider L, Fischer MT, Frischer JM, Bauer J, Hoftberger R, Botond G, et al. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134(7):1914–24. https://doi.org/10.1093/brain/awr128.
Mahad DJ, Ziabreva I, Campbell G, Lax N, White K, Hanson PS, et al. Mitochondrial changes within axons in multiple sclerosis. Brain. 2009;132(5):1161–74. https://doi.org/10.1093/brain/awp046.
Dutta R, Mcdonough J, Yin X, Peterson J, Chang A, Torres T, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Annals of Neurology. 2006;59(3):478–89. https://doi.org/10.1002/ana.20736.
Witte ME, Nijland PG, Drexhage JA, Gerritsen W, Geerts D, van Het Hof B, et al. Reduced expression of PGC-1alpha partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta Neuropathol. 2013;125(2):231–43. https://doi.org/10.1007/s00401-012-1052-y.
Campbell GR, Worrall JT, Mahad DJ. The central role of mitochondria in axonal degeneration in multiple sclerosis. Multiple Sclerosis Journal. 2014;20(14):1806–13. https://doi.org/10.1177/1352458514544537.
Correale J, Marrodan M, Ysrraelit M. Mechanisms of neurodegeneration and axonal dysfunction in progressive multiple sclerosis. Biomedicines. 2019;7(1):14. https://doi.org/10.3390/biomedicines7010014.
• Licht-Mayer S, Campbell GR, Canizares M, Mehta AR, Gane AB, McGill K, et al. Enhanced axonal response of mitochondria to demyelination offers neuroprotection: implications for multiple sclerosis. Acta Neuropathol. 2020;140(2):143–67. https://doi.org/10.1007/s00401-020-02179-xThis study supports the hypothesis that metabolic dysregulation contributes to axonal degeneration following demyelination, as well as proof of principle that correction can preserve axons.
Kuhlmann T, Ludwin S, Prat A, Antel J, Brück W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathologica. 2017;133(1):13–24. https://doi.org/10.1007/s00401-016-1653-y.
Zrzavy T, Hametner S, Wimmer I, Butovsky O, Weiner HL, Lassmann H. Loss of ‘homeostatic’ microglia and patterns of their activation in active multiple sclerosis. Brain. 2017;140(7):1900–13. https://doi.org/10.1093/brain/awx113.
Lassmann H, Van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nature Reviews Neurology. 2012;8(11):647–56. https://doi.org/10.1038/nrneurol.2012.168.
Guerrero BL, Sicotte NL. Microglia in multiple sclerosis: friend or foe? Frontiers in Immunology. 2020;11. https://doi.org/10.3389/fimmu.2020.00374.
• Berghoff SA, Spieth L, Sun T, Hosang L, Schlaphoff L, Depp C, et al. Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis, Nat Neurosci. 2021;24(1):47–60. https://doi.org/10.1038/s41593-020-00757-6This study provides evidence and a possible mechanism whereby microglia facilitate myelin repair.
Lloyd AF, Davies CL, Holloway RK, Labrak Y, Ireland G, Carradori D, et al. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nature Neuroscience. 2019;22(7):1046–52. https://doi.org/10.1038/s41593-019-0418-z.
Lassmann H. Multiple sclerosis: Lessons from molecular neuropathology. Experimental Neurology. 2014;262:2–7. https://doi.org/10.1016/j.expneurol.2013.12.003.
Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128(11):2705–12. https://doi.org/10.1093/brain/awh641.
De Groot CJ, Bergers E, Kamphorst W, Ravid R, Polman CH, Barkhof F, et al. Post-mortem MRI-guided sampling of multiple sclerosis brain lesions: increased yield of active demyelinating and (p)reactive lesions. Brain. 2001;124(Pt 8):1635–45. https://doi.org/10.1093/brain/124.8.1635.
Singh S, Metz I, Amor S, Van Der Valk P, Stadelmann C, Brück W. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathologica. 2013;125(4):595–608. https://doi.org/10.1007/s00401-013-1082-0.
Campbell GR, Ziabreva I, Reeve AK, Krishnan KJ, Reynolds R, Howell O, et al. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Annals of Neurology. 2011;69(3):481–92. https://doi.org/10.1002/ana.22109.
Correale J. The role of microglial activation in disease progression. Multiple Sclerosis Journal. 2014;20(10):1288–95. https://doi.org/10.1177/1352458514533230.
Gimenez MAT, Sim JE, Russell JH. TNFR1-dependent VCAM-1 expression by astrocytes exposes the CNS to destructive inflammation. Journal of Neuroimmunology. 2004;151(1-2):116–25. https://doi.org/10.1016/j.jneuroim.2004.02.012.
Sobel RA, Mitchell ME, Fondren G. Intercellular adhesion molecule-1 (ICAM-1) in cellular immune reactions in the human central nervous system. Am J Pathol. 1990;136(6):1309–16.
Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36(2):180–90. https://doi.org/10.1002/glia.1107.
Dewitt DA, Perry G, Cohen M, Doller C, Silver J. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer's disease. Experimental Neurology. 1998;149(2):329–40. https://doi.org/10.1006/exnr.1997.6738.
Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, et al. Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nature Medicine. 2014;20(10):1147–56. https://doi.org/10.1038/nm.3681.
Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. The Journal of Neuroscience. 2001;21(17):6480–91. https://doi.org/10.1523/jneurosci.21-17-06480.2001.
Hamby ME, Hewett JA, Hewett SJ. TGF-β1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia. 2006;54(6):566–77. https://doi.org/10.1002/glia.20411.
Stojanovic IR, Kostic M, Ljubisavljevic S. The role of glutamate and its receptors in multiple sclerosis. J Neural Transm (Vienna). 2014;121(8):945–55. https://doi.org/10.1007/s00702-014-1188-0.
Rossi S, Motta C, Studer V, Barbieri F, Buttari F, Bergami A, et al. Tumor necrosis factor is elevated in progressive multiple sclerosis and causes excitotoxic neurodegeneration. Multiple Sclerosis Journal. 2014;20(3):304–12. https://doi.org/10.1177/1352458513498128.
Sherman LS, Struve JN, Rangwala R, Wallingford NM, Tuohy TMF, Kuntz C. Hyaluronate-based extracellular matrix: keeping glia in their place. Glia. 2002;38(2):93–102. https://doi.org/10.1002/glia.10053.
Hammond R. Timothy, Gadea A, Dupree J, Kerninon C, Nait-Oumesmar B, Aguirre A, et al. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron. 2014;81(3):588–602. https://doi.org/10.1016/j.neuron.2013.11.015.
Back SA, Tuohy TMF, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nature Medicine. 2005;11(9):966–72. https://doi.org/10.1038/nm1279.
Gutowski N. Cuzner. Tenascin-R and C in multiple sclerosis lesions: relevance to extracellular matrix remodelling. Neuropathology and Applied Neurobiology. 1999;25(3):207–14. https://doi.org/10.1046/j.1365-2990.1999.00176.x.
Stoffels JMJ, De Jonge JC, Stancic M, Nomden A, Van Strien ME, Ma D, et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain. 2013;136(1):116–31. https://doi.org/10.1093/brain/aws313.
Feliu A, Mestre L, Carrillo-Salinas FJ, Yong VW, Mecha M, Guaza C. 2-arachidonoylglycerol reduces chondroitin sulphate proteoglycan production by astrocytes and enhances oligodendrocyte differentiation under inhibitory conditions. Glia. 2020;68(6):1255–73. https://doi.org/10.1002/glia.23775.
Lau LW, Keough MB, Haylock-Jacobs S, Cua R, Döring A, Sloka S, et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Annals of Neurology. 2012;72(3):419–32. https://doi.org/10.1002/ana.23599.
Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nature Reviews Immunology. 2006;6(3):205–17. https://doi.org/10.1038/nri1786.
Corsiero E, Nerviani A, Bombardieri M, Pitzalis C. Ectopic lymphoid structures: powerhouse of autoimmunity. Front Immunol. 2016;7:430. https://doi.org/10.3389/fimmu.2016.00430.
Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2006;130(4):1089–104. https://doi.org/10.1093/brain/awm038.
Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Annals of Neurology. 2010;68(4):477–93. https://doi.org/10.1002/ana.22230.
Makshakov G, Magonov E, Totolyan N, Nazarov V, Lapin S, Mazing A, et al. Leptomeningeal contrast enhancement is associated with disability progression and grey matter atrophy in multiple sclerosis. Neurology Research International. 2017;2017:1–7. https://doi.org/10.1155/2017/8652463.
Zivadinov R, Ramasamy DP, Vaneckova M, Gandhi S, Chandra A, Hagemeier J, et al. Leptomeningeal contrast enhancement is associated with progression of cortical atrophy in MS: A retrospective, pilot, observational longitudinal study. Multiple Sclerosis Journal. 2017;23(10):1336–45. https://doi.org/10.1177/1352458516678083.
Montalban X, Leist TP, Cohen BA, Moses H, Campbell J, Hicking C, et al. Cladribine tablets added to IFN-β in active relapsing MS. Neurology - Neuroimmunology Neuroinflammation. 2018;5(5):e477. https://doi.org/10.1212/nxi.0000000000000477.
Abell A. FDA approves two new oral drugs for relapsing forms of MS. Pharmacy Today. 2019;25(6):20–1. https://doi.org/10.1016/j.ptdy.2019.05.012.
FDA Online Label Repository. labels.fda.gov Accessed October 21 2020.
Gelfand JM, Cree BAC, Hauser SL. Ocrelizumab and other CD20+ B-cell-depleting therapies in multiple sclerosis. Neurotherapeutics. 2017;14(4):835–41. https://doi.org/10.1007/s13311-017-0557-4.
Behrangi N, Fischbach F, Kipp M. Mechanism of siponimod: anti-inflammatory and neuroprotective mode of action. Cells. 2019;8(1). https://doi.org/10.3390/cells8010024.
Pebay A, Toutant M, Premont J, Calvo CF, Venance L, Cordier J, et al. Sphingosine-1-phosphate induces proliferation of astrocytes: regulation by intracellular signalling cascades. Eur J Neurosci. 2001;13(12):2067–76.
Jackson SJ, Giovannoni G, Baker D. Fingolimod modulates microglial activation to augment markers of remyelination. J Neuroinflammation. 2011;8:76. https://doi.org/10.1186/1742-2094-8-76.
O'Sullivan C, Schubart A, Mir AK, Dev KK. The dual S1PR1/S1PR5 drug BAF312 (Siponimod) attenuates demyelination in organotypic slice cultures. J Neuroinflammation. 2016;13:31. https://doi.org/10.1186/s12974-016-0494-x.
Tiwari-Woodruff S, Yamate-Morgan H, Sekyi M, Lauderdale K, Hasselmann J, Schubart A. The sphingosine 1-phosphate (S1P) receptor modulator, siponimod decreases oligodendrocyte cell death and axon demyelination in a mouse model of multiple sclerosis (P5.325). Neurology. 2016;86(16 Supplement):P5.325.
Comi G, Cook S, Giovannoni G, Rieckmann P, Sørensen PS, Vermersch P, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Multiple Sclerosis and Related Disorders. 2019;29:168–74. https://doi.org/10.1016/j.msard.2019.01.038.
Giovannoni G. Cladribine to treat relapsing forms of multiple sclerosis. Neurotherapeutics. 2017;14(4):874–87. https://doi.org/10.1007/s13311-017-0573-4.
Mi S, Hu B, Hahm K, Luo Y, Kam Hui ES, Yuan Q, et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nature Medicine. 2007;13(10):1228–33. https://doi.org/10.1038/nm1664.
Cadavid D, Balcer L, Galetta S, Aktas O, Ziemssen T, Vanopdenbosch L, et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. The Lancet Neurology. 2017;16(3):189–99. https://doi.org/10.1016/s1474-4422(16)30377-5.
Cadavid D, Mellion M, Hupperts R, Edwards KR, Calabresi PA, Drulović J, et al. Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): a randomised, placebo-controlled, phase 2 trial. The Lancet Neurology. 2019;18(9):845–56. https://doi.org/10.1016/s1474-4422(19)30137-1.
Chen Y, Zhen W, Guo T, Zhao Y, Liu A, Rubio JP, et al. Histamine Receptor 3 negatively regulates oligodendrocyte differentiation and remyelination. PLOS ONE. 2017;12(12):e0189380. https://doi.org/10.1371/journal.pone.0189380.
Schwartzbach CJ, Grove RA, Brown R, Tompson D, Then Bergh F, Arnold DL. Lesion remyelinating activity of GSK239512 versus placebo in patients with relapsing-remitting multiple sclerosis: a randomised, single-blind, phase II study. Journal of Neurology. 2017;264(2):304–15. https://doi.org/10.1007/s00415-016-8341-7.
Mei F, Fancy SPJ, Shen Y-AA, Niu J, Zhao C, Presley B, et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nature Medicine. 2014;20(8):954–60. https://doi.org/10.1038/nm.3618.
• Hartley MD, Banerji T, Tagge IJ, Kirkemo LL, Chaudhary P, Calkins E, et al. Myelin repair stimulated by CNS-selective thyroid hormone action. Journal of Clinical Investigation. 2019;4(8):1097. https://doi.org/10.1172/jci.insight.126329This study details the ability of new thyromimetic drugs to stimulate myelin repair, which may be a new avenue for drug development.
Faissner S, Plemel JR, Gold R, Yong VW. Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nature Reviews Drug Discovery. 2019;18(12):905–22. https://doi.org/10.1038/s41573-019-0035-2.
Spain R, Powers K, Murchison C, Heriza E, Winges K, Yadav V, et al. Lipoic acid in secondary progressive MS. Neurology - Neuroimmunology Neuroinflammation. 2017;4(5):e374. https://doi.org/10.1212/nxi.0000000000000374.
Fiedler SE, Spain RI, Kim E, Salinthone S. Lipoic acid modulates inflammatory responses of monocytes and monocyte-derived macrophages from healthy and relapsing-remitting multiple sclerosis patients. Immunology & Cell Biology. 2020. https://doi.org/10.1111/imcb.12392.
Yang C, Hao Z, Zhang L, Zeng L, Wen J. Sodium channel blockers for neuroprotection in multiple sclerosis. Cochrane Database Syst Rev. 2015;10:CD010422. https://doi.org/10.1002/14651858.CD010422.pub2.
Acknowledgements
The authors would like to thank Ben Emery for reviewing the manuscript and providing insightful feedback contributing to the final version. The figure was created with BioRender.com.
Author information
Authors and Affiliations
Contributions
T.S. and D.B. devised the manuscript. T. S, G.D., and D.B wrote/revised the manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights
This article does not present in full the data from studies using animals/humans. However It does cite studies performed by some of the authors (Duncan and Bourdette).
Competing Interests
T.S. reports no conflict of interest. G.D. reports no conflict of interest. D.B. is a co-founder of Autobahn Therapeutics.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Demyelinating Disorders
Rights and permissions
About this article
Cite this article
Simkins, T.J., Duncan, G.J. & Bourdette, D. Chronic Demyelination and Axonal Degeneration in Multiple Sclerosis: Pathogenesis and Therapeutic Implications. Curr Neurol Neurosci Rep 21, 26 (2021). https://doi.org/10.1007/s11910-021-01110-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s11910-021-01110-5