Abstract
Purpose of Review
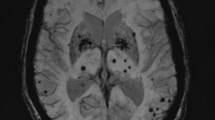
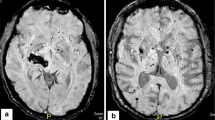
Cerebral microbleeds (CMB)—small round or ovoid lesions detected in hyposignal on blood-sensitive MRI sequences—are promising radiological biomarkers of cerebral small vessel disease. Their relations with ischaemic or haemorragic stroke and their potential contribution to dementia have been extensively addressed. This article reviews recent research on the clinical significance of CMB that remains to be determined.
Recent Findings
The presence, burden and location of CMB allow to obtain a more accurate estimate of intracerebral haemorrhage and ischaemic stroke risk. Most studies evaluating the association between CMB and dementia are hampered by methodological limitations and show conflicting results.
Summary
CMB mainly reflect the severity of the underlying small vessel disease and should not be interpreted independently of the others neuroimaging biomarkers or the clinical setting. Future large prospective longitudinal studies and randomized controlled trials in various settings are required to determine whether specific therapies are beneficial in case of incidental findings.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Salman RA-S, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol Elsevier Ltd. 2009;8:165–74. In this review, authors propose a procedural guide for identification of CMB and suggest possible future approaches for elucidating the role of these common lesions as markers for, and contributors to, small-vessel brain disease.
Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003.
Sepehry AA, Lang D, Hsiung GY, Rauscher A. Prevalence of brain microbleeds in Alzheimer disease: a systematic review and meta-analysis on the influence of neuroimaging techniques. AJNR Am J Neuroradiol. 2016;37:215–22.
• Ana M Daugherty PD, Naftali Raz PD. Incident risk and progression of cerebral microbleeds in healthy adults: a multi-occasion longitudinal study. Neurobiology of Aging. Elsevier Inc; 2017;1–38. The contributions of chronological age and vascular health indicators to risk of developing a CMB was examined in a prospective eight-year longitudinal study, as well as change in CMB size and iron content from susceptibility weighted imaging. Although developing a CMB is unlikely during normal aging, risk increases with declining vascular health, which is modifiable via behavioral and pharmaceutical intervention.
Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–14.
Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain. 2011;134:335–44.
Staekenborg SS, Koedam ELGE, Henneman WJP, Stokman P, Barkhof F, Scheltens P, et al. Progression of mild cognitive impairment to dementia. Stroke. 2009;40:1269–74.
•• Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, et al. Mixed-location cerebral hemorrhage/microbleeds: underlying microangiopathy and recurrence risk. Neurology. 2018;90:e119–26 The objective of the study was to assess the predominant type of cerebral small vessel disease and recurrence risk in patients who present with a combination of lobar and deep intracerebral hemorrhage (ICH) /microbleed locations (mixed ICH).
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701.
Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–9.
van Rooden S, van der Grond J, van den Boom R, Haan J, Linn J, Greenberg SM, et al. Descriptive analysis of the Boston criteria applied to a Dutch-type cerebral amyloid angiopathy population. Stroke. 2009;40:3022–7.
Charidimou A, Krishnan A, Werring DJ, Rolf Jäger H. Cerebral microbleeds: a guide to detection and clinical relevance in different disease settings. Neuroradiology. 2013;55:655–74.
Dierksen GA, Skehan ME, Khan MA, Jeng J, Nandigam RNK, Becker JA, et al. Spatial relation between microbleeds and amyloid deposits in amyloid angiopathy. Ann Neurol. 2010;68:545–8.
Gurol ME, Dierksen G, Betensky R, Gidicsin C, Halpin A, Becker A, et al. Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology. 2012;79:320–6.
Gregoire SM, Charidimou A, Gadapa N, Dolan E, Antoun N, Peeters A, et al. Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross-sectional magnetic resonance imaging study. Brain. 2011;134:2376–86.
Akoudad S, Portegies MLP, Koudstaal PJ, Hofman A, van der Lugt A, Ikram MA, et al. Cerebral microbleeds are associated with an increased risk of stroke. Circulation. 2015;132:509–16.
•• McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379(16):1519–28 The use of low-dose aspirin as a primary prevention strategy in older adults resulted in a significantly higher risk of major hemorrhage and did not result in a significantly lower risk of cardiovascular disease than placebo.
Strandberg TE, Tilvis RS. Interpretation of IST and CAST stroke trials. International Stroke Trial. Chinese Acute Stroke Trial. Lancet. 1997;350:442.
• Al-Shahi Salman R, Murray GD, Dennis MS, Newby DE, Sandercock PAG, Sprigg N, et al. The REstart or STop Antithrombotics Randomised Trial (RESTART) after stroke due to intracerebral haemorrhage: statistical analysis plan for a randomised controlled trial. Trials. 2019;20:183 RESTART is an investigator-led, parallel group, open, assessor-blind, randomised trial comparing starting versus avoiding antiplatelet therapy for adults surviving antithrombotic-associated ICH. Final results of RESTART will be analysed and disseminated in May 2019.
Lovelock CE, Cordonnier C, Naka H, Al-Shahi Salman R, Sudlow CLM. Edinburgh stroke study group, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010;41:1222–8.
Vernooij MW, Haag MDM, van der Lugt A, Hofman A, Krestin GP, Stricker BH, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009;66:714–20.
Lee S-H, Ryu W-S, Roh J-K. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology. 2009;72:171–6.
•• Wilson D, Ambler G, Shakeshaft C, Brown MM, Charidimou A, Al-Shahi Salman R, et al. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol. 2018;17:539–47 In patients with atrial fibrillation anticoagulated after recent ischaemic stroke or transient ischaemic attack, cerebral microbleed presence is independently associated with symptomatic intracranial haemorrhage risk and could be used to inform anticoagulation decisions.
O'Donnell MJ, Eikelboom JW, Yusuf S, Diener H-C, Hart RG, Smith EE, et al. Effect of apixaban on brain infarction and microbleeds: AVERROES-MRI assessment study. Am Heart J. 2016;178:145–50.
Charidimou A, Shoamanesh A, Al-Shahi Salman R, Cordonnier C, Perry LA, Sheth KN, et al. Cerebral amyloid angiopathy, cerebral microbleeds and implications for anticoagulation decisions: the need for a balanced approach. Int J Stroke. 2018;13:117–20.
Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and the risk of intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013;84:277–80.
Charidimou A, Shoamanesh A. International META-MICROBLEEDS Initiative. Clinical relevance of microbleeds in acute stroke thrombolysis: comprehensive meta-analysis. Neurology. 2016;87:1534–41.
• Tsivgoulis G, Zand R, Katsanos AH, Turc G, Nolte CH, Jung S, et al. Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden: a meta-analysis. JAMA Neurol. 2016;73:675–83 The objective of the study was to investigate the association of high CMB burden (>10 CMBs on a pre-intravenous thrombolysis (IVT) MRI scan) with the risk of symtomatic ICH following IVT for acute ischemic stroke (AIS). Presence of CMB and high CMB burdens on pretreatment MRI were independently associated with symptomatic ICH in patients with AIS treated with IVT. High CMB burden may be included in individual risk stratification scores predicting sICH risk following IVT for AIS.
Shoamanesh A, Kwok CS, Lim PA, Benavente OR. Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: a systematic review and meta-analysis. Int J Stroke. 2013;8:348–56.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110.
• Charidimou A, Imaizumi T, Moulin S, Biffi A, Samarasekera N, Yakushiji Y, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds. Neurology. 2017;89:820–9 CMB burden and distribution on MRI identify subgroups of ICH survivors with higher ICH recurrence risk, which may help to predict ICH prognosis with relevance for clinical practice and treatment trials.
Viswanathan A, Greenberg SM. Cerebral amyloid angiopathy in the elderly. Ann Neurol. 2011;70:871–80.
Viswanathan A, Rakich SM, Engel C, Snider R, Rosand J, Greenberg SM, et al. Antiplatelet use after intracerebral hemorrhage. Neurology. 2006;66:206–9.
Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology. 2001;56:773–7.
Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123–7.
Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–20.
Biffi A, Halpin A, Towfighi A, Gilson A, Busl K, Rost N, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. Lippincott Williams & Wilkins. 2010;75:693–8.
Charidimou A, Shams S, Romero JR, Ding J, Veltkamp R, Horstmann S, et al. Clinical significance of cerebral microbleeds on MRI: a comprehensive meta-analysis of risk of intracerebral hemorrhage, ischemic stroke, mortality, and dementia in cohort studies (v1). Int J Stroke. 2018;13:454–68.
Charidimou A, Linn J, Vernooij MW, Opherk C, Akoudad S, Baron J-C, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain. 2015;138:2126–39.
Moulin S, Casolla B, Kuchcinski G, Boulouis G, Rossi C, Hénon H, et al. Cortical superficial siderosis: a prospective observational cohort study. Neurology. 2018;91:e132–8.
Charidimou A, Peeters AP, Jäger R, Fox Z, Vandermeeren Y, Laloux P, et al. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology. 2013;81:1666–73.
Shoamanesh A, Pearce LA, Bazan C, Catanese L, McClure LA, Sharma M, et al. Microbleeds in the secondary prevention of small subcortical strokes trial: stroke, mortality, and treatment interactions. Ann Neurol. 2017;82:196–207.
Shoamanesh A, Morotti A, Romero JM, Oliveira-Filho J, Schlunk F, Jessel MJ, et al. Cerebral microbleeds and the effect of intensive blood pressure reduction on hematoma expansion and functional outcomes. JAMA Neurol. 2018;75:850–10.
Lim J-S, Hong K-S, Kim GM, Bang OY, Bae H-J, Kwon H-M, et al. Cerebral microbleeds and early recurrent stroke after transient ischemic attack: results from the Korean Transient Ischemic Attack Expression Registry. JAMA Neurol. 2015;72:301–8.
Lee S-H, Kim BJ, Roh J-K. Silent microbleeds are associated with volume of primary intracerebral hemorrhage. Neurology. 2006;66:430–2.
Heringa SM, Reijmer YD, Leemans A, Koek HL, Kappelle LJ, Biessels GJ, et al. Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer's disease. J Alzheimers Dis. 2014;38:211–21.
Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, et al. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265–75.
Poels MMF, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78:326–33.
Lei C, Lin S, Tao W, Hao Z, Liu M, Wu B. Association between cerebral microbleeds and cognitive function: a systematic review. J Neurol Neurosurg Psychiatry. 2013;84:693–7.
• Haller S, Montandon M-L, Lazeyras F, Scheffler M, Meckel S, Herrmann FR, et al. Radiologic-histopathologic correlation of cerebral microbleeds using pre-mortem and post-mortem MRI. Jiang Q, editor. PLoS One. 2016;11:e0167743 Authors demonstrated that routine clinical brain MRI underestimates the prevalence of CMBs by approximately 50%, and that 12% of radiologic pre-mortem MRI CMBs were false positives. Post-mortem MRI confirmed that this discordance is not explained by microbleeds occurring after the pre-mortem MRI.
Haller S, Vernooij MW, Kuijer JPA, Larsson E-M, Jäger HR, Barkhof F. Cerebral microbleeds: imaging and clinical significance. Radiology. 2018;287:11–28.
Yakushiji Y, Nishiyama M, Yakushiji S, Hirotsu T, Uchino A, Nakajima J, et al. Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke. 2008;39:3323–8.
Miwa K, Tanaka M, Okazaki S, Yagita Y, Sakaguchi M, Mochizuki H, et al. Multiple or mixed cerebral microbleeds and dementia in patients with vascular risk factors. Neurology. 2014;83:646–53.
Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73:934–43 This study shows that in the general population, a high microbleed count was associated with an increased risk for cognitive deterioration and dementia. Microbleeds thus mark the presence of diffuse vascular and neurodegenerative brain damage.
Romero JR, Beiser A, Himali JJ, Shoamanesh A, DeCarli C, Seshadri S. Cerebral microbleeds and risk of incident dementia: the Framingham Heart Study. Neurobiol Aging. 2017;54:94–9.
Ding J, Sigurðsson S, Jónsson PV, Eiriksdottir G, Meirelles O, Kjartansson O, et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88:2089–97.
Bos D, Wolters FJ, Darweesh SKL, Vernooij MW, de Wolf F, Ikram MA, et al. Cerebral small vessel disease and the risk of dementia: a systematic review and meta-analysis of population-based evidence. Alzheimers Dement Elsevier Inc. 2018;14:1482–92.
Debette S, Schilling S, Duperron M-G, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury. JAMA Neurol. 2019;76:81–14 Authors report evidence that MRI markers of vascular brain injury have major clinical significance. This research prompts careful evaluation of the benefit–risk ratio for available prevention strategies in individuals with covert vascular brain injury.
Lawrence AJ, Patel B, Morris RG, MacKinnon AD, Rich PM, Barrick TR, et al. Mechanisms of cognitive impairment in cerebral small vessel disease: multimodal MRI results from the St George’s cognition and neuroimaging in stroke (SCANS) study. Baron J-C, editor. PLoS One. 2013;8:e61014–9.
Lawrence AJ, Chung AW, Morris RG, Markus HS, Barrick TR. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology. 2014;83:304–11.
Tuladhar AM, van Uden IWM, Rutten-Jacobs LCA, Lawrence A, van der Holst H, van Norden A, et al. Structural network efficiency predicts conversion to dementia. Neurology. 2016;86:1112–9.
Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66:1356–60.
Pettersen JA, Sathiyamoorthy G, Gao F-Q, Szilagyi G, Nadkarni NK, St George-Hyslop P, et al. Microbleed topography, leukoaraiosis, and cognition in probable Alzheimer disease from the Sunnybrook dementia study. Arch Neurol. 2008;65:790–5.
Martinez-Ramirez S, Romero J-R, Shoamanesh A, McKee AC, Van Etten E, Pontes-Neto O, et al. Diagnostic value of lobar microbleeds in individuals without intracerebral hemorrhage. Alzheimers Dement. 2015;11:1480–8.
van der Vlies AE, Goos JDC, Barkhof F, Scheltens P, van der Flier WM. Microbleeds do not affect rate of cognitive decline in Alzheimer disease. Neurology. 2012;79:763–9.
Boyle PA, Yu L, Nag S, Leurgans S, Wilson RS, Bennett DA, et al. Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology. 2015;85:1930–6.
Moulin S, Labreuche J, Bombois S, Rossi C, Boulouis G, Hénon H, et al. Dementia risk after spontaneous intracerebral haemorrhage: a prospective cohort study. Lancet Neurol. 2016;15:820–9 This observational prospective study reports a substantial risk of incident dementia in dementia-free survivors of spontaneous intracerebral haemorrhage; our results suggest that underlying cerebral amyloid angiopathy is a contributing factor to the occurrence of new-onset dementia. Future clinical trials including patients with intracerebral haemorrhage should assess cognitive endpoints.
Biffi A, Bailey D, Anderson CD, Ayres AM, Gurol EM, Greenberg SM, et al. Risk factors associated with early vs delayed dementia after intracerebral hemorrhage. JAMA Neurol. 2016;73:969–76.
Meier IB, Gu Y, Guzaman VA, Wiegman AF, Schupf N, Manly JJ, et al. Lobar microbleeds are associated with a decline in executive functioning in older adults. Cerebrovasc Dis. 2014;38:377–83.
Xiong L, Boulouis G, Charidimou A, Roongpiboonsopit D, Jessel MJ, Pasi M, et al. Dementia incidence and predictors in cerebral amyloid angiopathy patients without intracerebral hemorrhage. J Cereb Blood Flow Metab. 2017;38:241–9.
Reijmer YD, Fotiadis P, Martinez-Ramirez S, Salat DH, Schultz A, Shoamanesh A, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain. 2015;138:179–88.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Solène Moulin and Charlotte Cordonnier each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stroke
Rights and permissions
About this article
Cite this article
Moulin, S., Cordonnier, C. Role of Cerebral Microbleeds for Intracerebral Haemorrhage and Dementia. Curr Neurol Neurosci Rep 19, 51 (2019). https://doi.org/10.1007/s11910-019-0969-0
Published:
DOI: https://doi.org/10.1007/s11910-019-0969-0