Abstract
Purpose of Review
Status epilepticus (SE) is a multisystem disorder. Initially, complications of a massive catecholamine release followed by the side effects of medical therapies, impact patients’ outcomes. The aim of this article is to provide an updated summary of the systemic complications following SE.
Recent Findings
In recent years, the importance of the multifaceted nature of SE and its relationship with clinical outcomes has been increasingly recognized. The cumulative systemic effects of prolonged seizures and their treatment contribute to morbidity and mortality in this condition.
Summary
Most systemic complications after SE are predictable. Anticipating their occurrence and respecting a number of simple guidelines may improve the prognosis of these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Status epilepticus (SE) encompasses a heterogeneous group of disorders with various clinical presentations and etiologies. A significant proportion of patients do not respond to first- and second-line treatments and require more aggressive management in the intensive care unit (ICU). Their prognosis depends on the type of SE [1], refractoriness of seizures [2, 3], complications of medical care [4, 5], and maybe, use of anesthetic drugs [6, 7, 8••, 9, 10]. While most early complications of SE are a consequence of catecholaminergic release [11], sustained muscle contraction, and mechanical injury, side effects of antiseizure medications and prolonged immobility are major contributors later on in the course of SE. Hence, optimal management of this condition relies on the anticipation of medical complications and side effect of medical therapies.
Given the great heterogeneity of SE, we will focus this review on the pathophysiologic basis, clinical presentation, and prevention of the systemic complications of generalized convulsive SE.
Physiologic Changes During Status Epilepticus
Sustained convulsive SE causes profound physiologic derangements which occur in order to compensate for a high metabolic demand and which result in widespread systemic consequences [11, 12]. Catecholamine release disturbs the cardiovascular function by increasing the blood pressure, heart rate, and cardiac output [13]. The pressures in the pulmonary circulation and left atrium also rise, while the threshold for cardiac arrhythmias decreases.
As a consequence of systemic hypertension, there is an increase in the cerebral perfusion pressure. This, in combination with impaired cerebral autoregulation, leads to uncontrolled increases in cerebral blood flow and intracranial pressure.
The hyperadrenergic state also causes hyperglycemia, demargination of neutrophils, and hyperpyrexia, and these are perpetuated by sustained muscle contraction. While hyperglycemia is usually transient and well tolerated, hyperpyrexia warrants aggressive medical intervention because it is associated with the release of proconvulsant cytokines, neuropathological sequelae, and increased mortality [14, 15•]. After approximately after 30 min of persistent seizures, compensatory mechanisms are insufficient to maintain cellular homeostasis and irreversible neuronal injury occurs [12].
Systemic Complications of Status Epilepticus
Metabolic Complications
Sustained muscle contraction depletes glycogen reserves, shifting energy production from aerobic respiration to anaerobic glycolysis. The production of lactic acid increases leading to development of an anion-gap metabolic acidosis which usually resolves without further interventions and is not associated with life-threatening cardiac arrhythmias (especially with pH greater than 7.2). Treatment with sodium bicarbonate is therefore not warranted [16]. While metabolic acidosis has been traditionally described as the main metabolic derangement in SE, respiratory acidosis, due to the increased production of carbon dioxide from seizures and decreased removal from alveolar hypoventilation, is in fact the most common metabolic imbalance [16].
Pulmonary Complications
Respiratory failure occurs in one third of SE episodes and is associated with poor outcome [17•]. It can result from multiple factors including apnea, asynchronic diaphragmatic contractions, upper airway obstruction, aspiration of gastric contents, mucous plugging, and neurocardiogenic pulmonary edema. The administration of antiseizure medications may contribute to respiratory depression; however, available randomized controlled trial data indicate that underdosing of benzodiazepines and consequent continued seizure activity is more likely to lead to airway complications and hypoxemic respiratory failure than is an appropriate dose of benzodiazepine [18, 19].
Endotracheal intubation has been reported in 21% of patients with SE, frequently in elderly patients or those with refractory seizures. Intubation and late intubation are both associated with worse mortality (3% and 14% respectively) [20•]. While the increased mortality with late intubation highlights the need for rapid recognition and treatment of the pulmonary complications of SE, patients intubated late are more likely intubated for control of refractory SE with an anesthetic drug rather than for respiratory failure. Thus, it is likely the refractoriness of the seizures and a more severe underlying pathology impact mortality and not the delay in intubation per se.
Up to one third of patients with SE develop neurocardiogenic pulmonary edema [21]. It has also been found in 80 to 100% of epileptic patients who die unexpectedly of seizures [22]. While this complication usually presents within minutes to hours, delayed forms have been described 12 to 24 h after the initial insult. Symptoms are present in the context of sympathetic activation (fever, tachycardia, hypertension, and leukocytosis) and usually resolve within 24 to 48 h of seizure control. Chest radiograph will reveal bilateral infiltrates (Fig. 1) [23].
Four main theories have been proposed to explain the development of neurocardiogenic pulmonary edema, each involving an acute catecholaminergic surge [23].
-
1)
Neurocardiogenic theory: the catecholaminergic surge causes myocardial stunning and increases the pressures on the left heart resulting in pulmonary edema.
-
2)
Neurohemodynamic theory: a sudden increase in the systemic vascular resistance produces a shift of blood to the pulmonary circulation with fluid extravasation to the alveoli.
-
3)
Blast theory: the abrupt rise in the pulmonary pressure causes barotrauma, disrupting the capillary walls, and causing fluid to leak into the alveoli. This theory also explains the exudative characteristic of the pulmonary edema.
-
4)
Pulmonary venule adrenergic hypersensitivity theory: sympathetic surge directly stimulates α- and β-adrenergic receptors in the pulmonary vascular bed increasing the endothelial permeability causing pulmonary edema regardless of any systemic changes.
While all these mechanisms may play a role at the same time, understanding the degree of cardiogenic involvement has clear therapeutic implications as explained in the next section.
Cardiac Complications
Two thirds of patients with SE have markers of cardiac injury, the presence of which are associated with poor outcomes [24]. A reversible myocardial stunning from hyperadrenergic stimulation (also known as stress-induced or Takotsubo cardiomyopathy) is classically described after aneurysmal subarachnoid hemorrhage [25] and may affect up to 50% of patients with SE [26••]. These patients will present with hypotension, pulmonary edema, a modest elevation of the cardiac troponin, and less commonly cardiogenic shock. Characteristic echocardiographic findings are reversible apical ballooning and reduced left ventricular function. Nonetheless, atypical presentations do occur and can include any combination of akinesis, hypokinesis, or dyskinesis of the left ventricle extending beyond the territory of a single coronary distribution. Most of these patients will respond to treatment with inotropes (dobutamine or milrinone), while intraarterial balloon pump can be considered for refractory cases [27, 28]. Autopsy studies have shown subendocardial and myocyte contraction band necrosis, a reflection of hyperadrenergic damage. This anatomic substrate increases the susceptibility for cardiac arrhythmias [29].
Demand ischemia should be considered in patients with pre-existent coronary artery disease. The use of cardio depressant anesthetics drugs, as propofol or pentobarbital, can precipitate acute heart failure in patients with borderline cardiac function and should be avoided. The most frequent cardiac arrhythmias in patients with SE are sinus tachycardia (65.7%), bradycardia (48.6%), and atrial fibrillation or flutter (20.0%). Remarkably, life-threatening arrhythmias like ventricular tachycardia or fibrillation (11.4%) and atrioventricular block (2.9%) are not uncommon. The initial electrocardiogram shows T-wave inversion and nonspecific ST segment changes in almost 40% of patients. ST segment elevation and depression can be found in 11 and 6% of patients respectively, and the QTc interval can be prolonged in one quarter of patients [24].
Infectious Complications
Infections can complicate up to 50% of SE [5, 30•, 31]. They are associated with a longer duration of SE, need for mechanical ventilation, unfavorable hospital discharge disposition, and poor recovery [30•]. The diagnosis of any infection at the onset of the SE also increases the likelihood of progression to refractory SE, prolonged mechanical ventilation, longer ICU and hospital stay, poor functional outcome, and death [32••].
The most common infectious complications are pneumonia (10–50%), sepsis (7–10%), and urinary tract infections (5–7%) [5, 17•, 30•, 33]. Aspiration of oropharyngeal and gastric contents caused by a reduced level of consciousness, upper airway weakness, recurrent seizures, and the sedative effect of antiseizure medications may contribute to the high frequency of pneumonia in this population. Prolonged sedation, invasive ventilation with a weak cough, and recurrent mucus plugging are additional risk factors for pneumonia development in patients with prolonged hospitalizations. The use of indwelling urinary catheters, sometimes for weeks to months, increases the risk for urinary tract infections.
Renal Complications
Rhabdomyolysis may complicate prolonged seizures. Reddish-gold pigmented urine may be the first sign of rhabdomyolysis. As the rise of serum creatine-phospho-kinase (CPK) begins within 2 to 12 h following the muscular injury, empiric hydration is warranted until the CPK plateaus. Hyperkalemia, hyperphosphatemia, and hypocalcemia are additional findings in patients with rhabdomyolysis, mostly in those with severe acute kidney injury [34]. Hypovolemia (from a high metabolic demand and the shift of fluid into injured muscles) and acidosis also favor the precipitation of myoglobin in the renal tubules.
Acute urate nephropathy, while rare, has been described in patients with SE. Severe hyperuricemia may develop when the nucleosides released by muscle cells are converted to uric acid in the liver. Lactic acidosis, another frequent finding in these patients, would further contribute by decreasing the tubular secretion of urate [35]. Hyperuricemia can lead to acute kidney injury by precipitation of uric acid crystals, renal vasoconstriction, and direct microvascular injury [35].
Hematological Complications
Peripheral leukocytosis is a common finding in more than 60% of patients with SE. It may result from demargination of neutrophils due to the physiologic stress of the seizures when it occurs at presentation, or it may point towards either an infectious cause of SE or a superimposed infection resulting from aspiration. Given the possible co-existence with infections, the threshold to screen for infections, including a lumbar puncture, should be low. In general, a lumbar puncture should be performed as soon as the etiology is not defined after completion of a focused history, non-contrast head CT scan, and basic laboratory evaluation, irrespective of the presence or persistence of fever or leukocytosis.
Disseminated intravascular coagulation (DIC) is a rare complication of SE, and data are limited to case reports and case series. Hyperpyrexia resulting in endothelial damage with activation of coagulation and fibrinolysis has been proposed as the responsible mechanism [36]. DIC presents as both hemorrhagic and thrombotic disorders. Thrombosis can be venous or arterial, and hemorrhage includes any combination of ecchymosis, petechiae, oozing from mucosal surfaces or intravenous lines and major bleeding from the gastrointestinal, pulmonary, or central nervous systems. The diagnosis relies on a high level of suspicion, coagulation studies (prolongation of the prothrombin and activated partial thromboplastin times, hypofibrinogenemia, increased D-dimer, anemia and thrombocytopenia) and a peripheral blood smear (microangiopathic hemolytic anemia with schistocytes and helmet cells). The mainstays for treatment are the correction of the underlying cause, in this case hyperpyrexia, and supportive care. Platelets should be transfused to maintain a count > 20 × 109/l and > 50 × 109/l if there is active bleeding. Thromboprophylaxis with low-molecular weight heparin is warranted unless there is active bleeding or the platelet count is < 30 × 109/l [37].
Musculoskeletal Complications
Early identification of musculoskeletal complications of SE may allow for proper consultation and treatment. Injuries to the tongue and soft tissues of the mouth can be found in 20% of patients with seizures [38]. Around 1% of patients hospitalized after seizures have fractures. One half of them can be explained by trauma; the remainder likely occurs as a direct result of the violent muscular contractions [39]. Skull, nasal bones, and clavicle are the most frequent sites of traumatic fractures. Non-traumatic fractures usually affect the proximal humerus and femur and can be unilateral or bilateral, with or without dislocation of the shoulder [40,41,42]. Shoulder dislocations are typically posterior [43]. Vertebral compression fractures, bilateral fibular fractures, and more rarely traumatic arterial dissection with stroke have also been reported [44, 45]. As the clinical examination is often limited in this population of patients who are frequently comatose or encephalopathic, a high index of suspicion is needed to recognize and treat these conditions.
When rhabdomyolysis is severe, compartment syndrome may develop after fluid resuscitation, due to shift of fluid into the damaged muscle cells. When suspected, it can be confirmed by measuring compartment pressures because clinical findings such as pain, parenthesis, decreased sensation, and muscle weakness are difficult to assess in comatose patients and may lead to a delay in recognition and increased morbidity, including amputation of the limb [46].
Complications of Medical Therapies
Complications resulting from treatment typically occur later in the hospital course and can be divided into two broad categories: (1) adverse effects of the interventions used to treat SE and (2) sequelae of critical illness, immunosuppression, and prolonged immobilization.
Adverse Effects of SE Treatments
First- and Second-Line Antiepileptic Drugs
High doses of benzodiazepines are associated with sedation, muscle relaxation, and both respiratory and cardiovascular depression. However, doses exceeding those used for SE have been demonstrated to be safe in human patients [47]. Indeed, as discussed above, respiratory complications associated with prolonged seizures are more prominent than those caused by benzodiazepines [48, 49]. When benzodiazepines fail, fosphenytoin and valproic acid are the preferred second line options [50, 51].
Phenytoin is associated with severe hypotension, cardiac arrhythmias, and serious infusion site reactions. Its prodrug, fosphenytoin, lacks propylene glycol as a carrier allowing for three to four times faster infusion rates and less side effects. Hypotension does occur in 40% of patients during the infusion of fosphenytoin, and the severity is rate and volume dependent; thus, it typically responds well to a combination of slowing the rate of infusion and fluid administration. Yet, one half of these patients will require support with vasopressors [28, 52]. The FDA recommendation for continuous cardiac monitoring during and after fosphenytoin infusion is supported by the report of 29 serious cardiac events including death, high-grade atrioventricular block, and transient sinus arrest [53]. While it has been questioned [54], reports of serious toxicity continue to appear [55••].
Valproic acid is as effective as fosphenytoin for seizure termination [51], with neither arrhythmogenic nor sedative potential [56]. The risk of hepatic toxicity and hyperammonemic encephalopathy are the main concerns with this drug however in practice they are rare. Caution is also advised in patients with active bleeding or a recent neurosurgical procedure due to theoretical risks of thrombocytopenia and platelet dysfunction [57, 58].
In our experience, levetiracetam may be quite sedating at the high doses required in SE but is otherwise well tolerated and without early significant adverse effects.
Treatment-related adverse events with lacosamide may include mild to moderate dizziness and sedation. As lacosamide targets sodium channels, serious cardiac side effects are a major concern when high doses are administered rapidly, but they are uncommon at the recommended doses and infusion rates [59••, 60].
Third-Line Antiepileptic Drugs
Midazolam has a large volume of distribution and accumulates in peripheral tissues, causing prolonged sedation if administered over a long period of time. This is more pronounced in elderly patients, patients with impaired hepatic or renal function, obese patients, and those receiving CYP3A4 inhibitors. Tachyphylaxis necessitates higher doses and increases the cumulative volume of intravenous fluid administered to the patient (Table 1), limiting its prolonged use.
Propofol causes hypotension by peripheral vasodilation and cardiac depression; hence, it should be used with caution in patients with heart failure. The propofol infusion syndrome (PRIS) causes lactic acidosis, rhabdomyolysis, lipemia, hyperkalemia, renal failure, and rapid cardiovascular collapse. The risk of PRIS is greater in young patients, with high doses of propofol (> 80 μg/kg/min), treatment for more than 48 h, and the concurrent use of vasopressors, steroids, or ketogenic diet [61, 62]. For this reason propofol is often avoided altogether in children. Although its incidence is low (around 1%), its mortality is high at 30% [63, 64]. Management involves immediate discontinuation of propofol, supportive care, and initiation of continuous dialysis and extracorporal membrane oxygenation if indicated [65].
Ketamine is more likely to cause hypertension and tachycardia at therapeutic doses. While useful in patients with hypotension, caution is advised when used in patients with comorbid cardiovascular disease. Hypotension and bradycardia have also been reported. Idiosyncratic side effects include laryngospasm, hypersalivation, and bronchorrhea. In a retrospective review that included 58 patients with SE, ketamine, in combination with additional anesthetics, terminated seizures in 30% of cases. Four patients had treatment-related adverse events. Two patients had supraventricular tachycardia and one atrial fibrillation, and one patient had a syndrome similar to the PRIS after 4 days of ketamine at high doses [66]. Like midazolam, prolonged use of ketamine at the doses required for control of SE can result in significant volume accumulation and may require concentration of the drug (Table 1).
While highly effective, barbiturates are associated with profound peripheral vasodilation leading to hypotension and distributive shock, usually requiring intravenous vasopressors. Severe immunosuppression with a high risk of developing hospital acquired infections can also be anticipated. Paralytic ileus occurs in about 10% of patients and can be resistant to treatment. Refractory ileus may lead to microvascular ischemia with bowel perforation [67]. Severe angioedema with airway obstruction has also been reported and resolves upon discontinuation of the drug [68]. Similar to midazolam and ketamine, continuous infusion of pentobarbital results in administration of high volumes of fluid at typical concentrations (Table 1) which frequently leads to the development of pleural effusions and anasarca. With prolonged use of high doses, patients can develop propylene glycol toxicity due to the solvent in which both phenobarbital and pentobarbital are mixed. In toxic levels, it causes high osmolality lactic acidosis, progressive renal failure, cardiac dysfunction, central nervous system depression, hypotension, and seizures. As symptoms are non-specific and frequent in ICU patients, a high index of suspicion and frequent measurement of osmolar gap are needed for an early diagnosis. Patients with renal and hepatic insufficiency are at higher risk of developing this complication [69]. Osmolar gap has been shown to correlate well with propylene glycol levels, being > 10 mmol/L suggestive of toxicity [70]. Treatment of propylene glycol toxicity consists of discontinuing the offending agent, supportive treatment, and dialysis in severe cases.
Data on the side effects of the inhalational halogenated anesthetics (isoflurane and desflurane) is restricted to 46 patients (28 adults). The main reported complications are hypotension needing vasopressors, infections, and ileus [71•].
Non-pharmacologic Therapies
Hypothermia has been used as an adjunct to pharmacologic therapies in SE. Recently, the Hypothermia for Brain Enhancement Recovery by Neuroprotective and Anticonvulsivant Action after Convulsive Status Epilepticus (HYBERNATUS) trial found no benefit from hypothermia in patients with SE, with a higher incidence aspiration pneumonia (51 vs 45%) and ICU-acquired weakness, with a slightly lower incidence serious adverse events in the hypothermia group [72••]. It is unknown whether therapeutic normothermia, still believed to be important in nearly all acute neurologic emergencies, has a similar adverse effect profile.
Ketogenic diet has shown promising results in adults with SRSE according to a recent phase II clinical trial [73••]. The most common side effect was metabolic acidosis in almost 30% of patients. For this reason, close monitoring and aggressive supplementation of bicarbonate is warranted. The risk of acidosis may be higher when the diet is combined with propofol administration and thus the combination should be avoided [63]. Hypoglycemia, hyperlipidemia, constipation, hyponatremia, and weight loss were also reported [73••]. In our experience, a comprehensive review of all patients’ medications and feeding formulas is needed to effectively achieve ketosis. Since carbohydrate-containing medications are a cause of treatment failure, they must be appropriately replaced.
Complications of Prolonged Immobilization and Critical Illness
Because patients with refractory and super refractory SE can be immobilized for weeks to months, they are at increased risk of developing deep venous thrombosis, pulmonary embolism, skin breakdown, muscle atrophy, and ICU-acquired weakness (neuropathy and/or myopathy). Immobility also predisposes to recurrent mucus plugging with atelectasis (leading to repeated therapeutic bronchoscopies) and bacterial infections. Patients who need mechanical ventilation have further risks of ventilator associated pneumonia. Up to 30% of them will require tracheostomy, often combined with percutaneous gastrostomy tube placement due to ventilator dependence and dysphagia [27]. The prolonged use of indwelling urinary catheters is also associated with urinary tract infections.
Conclusions
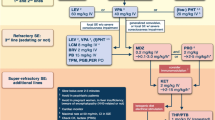
Most systemic complications of SE can be anticipated and many can be prevented. Understanding the physiologic changes triggered by SE, following screening protocols designed to prevent and detect medical complications early (Fig. 2), and mastering the side effect profiles of antiseizure drugs may reduce the mortality and improve the functional outcomes for survivors.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sutter R, Kaplan PW, Ruegg S. Outcome predictors for status epilepticus—what really counts. Nat Rev Neurol. 2013;9(9):525–34. https://doi.org/10.1038/nrneurol.2013.154.
Drislane FW, Blum AS, Lopez MR, Gautam S, Schomer DL. Duration of refractory status epilepticus and outcome: loss of prognostic utility after several hours. Epilepsia. 2009;50(6):1566–71. https://doi.org/10.1111/j.1528-1167.2008.01993.x.
Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia. 2010;51(2):251–6. https://doi.org/10.1111/j.1528-1167.2009.02323.x.
Hocker SE, Britton JW, Mandrekar JN, Wijdicks EF, Rabinstein AA. Predictors of outcome in refractory status epilepticus. JAMA Neurol. 2013;70(1):72–7. https://doi.org/10.1001/jamaneurol.2013.578.
Amare A, Zenebe G, Hammack J, Davey G. Status epilepticus: clinical presentation, cause, outcome, and predictors of death in 119 Ethiopian patients. Epilepsia. 2008;49(4):600–7. https://doi.org/10.1111/j.1528-1167.2008.01556.x.
Marchi NA, Novy J, Faouzi M, Stahli C, Burnand B, Rossetti AO. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med. 2015;43(5):1003–9. https://doi.org/10.1097/CCM.0000000000000881.
Kowalski RG, Ziai WC, Rees RN, Werner JK Jr, Kim G, Goodwin H, et al. Third-line antiepileptic therapy and outcome in status epilepticus: the impact of vasopressor use and prolonged mechanical ventilation. Crit Care Med. 2012;40(9):2677–84. https://doi.org/10.1097/CCM.0b013e3182591ff1.
•• Alvarez V, Lee JW, Westover MB, Drislane FW, Novy J, Faouzi M, et al. Therapeutic coma for status epilepticus: differing practices in a prospective multicenter study. Neurology. 2016;87(16):1650–9. Provides evidence that the association between anesthetic drug use and outcomes in SE is dependent on the refractoriness of the seizures and that when refractory SE is controlled for, there is no longer an association. https://doi.org/10.1212/WNL.0000000000003224.
Sutter R, De Marchis GM, Semmlack S, Fuhr P, Ruegg S, Marsch S, et al. Anesthetics and outcome in status epilepticus: a matched two-center cohort study. CNS Drugs. 2017;31(1):65–74. https://doi.org/10.1007/s40263-016-0389-5.
Hocker SE, Shorvon S. Anesthetic drugs in status epilepticus: risk or rescue? A 6-year cohort study. Neurology. 2014;83(9):866. https://doi.org/10.1212/WNL.0000000000000723.
Walton NY. Systemic effects of generalized convulsive status epilepticus. Epilepsia. 1993;34(Suppl 1):S54–8. https://doi.org/10.1111/j.1528-1157.1993.tb05906.x.
Lothman E. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40(5 Suppl 2):13–23.
Simon RP, Aminoff MJ, Benowitz NL. Changes in plasma catecholamines after tonic-clonic seizures. Neurology. 1984;34(2):255–7. https://doi.org/10.1212/WNL.34.2.255.
Suchomelova L, Lopez-Meraz ML, Niquet J, Kubova H, Wasterlain CG. Hyperthermia aggravates status epilepticus-induced epileptogenesis and neuronal loss in immature rats. Neuroscience. 2015;305:209–24. https://doi.org/10.1016/j.neuroscience.2015.08.006.
• Vooturi S, Jayalakshmi S, Sahu S, Mohandas S. Prognosis and predictors of outcome of refractory generalized convulsive status epilepticus in adults treated in neurointensive care unit. Clin Neurol Neurosurg. 2014;126:7–10. Refractoriness of seizures and fever predicts mortality in SE. Stresses the benefit of prevention of systemic complications to improve outcomes. https://doi.org/10.1016/j.clineuro.2014.07.038.
Wijdicks EFM, Hubmayr RD. Acute acid-base disorders associated with status epilepticus. Mayo Clin Proc. 1994;69(11):1044–6. https://doi.org/10.1016/S0025-6196(12)61370-6.
• Tiamkao S, Pranboon S, Thepsuthammarat K, Sawanyawisuth K. Incidences and outcomes of status epilepticus: a 9-year longitudinal national study. Epilepsy Behav. 2015;49:135–7. National database including 12000 patients with SE. Age, gender, hospital level, comorbid conditions, complications of SE, and procedural intervention were associated with an increased mortality. Factors associated with poor outcome in patients admitted with SE by national data were age, gender, hospital level, comorbid conditions, complications of SE, and procedural intervention. https://doi.org/10.1016/j.yebeh.2015.04.040.
Chamberlain JM, Altieri MA, Futterman C, Young GM, Ochsenschlager DW, Waisman Y. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatr Emerg Care. 1997;13(2):92–4. https://doi.org/10.1097/00006565-199704000-00002.
Silbergleit R, Biros MH, Harney D, Dickert N, Baren J. Implementation of the exception from informed consent regulations in a large multicenter emergency clinical trials network: the RAMPART experience. Acad Emerg Med Off J Soc Acad Emerg Med. 2012;19(4):448–54. https://doi.org/10.1111/j.1553-2712.2012.01328.x.
• Vohra TT, Miller JB, Nicholas KS, Varelas PN, Harsh DM, Durkalski V, et al. Endotracheal intubation in patients treated for prehospital status epilepticus. Neurocrit Care. 2015;23(1):33–43. Subanalysis of the RAMPART trial showing that endotracheal intubation occurs in 20% of patients with status epilepticus, particularly among the elderly or those with refractory seizures. https://doi.org/10.1007/s12028-014-0106-5.
Simon RP. Neurogenic pulmonary edema. Neurol Clin. 1993;11(2):309–23.
Wayne SL, O'Donovan CA, McCall WV, Link K. Postictal neurogenic pulmonary edema: experience from an ECT model. Convuls Ther. 1997;13(3):181–4.
Davison DL, Terek M, Chawla LS. Neurogenic pulmonary edema. Crit Care. 2012;16(2):212. https://doi.org/10.1186/cc11226.
Hocker S, Prasad A, Rabinstein AA. Cardiac injury in refractory status epilepticus. Epilepsia. 2013;54(3):518–22. https://doi.org/10.1111/epi.12017.
Abd TT, Hayek S, Cheng JW, Samuels OB, Wittstein IS, Lerakis S. Incidence and clinical characteristics of takotsubo cardiomyopathy post-aneurysmal subarachnoid hemorrhage. Int J Cardiol. 2014;176(3):1362–4. https://doi.org/10.1016/j.ijcard.2014.07.279.
•• Belcour D, Jabot J, Grard B, Roussiaux A, Ferdynus C, Vandroux D, et al. Prevalence and risk factors of stress cardiomyopathy after convulsive status epilepticus in ICU patients. Crit Care Med. 2015;43(10):2164–70. Stress cardiomyopathy occurs in a large proportion of patients admitted to the ICU for convulsive status epilepticus (56%) demonstrating that patients with hemodynamic compromise should be screened for stress cardiomyopathy. https://doi.org/10.1097/CCM.0000000000001191.
Wijdicks EF. The multifaceted care of status epilepticus. Epilepsia. 2013;54(Suppl 6):61–3. https://doi.org/10.1111/epi.12280.
Hocker S. Systemic complications of status epilepticus—an update. Epilepsy Behav: E&B. 2015;49:83–7. https://doi.org/10.1016/j.yebeh.2015.04.024.
Manno EM, Pfeifer EA, Cascino GD, Noe KH, Wijdicks EF. Cardiac pathology in status epilepticus. Ann Neurol. 2005;58(6):954–7. https://doi.org/10.1002/ana.20677.
• Zelano J, Moller F, Dobesberger J, Trinka E, Kumlien E. Infections in status epilepticus: a retrospective 5-year cohort study. Seizure. 2014;23(8):603–6. Showed that infections in patients with SE are associated with a longer SE duration and an unfavorable outcome. https://doi.org/10.1016/j.seizure.2014.04.012.
Sutter R, Marsch S, Fuhr P, Ruegg S. Mortality and recovery from refractory status epilepticus in the intensive care unit: a 7-year observational study. Epilepsia. 2013;54(3):502–11. https://doi.org/10.1111/epi.12064.
•• Semmlack S, Tschudin-Sutter S, Widmer AF, Valenca M, Ruegg S, Marsch S, et al. Independent impact of infections on the course and outcome of status epilepticus: a 10-year cohort study. J Neurol. 2016;263(7):1303–13. Infections at SE onset are frequent and associated with prolonged medical care, treatment refractory SE, higher morbidity, and mortality. https://doi.org/10.1007/s00415-016-8140-1.
Sutter R, Tschudin-Sutter S, Grize L, Fuhr P, Bonten MJ, Widmer AF, et al. Associations between infections and clinical outcome parameters in status epilepticus: a retrospective 5-year cohort study. Epilepsia. 2012;53(9):1489–97. https://doi.org/10.1111/j.1528-1167.2012.03576.x.
Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis—an overview for clinicians. Crit Care (Lond, Engl). 2005;9(2):158–69.
Makki N, Hajj G, Schmidt GA. Seizure-induced acute urate nephropathy: case report and review. Chest. 2013;144(2):666–9. https://doi.org/10.1378/chest.12-2129.
Felcher A, Commichau C, Cao Q, Brown MJ, Torres A, Francis CW. Disseminated intravascular coagulation and status epilepticus. Neurology. 1998;51(2):629–31. https://doi.org/10.1212/WNL.51.2.629.
Squizzato A, Hunt BJ, Kinasewitz GT, Wada H, Ten Cate H, Thachil J, et al. Supportive management strategies for disseminated intravascular coagulation. An international consensus. Thromb Haemost. 2016;115(5):896–904. https://doi.org/10.1160/TH15-09-0740.
Gawlak D, Luniewska J, Stojak W, Hovhannisyan A, Strozynska A, Manka-Malara K, et al. The prevalence of orodental trauma during epileptic seizures in terms of dental treatment—survey study. Neurol Neurochir Pol. 2017;51(5):361–5. https://doi.org/10.1016/j.pjnns.2017.06.004.
Finelli PF, Cardi JK. Seizure as a cause of fracture. Neurology. 1989;39(6):858–60. https://doi.org/10.1212/WNL.39.6.858.
Pushpakumara J, Sivathiran S, Roshan L, Gunatilake S. Bilateral posterior fracture-dislocation of the shoulders following epileptic seizures: a case report and review of the literature. BMC Res Notes. 2015;8(1):704. https://doi.org/10.1186/s13104-015-1674-y.
Heckmann JG, Stangl R, Erbguth F, Rutherford H, Neundorfer B. Nontraumatic seizure-associated bilateral fractures of the head of the humerus. Intensive Care Med. 1999;25(5):548–9.
Brackstone M, Patterson SD, Kertesz A. Triple “E” syndrome: bilateral locked posterior fracture dislocation of the shoulders. Neurology. 2001;56(10):1403–4. https://doi.org/10.1212/WNL.56.10.1403.
Aldebeyan S, Aoude A, Van Lancker H. Traumatic posterior shoulder dislocation with a large engaging Hill-Sachs lesion: splinting technique. Am J Emerg Med. 2016;34(3):682. e1-.e3
Rawes ML, Roberts J, Dias JJ. Bilateral fibula head fractures complicating an epileptic seizure. Injury. 1995;26(8):562. https://doi.org/10.1016/0020-1383(95)00086-O.
Nunes-de Oliveira S, Moniz JC, Bandeira-Costa JC. Status epilepticus, dissection of the vertebral artery and ischaemic vascular accident in the pons. Rev Neurol. 2007;44(10):635–6.
Konda SR, Kester BS, Fisher N, Behery OA, Crespo AM, Egol KA. Acute compartment syndrome of the leg. J Orthop Trauma. 2017;31(Suppl 3):S17–s8. https://doi.org/10.1097/BOT.0000000000000894.
Spatola M, Alvarez V, Rossetti AO. Benzodiazepine overtreatment in status epilepticus is related to higher need of intubation and longer hospitalization. Epilepsia. 2013;54(8):e99–e102. https://doi.org/10.1111/epi.12235.
Cascino GD, Hesdorffer D, Logroscino G, Hauser WA. Treatment of nonfebrile status epilepticus in Rochester, Minn, from 1965 through 1984. Mayo Clin Proc. 2001;76(1):39–41. https://doi.org/10.4065/76.1.39.
Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345(9):631–7. https://doi.org/10.1056/NEJMoa002141.
Misra UK, Kalita J, Patel R. Sodium valproate vs phenytoin in status epilepticus: a pilot study. Neurology. 2006;67(2):340–2. https://doi.org/10.1212/01.wnl.0000224880.35053.26.
Agarwal P, Kumar N, Chandra R, Gupta G, Antony AR, Garg N. Randomized study of intravenous valproate and phenytoin in status epilepticus. Seizure. 2007;16(6):527–32. https://doi.org/10.1016/j.seizure.2007.04.012.
Hocker SE. Status Epilepticus. Continuum (Minneap Minn). 2015;21(5 Neurocritical Care):1362–83. https://doi.org/10.1212/CON.0000000000000225.
Adams BD, Buckley NH, Kim JY, Tipps LB. Fosphenytoin may cause hemodynamically unstable bradydysrhythmias. J Emerg Med. 2006;30(1):75–9. https://doi.org/10.1016/j.jemermed.2005.01.034.
Siebert WJ, McGavigan AD. Requirement for cardiac telemetry during intravenous phenytoin infusion: guideline fact or guideline fiction? Intern Med J. 2013;43(1):7–17. https://doi.org/10.1111/j.1445-5994.2012.02935.x.
•• Parsai S, Hariri I, Taleb M, Yoon Y. A literature review revisiting phenytoin-induced sinus arrest. Am J Ther. 2016;23(4):e1091–3. Recent literature review of the cardiac sides effects of IV phenytoin infusion. https://doi.org/10.1097/MJT.0000000000000159.
Limdi NA, Shimpi AV, Faught E, Gomez CR, Burneo JG. Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology. 2005;64(2):353–5. https://doi.org/10.1212/01.WNL.0000149527.47600.5A.
Gerstner T, Teich M, Bell N, Longin E, Dempfle CE, Brand J, et al. Valproate-associated coagulopathies are frequent and variable in children. Epilepsia. 2006;47(7):1136–43. https://doi.org/10.1111/j.1528-1167.2006.00587.x.
Nasreddine W, Beydoun A. Valproate-induced thrombocytopenia: a prospective monotherapy study. Epilepsia. 2008;49(3):438–45. https://doi.org/10.1111/j.1528-1167.2007.01429.x.
•• Strzelczyk A, Zollner JP, Willems LM, Jost J, Paule E, Schubert-Bast S, et al. Lacosamide in status epilepticus: systematic review of current evidence. Epilepsia. 2017;58(6):933–50. Updated review on the effectiveness and side effects of lacosamide in the treatment of SE. https://doi.org/10.1111/epi.13716.
Hofler J, Trinka E. Lacosamide as a new treatment option in status epilepticus. Epilepsia. 2013;54(3):393–404. https://doi.org/10.1111/epi.12058.
Baumeister FA, Oberhoffer R, Liebhaber GM, Kunkel J, Eberhardt J, Holthausen H, et al. Fatal propofol infusion syndrome in association with ketogenic diet. Neuropediatrics. 2004;35(4):250–2. https://doi.org/10.1055/s-2004-820992.
Diedrich DA, Brown DR. Analytic reviews: propofol infusion syndrome in the ICU. J Intensive Care Med. 2011;26(2):59–72. https://doi.org/10.1177/0885066610384195.
Iyer VN, Hoel R, Rabinstein AA. Propofol infusion syndrome in patients with refractory status epilepticus: an 11-year clinical experience. Crit Care Med. 2009;37(12):3024–30. https://doi.org/10.1097/CCM.0b013e3181b08ac7.
Rossetti AO, Reichhart MD, Schaller MD, Despland PA, Bogousslavsky J. Propofol treatment of refractory status epilepticus: a study of 31 episodes. Epilepsia. 2004;45(7):757–63. https://doi.org/10.1111/j.0013-9580.2004.01904.x.
Mayette M, Gonda J, Hsu JL, Mihm FG. Propofol infusion syndrome resuscitation with extracorporeal life support: a case report and review of the literature. Ann Intensive Care. 2013;3(1):32. https://doi.org/10.1186/2110-5820-3-32.
Gaspard N, Foreman B, Judd LM, Brenton JN, Nathan BR, McCoy BM, et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia. 2013;54(8):1498–503. https://doi.org/10.1111/epi.12247.
Cereda C, Berger MM, Rossetti AO. Bowel ischemia: a rare complication of thiopental treatment for status epilepticus. Neurocrit Care. 2009;10(3):355–8. https://doi.org/10.1007/s12028-008-9168-6.
Ji T, Zubkov AY, Wijdicks EFM, Manno EM, Rabinstein AA, Kotagal S. Massive tongue swelling in refractory status epilepticus treated with high-dose pentobarbital. Neurocrit Care. 2009;10(1):73–5. https://doi.org/10.1007/s12028-008-9072-0.
Pillai U, Hothi JC, Bhat ZY. Severe propylene glycol toxicity secondary to use of anti-epileptics. Am J Ther. 2014;21(4):e106–9. https://doi.org/10.1097/MJT.0b013e31824c407d.
Barnes BJ, Gerst C, Smith JR, Terrell AR, Mullins ME. Osmol gap as a surrogate marker for serum propylene glycol concentrations in patients receiving lorazepam for sedation. Pharmacotherapy. 2006;26(1):23–33. https://doi.org/10.1592/phco.2006.26.1.23.
• Zeiler FA, Zeiler KJ, Teitelbaum J, Gillman LM, West M. Modern inhalational anesthetics for refractory status epilepticus. Can J Neurol Sci. 2015;42(2):106–15. Systematic review of the literature on the use of modern inhalational anesthetic agents for refractory status epilepticus. https://doi.org/10.1017/cjn.2014.121.
•• Legriel S, Lemiale V, Schenck M, Chelly J, Laurent V, Daviaud F, et al. Hypothermia for neuroprotection in convulsive status epilepticus. N Engl J Med. 2016;375(25):2457–67. Randomized control trial demonstrating that induced hypothermia (32-34 °C for 24 h) is not associated with better 90-day outcomes in patients with convulsive status epilepticus. https://doi.org/10.1056/NEJMoa1608193.
•• Cervenka MC, Hocker S, Koenig M, Bar B, Henry-Barron B, Kossoff EH, et al. Phase I/II multicenter ketogenic diet study for adult superrefractory status epilepticus. Neurology. 2017;88(10):938–43. Phase I/II multicenter study providing class IV evidence on the feasibility and safety of ketogenic diet for adults with super refractory status epilepticus. https://doi.org/10.1212/WNL.0000000000003690.
Acknowledgments
The authors thank Sarah L. Clark Pharm.D., R.Ph., for revising the concentration of anesthetic agents presented in this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Maximiliano A. Hawkes declares that he has no conflict of interest.
Sara Hocker has received compensation for consulting for SAGE Therapeutics and has received an honorarium from Continuum.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neurology of Systemic Diseases
Rights and permissions
About this article
Cite this article
Hawkes, M.A., Hocker, S.E. Systemic Complications Following Status Epilepticus. Curr Neurol Neurosci Rep 18, 7 (2018). https://doi.org/10.1007/s11910-018-0815-9
Published:
DOI: https://doi.org/10.1007/s11910-018-0815-9