Abstract
Treatment options for managing traumatic brain injury remain limited. Therapies that limit the development of secondary brain injury—the delayed injury that can occur days to weeks after initial presentation—would have a major impact on outcomes and reduce the medical, social, and economic burden of this devastating disease. A growing body of evidence suggests that inflammation and activation of the immune system is a central driver of secondary brain injury. This article reviews the evidence for inflammation mediating secondary injury after head trauma and outlines potential approaches for immunomodulatory therapies after traumatic brain injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite intense study and improvements in critical care, outcomes after traumatic brain injury (TBI) continue to be poor and difficult to predict. TBI remains the commonest cause of death in individuals younger than 40 years in the developed world, and in developing countries the incidence and societal costs of TBI are rising [1]. Long-term complications including epilepsy, dementia, and other neurocognitive disabilities such as depression, impulsivity, and poor executive function result in significant individual disabilities and societal costs for survivors [2]. Novel therapies that limit the progression of injury after head trauma are thus sorely needed.
Brain damage induced by traumatic injury occurs in distinct phases [2]. “Primary injury” refers to the initial damage produced by external trauma such as hemorrhage from ruptured blood vessels, shearing of axons, and direct compression of neurons and glia. Little can be done to treat primary injury except for preventing the traumatic insult. A second, delayed phase of damage, termed “secondary injury,” occurs hours to days and even weeks after the initial event [2, 3]. Prevention of secondary brain injury is a major focus of care in patients admitted to the hospital with TBI. Current approaches for limiting secondary injury focus on treating physiological and metabolic derangements (such as hypotension, acidosis, hypoxia, fever, intracranial hypertension, and seizures) that impair brain tissue oxygenation and cerebral blood flow [3]. Such measures, although undoubtedly important, fail to target important molecular signaling cascades that mediate secondary damage.
Numerous lines of evidence suggest that immune system activation and inflammation are central mediators of secondary injury after brain trauma. Targeted therapies that limit inflammation thus hold great promise for improving mortality and functional outcome after TBI. This review will outline how the immune system is activated after TBI and discuss novel strategies for inflammation-targeted therapy in TBI. Finally, new experimental approaches and animal models that hold promise in providing greater insight into the immune responses that occur after brain injury will be discussed.
The Innate Immune Response After Infection and Sterile Tissue Injury
The first line of defense against infections involves activation of the innate immune system [4]. Myeloid-derived cells, including macrophages and dendritic cells, express unique receptors known as pattern recognition receptors (PRRs) [5] that recognize conserved molecular motifs expressed by broad classes of infectious organisms. These motifs, known as pathogen-associated molecular patterns (PAMPs), include bacterial and fungal cell wall components, bacterial DNA, and viral coat proteins. PRRs include the Toll-like receptors (TLRs), C-type lectin receptors, NOD-like receptors (NLRs), and retinoic acid inducible gene I like receptors [5]. Different members of these PRR families localize to distinct cellular compartments, such as the plasma membrane, endosome, and cytoplasm, and recognize distinct classes of PAMPs. PRR activation induces the transcription of inflammatory cytokines, including tumor necrosis factor (TNF) α, interleukins (such as IL-1β, IL-6, and IL-18), and interferons, which, by altering gene expression in immune cells and recruiting leukocytes to sites of infection, orchestrate a systemic inflammatory response [5–7]. Some classes of PRRs work synergistically to upregulate cytokine production. For example, activation of TLR4 induces a signaling cascade that ultimately activates the transcriptional regulator nuclear factor κB, resulting in increased expression of pro-IL-1β and pro-IL-18. These proproteins are, however, biologically inactive until they are processed by the enzyme caspase 1. Coincident activation of NLRs results in the assembly of a multimeric complex composed of NLRs, caspase 1, and other adaptor proteins known as the inflammasome [6, 7] that ultimately cleave pro-IL-1β and pro-IL-18, activating these interleukins for secretion. In addition to upregulating cytokine expression, PRRs also induce the expression of co-stimulatory molecules on the surface of macrophages and dendritic cells that activate T and B lymphocytes, thus regulating clonal expansion and the adaptive immune system [4, 5].
Once initiated by PRR activation, the inflammatory response produces a characteristic pattern of tissue-level and systemic changes, including fever, increased local blood flow, and increased tissue permeability that help to promote leukocyte infiltration and clearance of infection in affected tissues. Although the inflammatory response is essential to contain and eradicate infections, excess inflammation and immune system activation causes collateral tissue damage that leads to organ dysfunction. Inflammation-mediated tissue injury occurs through a variety of mechanisms—including direct injury from leukocytes and upregulation of the coagulation cascade—and underlies the multiorgan failure that occurs with severe infection and sepsis [8].
Remarkably, in addition to recognizing external microbial pathogens, PRRs also recognize and respond to intracellular molecules released by damaged or stressed cells within the body. Termed “damage-associated molecular patterns” (DAMPs) or “alarmins,” these molecules include nuclear and cytoplasmic proteins, such as high mobility group box 1 (HMGB1), histones, S100B, and heat shock proteins, DNA, and other small molecules released during cellular stress, including reactive oxygen species, uric acid, and ATP [6, 9]. Although structurally distinct from microbe-associated molecular patterns, DAMPs and PAMPs share the important characteristic that they are not found in the extracellular space under normal conditions. Recognition of self-molecules released during tissue damage explains how inflammatory responses are amplified after infection, but also provides a mechanism for immune system activation after sterile tissue injury. Similarly to the inflammatory response after infection, inflammation in response to noninfectious tissue injury has both protective and deleterious effects; however, a growing body of evidence supports the notion that limiting inflammation may improve outcomes in many disease states, including autoimmune diseases, coronary artery disease, acute respiratory distress syndrome, sepsis, and multisystem trauma [9].
Immune Responses in the Brain
Because of highly regulated trafficking across the blood–brain barrier (BBB), and since direct inoculation of the brain parenchyma with antigen fails to elicit a robust immune response, the brain has traditionally been thought of as an “immune-privileged” organ. In spite of its relative immune privilege, however, robust trafficking of immune cells (especially memory T cells) occurs between the periphery and the central nervous system (CNS) under normal circumstances across the BBB and at the choroid plexus epithelium [blood–cerebrospinal fluid (CSF) barrier] [10, 11]. CSF also drains directly into cervical lymph nodes, providing a pathway for egress of antigens from the CNS to the periphery, where they can be processed by antigen-presenting cells [12]. Although the brain parenchyma lacks the traditional sentinel cells of the innate immune system found in the periphery, a unique class of myeloid-derived cells—microglia—provides crucial immune surveillance and can initiate brain-specific inflammatory responses [13, 14].
Microglia make up about 10 % of the total brain volume, and support multiple essential functions both under normal conditions and after injury. At rest, microglia are highly ramified cells whose processes continually sample the local microenvironment [15, 16]. Microglial processes make transient contacts with synapses [17], and recent studies suggest they the play important roles in synaptic pruning during development [18] and activity-dependent synaptic plasticity and learning in adulthood [19•]. The resting phenotype is promoted by “calming,” inhibitory signals released by neurons and astrocytes [13, 14]. After focal injury, DAMP release from injured cells and a reduction in inhibitory signals from healthy cells induce microglia to take on an “activated” phenotype. Activated microglia retract their fine processes and rapidly converge on sites of injury to promote inflammation and tissue repair [14, 15].
Activated microglial cells acquire distinct phenotypes based on the type, duration, and intensity of the activating stimulus. The classic, macrophage-like phenotype, termed M1, has increased transcription of nuclear factor κB, produces proinflammatory cytokines (including TNF-α, IL-1β, IL-6, and IL-23), has the ability to phagocytose extracellular debris, and may play a role in antigen presentation to circulating lymphocytes [14]. Other phenotypes, termed M2, may have more prominent roles in tissue repair [14]. Most studies investigating phenotype switching of activated microglia have used cell culture systems; therefore, the relative importance and regulation of these phenotypes in vivo are not well understood. However, multiple lines of evidence support a vital role for microglia in mediating brain-specific inflammatory and immune responses.
Like macrophages and dendritic cells in the periphery, microglia express PRRs, including TLR2, TLR4 [14], and components of the NLRP3 inflammasome [20]. Activation of these PRRs induces cytokine release in the brain, and these cytokines induce multiple downstream effects, including changes in cerebral blood flow and increases in BBB permeability, and influx of peripheral leukocytes into the brain. Microglia also express purinergic receptors [14, 15] and NMDA-type glutamate receptors [21••]. Thus, in addition to inducing inflammatory responses through PRR signaling, microglial activation can occur through ATP and glutamate released by damaged, depolarized neurons. Recent studies implicate microglia-mediated inflammation in the pathogenesis of a wide variety of neurologic diseases, including neurodegenerative diseases such as Alzheimer’s disease and amyotrophic lateral sclerosis, neurodevelopmental disorders, ischemic and hemorrhagic stroke, and TBI [14]. Although microglia are central in mediating brain inflammation, other CNS cell types, including neurons and astrocytes, may also have important roles. These cell types express unique inflammasomes, and may also interact with circulating lymphocytes [22]. The regulation of immune signaling in the brain by neurons and astrocytes is only beginning to be understood.
Evidence for Inflammation After TBI
Early rodent studies documented two distinct phases of BBB disruption after experimental TBI: an initial phase occurring immediately after head injury and persisting for about 24 h, and a second, delayed, phase that peaks 3–5 days after the event [23, 24]. Although early BBB disruption is likely largely mechanical (e.g., from sheared blood vessels), the factors responsible for the delayed BBB opening were unclear, but coincided with the timing of secondary brain injury [25]. Studies in humans failed to show distinct phases of BBB disruption; however, it is clear that there is prolonged BBB dysfunction that can occur days to weeks [25–27] and even months to years [28] after the primary event. It is now evident that much of this prolonged BBB dysfunction is mediated by inflammation and activation of the immune system.
Studies in both experimental animals and humans have shown that isolated head injury produces the key features of the inflammatory cascade outlined earlier, including DAMP release, activation of PRRs, upregulation of cytokines, and leukocyte infiltration. Brain tissue samples taken from animals with experimental traumatic injury [29, 30] and humans [31] show a robust inflammatory response with activated microglia and infiltrating leukocytes (including neutrophils, B and T lymphocytes, and mononuclear cells). In these studies, brain tissue samples from humans taken several days after primary injury show a greater degree of inflammation than samples taken within 24 h of the traumatic insult, corresponding to the timing of the secondary, delayed, increase in BBB permeability that occurs in animal models of TBI.
What triggers the inflammatory response after TBI? Severe TBI induces DAMP release into both the CSF and serum. A recent study comparing 106 patients with severe TBI admitted to a single institution with an equal number of age-matched controls showed that plasma HMGB1 levels on admission were markedly higher in TBI patients, and that serum HMGB1 levels correlated with 1-year outcome [32]. HMGB1 levels are also elevated in the CSF after TBI [33•]. A nuclear DNA-binding protein expressed in neurons and glial cells that is involved under normal conditions in transcriptional control, HMGB1 can be released passively after cell necrosis or actively from intact, but stressed, cells using a nonclassic mechanism involving the secretory lysosome. Once released, it can bind to multiple PRRs, including TLR2 and TLR4 [34, 35].
Other recent studies showed that brain injury results in the release of the astrocyte-specific proteins GFAP and S100B into the bloodstream, ultimately leading to the development of specific antibodies against these proteins in the serum [36, 37]. Anti-GFAP antibody production peaks 5–10 days after initial injury, corresponding to the time course of secondary injury development [37]. In animal models of TBI, DAMP release after injury activates multiple classes of PRRs. HMGB1 released in the brain after controlled cortical impact (a validated animal model of focal contusion) in mice activates TLR4, which subsequently upregulates aquaporin 4 expression, leading to increased cerebral edema [33•]. Another recent study in rats showed marked increased expression of the NLRP3 inflammasome after experimental focal contusion [38]. Blocking PRR signaling reduces the development of secondary brain injury after experimental TBI. Inhibiting TLR2 and TLR4 activation reduces the development of secondary brain injury, since mutant mice lacking functional TLR4 [33•] and transgenic mice lacking either TLR2 [39] or TLR4 [40] show decreased lesion volume and secondary apoptosis.
A multitude of studies in experimental animals and humans show robust expression of cytokines and other immune modulators in the brain (from both CSF and brain interstitial fluid taken from microdialysis samples) and the periphery (from serum) after head trauma [41, 42]. CSF and microdialysate samples likely reflect brain-specific cytokine production. Consistent with their role as sentinel immune cells in the brain, microglia are potent producers of multiple classes of cytokines, such as IL-1 and TNF, and likely provide most of the early wave of cytokine release that occurs within the first hours to days after injury [14, 41]. Other CNS cell types, including neurons and astrocytes, may also produce immune-stimulating molecules; however, the timing and relative contribution of these cell types to the inflammatory response is not clear. The source of cytokines measured in the serum is more difficult to assess, and likely reflects a combination of brain and peripheral production. Although many of the expressed cytokines are proinflammatory (such as TNF-α and IL1-β), some molecules likely have anti-inflammatory effects (such as transforming growth factor β) and may promote tissue repair (such as IL-6) [41]. Cytokine function is context specific; thus, the same molecule can have both proinflammatory and anti-inflammatory effects depending on its concentration, its pattern of release, and the overall cellular milieu. For example, although IL-1β is largely thought of as a proinflammatory molecule that worsens brain injury, it can exert neuroprotective effects through upregulation of astrocyte-derived nerve growth factor, depending on the local availability of oxygen and glucose [41]. Similarly, although transforming growth factor β is normally thought to foster immunosuppression, in concert with IL-6 it drives differentiation of the proinflammatory TH17 subset of CD4+ T lymphocytes [43]. At this point, the specific functions of different cytokines in driving immune and inflammatory responses after TBI are not clear. Indeed, a particular cytokine likely has multiple divergent functional roles depending on when it is released after the initial traumatic event, other co-released factors, and differences in the physiological microenvironment that occur with different injury mechanisms (such as diffuse axonal injury, focal contusion, and blast-related injury).
Different cytokines have distinct temporal profiles of release from the brain after head injury. TNF-α, IL-1β, and IL-8 are released early (hours to 2 days) after the traumatic event, reflecting their putative roles as inflammatory response initiators [41]. Molecules involved in promoting leukocyte infiltration, such as the chemoattractant CCL5 (also known as RANTES), and lymphocyte differentiation, such as IL-6, are released later, and brain levels can stay elevated for up to 1 week after injury [41]. This is consistent with the known delayed time course of leukocyte infiltration into the brain after TBI, and also agrees with human studies showing elevated CSF levels of soluble intercellular cell adhesion molecule 1 (a protein required for lymphocyte translocation across the BBB) persist for up to 10 days after severe TBI [44].
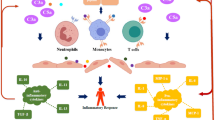
Taken together, the studies described above suggest that TBI induces a stereotyped pattern of inflammation in the brain (Fig. 1). First, damaged or stressed (e.g., from prolonged depolarization or energy failure) cells in the CNS release DAMPs, which activate multiple classes of PRRs on microglial cells (and possibly neurons and astrocytes as well). PRR activation induces cytokine release, which initiates an inflammatory cascade that results in disruption of the BBB, microvascular dysfunction, cerebral edema, and leukocyte infiltration that leads to worsening secondary brain injury. Cytokines released early, such as TNF-α and IL-1β, most likely serve as master initiators of the inflammatory response, ultimately inducing the release of other immune-modulating molecules from the brain and activating cells of the adaptive immune system (B and T lymphocytes) that recognize epitopes from DAMPs processed by microglia in the brain and by professional antigen-presenting cells in the periphery. Further study is needed to understand the fine details of the immune response after sterile brain injury, such as the specific functions of different classes of infiltrating leukocytes in promoting secondary injury and tissue repair. This detailed knowledge will hopefully result in therapies that limit the untoward effects of inflammation while maximizing the potential for repair and healing.
Pathways that initiate inflammatory responses after primary brain injury. Direct trauma and various metabolic stressors that occur during traumatic brain injury induce the release of damage-associated molecular patterns (DAMP), or alarmins, from neurons and astrocytes. These include intracellular proteins such as high mobility group box 1 (HMGB1) and S100B and small molecules such as adenosine triphosphate (ATP). Abnormal excitation can also increase glutamate levels, which can also induce N-methyl-D-aspartate (NMDA) receptor activation on microglial cells. Microglia express pattern recognition receptors which recognize DAMPs, and microglial activation in turn induces cytokine release, which upregulates inflammation. On the right, potential targeted treatments are outlined. BBB blood–brain barrier, CCL5 chemokine (C-C motif) ligand 5, HSP heat shock protein, IL1β interleukin 1β, IL6 interleukin 6, P2Y metabotropic purinergic receptor, TNFα tumor necrosis factor α, TLR Toll-like receptor
Immunotherapeutic Strategies
The vast body of knowledge documenting a robust inflammatory response after TBI suggests that targeting the immune system should limit the development of secondary brain injury. Unfortunately, no efficacious immunomodulatory or immunosuppressive treatments currently exist. Traditional immunosuppressive treatments, such as glucocorticoids, may actually worsen outcome because of other side effects of these medications [45, 46]. Despite this, multiple promising avenues exist for the development of targeted immunomodulatory therapies. Potential targets for intervention include (1) blocking the delayed leukocyte infiltration into the brain, (2) neutralizing proinflammatory cytokine activity, (3) inhibiting microglial activation, (4) blocking PRR activation, and (5) directly inhibiting the release and activity of DAMPs. Many of these options are being actively investigated as potential therapeutic strategies.
Although the efficacy of blocking leukocyte infiltration on secondary injury prevention after TBI has not been rigorously studied, therapeutic strategies used to treat other autoimmune neurologic diseases such as multiple sclerosis may be effectively co-opted as treatments for TBI. In particular, natalizumab—which blocks T-cell entry across the BBB by inhibiting lymphocyte α4 integrin signaling [47–49]—may be an effective inhibitor of secondary brain injury. A prior study in rodents showed that experimental seizures (induced by pilocarpine infusion) caused leukocyte infiltration into the brain, and that a single infusion of a monoclonal antibody against integrins, even after the initial seizure, reduced subsequent seizures and blocked epileptogenesis [50]. Thus, this treatment may be particularly effective at reducing late complications induced by immune-mediated neural circuit reorganization such as epilepsy. The risk of developing progressive multifocal leukoencephalopathy, a major adverse effect of long-term natalizumab use caused by reactivation of latent JC virus in the brain [51, 52], would likely be minimal with the short time course of treatment required for TBI patients. Fingolimod, an immunomodulating therapy used for multiple sclerosis treatment that sequesters lymphocytes in lymph nodes [53–55], may also prove useful in targeting inflammation after brain injury.
Blocking the activity of cytokines may be an effective way of reducing inflammation after TBI. Targeted inhibitors of both TNF-α and IL-1β are in current clinical use for treating autoimmune diseases such as rheumatoid arthritis, Crohn’s disease, and psoriasis, opening up the possibility for clinical trials of these medications in TBI. A randomized phase II clinical trial of one of these medications, a recombinant soluble receptor antagonist of IL-1 (rIL-1ra, Anakinra), has just recently been performed. Subcutaneously administered rIL-1ra penetrated into the plasma and brain extracellular fluid, and directly altered the cerebral cytokine profile measured with microdialysis [56••]. The results of a larger randomized controlled trial are eagerly awaited, since other recent studies showed that administration of an IL-1β neutralizing antibody (either by direct intracerebroventricular injection prior to experimental trauma [57] or intraperitoneally after primary injury [58]) can inhibit microglial activation, reduce the number of infiltrating leukocytes, and improve cerebral edema and functional outcome in rodents.
Blocking microglial activation directly is another attractive option for targeted treatment of inflammation after TBI. Minocycline, a widely used antibiotic related to tetracycline, can inhibit microglial activation and limit inflammatory responses in the brain. Multiple studies show that minocycline administration suppresses microglial activation, reduces cerebral edema and lesion volume, and suppresses caspase 1 activation after experimental TBI in rodents [59–61]. Microglial inhibition seems to occur through the activation of endocannabinoid receptors [62]. Given the long history of its clinical use and its favorable safety profile, minocycline is an appealing candidate drug that may be easily translatable from the laboratory to clinical use.
Studies evaluating PRR inhibitors as medications for TBI are lacking; however, several selective TLR inhibitors exist. The clinical safety profile and ability to cross the BBB of these medications has not been determined. Since DAMPs initiate the inflammatory response after sterile injury, inhibiting the biological activity of DAMPs could be an especially effective way of treating inflammation after TBI. A recent study in rodents gave an exciting proof of principle for this concept [63••]. Administration of an anti-HMGB1 monoclonal antibody 5 min and 6 h after fluid percussion injury in rats produced dramatic reductions in cytokine release, cerebral edema, and lesion volume along with significant improvements in motor function assessed by rotarod testing. Anti-HMGB1 antibodies appear to block the release of HMGB1 from neurons, rather than neutralizing already released HMGB1. Anti-HMGB1 antibody therapy also improves mortality in animal models of severe sepsis [64], suggesting that inhibiting DAMP function may be a general method for limiting the deleterious effects of inflammation in a variety of disparate disease states.
Future Directions
Recent studies using novel model organisms and sophisticated imaging techniques continue to refine our knowledge of how the immune system becomes activated after brain injury, and these approaches will undoubtedly allow significant advances in our understanding of brain-injury-triggered inflammation.
A recently developed Drosophila model of TBI is particularly exciting, since this will allow the power of forward genetic approaches to rapidly identify conserved genes that are protective after TBI [65•]. Early results from these studies show a clear upregulation of genes involved in the innate immune system, further confirming the importance of immune signaling pathways in TBI, and also show remarkable differences in mortality in different genetic strains of fruit flies. A recent study in rodents also demonstrated differences in TBI-induced inflammation in different genetic strains [66]. It is likely that genetic background impacts injury progression in humans as well, and studying different genetic factors in patients that affect clinical outcome will allow personalization of care after TBI.
Finally, new optical imaging techniques, particularly in vivo multiphoton microscopy, are allowing investigators to view the inflammatory response in brain tissue in real time with extraordinary spatial resolution [15–17, 67••]. A recent study documented the spatiotemporal dynamics of microglial activation and neutrophil infiltration in live anesthetized mice after a cranial compression injury [67••]. Application of the reactive oxygen species scavenger glutathione to the surface of the skull (which was thinned to produce mild TBI and provide a window for imaging) after injury almost completely abolished the inflammatory response. Such studies will allow a detailed understanding of the molecular and cellular events that occur during brain inflammatory responses that will undoubtedly provide new insights to allow the development of novel therapies for this currently devastating disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Roozenbeek B, Maas AIR, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol. 2013;9:231–6.
Maas AIR, Stochetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–41.
Rosenfeld JV, Maas AIR, Bragge P, Morganti-Kossman MC, Manley GT, Gruen RL. Early management of severe traumatic brain injury. Lancet. 2012;380:1088–98.
Medzhitov R, Janeway C. Innate immunity. N Engl J Med. 2000;343:338–44.
Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20.
Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–61.
Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–86.
Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51.
Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, et al. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–9.
Ransohoff RM, Kivisäkk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81.
Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–35.
Weller RO, Engelhardt B, Phillips MJ. Lymphocyte targeting of the central nervous system: a review of afferent and efferent CNS-immune pathways. Brain Pathol. 1996;6:275–88.
Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–62.
Benarroch EE. Microglia: multiple roles in surveillance, circuit shaping, and response to injury. Neurology. 2013;81:1079–88.
Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8.
Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8.
Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80.
Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–89.
Parkhurst CN, Yang G, Ninan I, Savas JN, Yates 3rd JR, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–609. Inducible expression of diphtheria toxin in microglia using transgenic technology allowed the authors to selectively ablate microglial cells in adult mice. Removing microglial cells caused deficits in learning that are mediated by brain-derived neurotrophic factor secreted by microglial cells.
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–65.
Kaindl AM, Degos V, Peineau S, Gouadon E, Chhor V, Loron G, et al. Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol. 2012;72:536–49. This is an intriguing study showing that microglia express functional NMDA receptors, and that microglial NMDA receptor activation induces cytokine release. Selective knockout of NMDA receptors in microglia protected mice from NMDA-receptor-mediated damage caused by intracerebral ibotenate injection.
de Rivero Vaccari JP, Dietrich WD, Keane RW. Activation and regulation of cellular inflammasomes: gaps in our knowledge for central nervous system injury. J Cereb Blood Flow Metab. 2014;34:369–75.
Başkaya MK, Rao AM, Doğan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett. 1997;226:33–6.
Holmin S, Mathiesen T. Biphasic edema development after experimental brain contusion in rat. Neurosci Lett. 1995;194:97–100.
Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6393-403.
Kirchhoff C, Stegmaier J, Bogner V, Buhmann S, Mussack T, Kreimeier U, et al. Intrathecal and systemic concentration of NT-proBNP in patients with severe traumatic brain injury. J Neurotrauma. 2006;23:943–9.
Lenzlinger PM, Marx A, Trentz O, Kossmann T, Morganti-Kossmann MC. Prolonged intrathcal release of soluble Fas following severe traumatic brain injury in humans. J Neuroimmunol. 2002;122:167–74.
Tomkins O, Shelef I, Kaizerman I, Eliushin A, Afawi Z, Misk A, et al. Blood-brain barrier disruption in post-traumatic epilepsy. J Neurol Neurosurg Psychiatry. 2008;79:774–7.
Holmin S, Mathiesen T, Shetye J, Biberfeld P. Intracerebral inflammatory response to experimental brain contusion. Acta Neurochir. 1995;132:110–9.
Holmin S, Mathiesen T. Long-term intracerebral inflammatory response after experimental focal brain injury in rat. Neuroreport. 1999;10:1889–91.
Holmin S, Söderlund J, Biberfeld P, Mathiesen T. Intracerebral inflammation after human brain contusion. Neurosurgery. 1998;42:291–8.
Wang KY, Yu GF, Zhang ZY, Huang Q, Dong XD. Plasma high-mobility group box 1 levels and prediction of outcome in patients with traumatic brain injury. Clin Chim Acta. 2012;413:1737–41.
Laird MD, Shields JS, Sukumari-Ramesh S, Kimbler DE, Fessler RD, Shakir B et al. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of Toll-like receptor 4. Glia. 2014;26-38. This study showed NMDA-receptor-dependent HMGB-1 release from neurons after experimental TBI. HMGB1 binding to TLR4 induced cerebral edema by upregulating aquaporin 4 expression.
Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–42.
Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202.
Marchi N, Bazarian JJ, Puvenna V, Janigro M, Ghosh C, Zhong J, et al. Consequences of repeated blood-brain barrier disruption in football players. PLoS One. 2013;8:e56805.
Zhang Z, Zoltewicz JS, Mondello S, Newsom KJ, Yang Z, Yang B, et al. Human traumatic brain injury induces autoantibody response against glial fibrillary acidic protein and its breakdown products. PLoS One. 2014;9:e92698.
Liu HD, Li W, Chen ZR, Hu YC, Zhang DD, Shen W, et al. Expression of the NLRP3 inflammasome in cerebral cortex after traumatic brain injury in a rat model. Neurochem Res. 2013;38:2072–83.
Yu ZQ, Zha JH. Genetic ablation of Toll-like receptor 2 reduces secondary brain injury caused by cortical contusion in mice. Ann Clin Lab Sci. 2012;42:26–33.
Ahmad A, Crupi R, Campolo M, Genovese T, Esposito E, Cuzzocrea S. Absence of TLR4 reduces neurovascular unit and secondary inflammatory process after traumatic brain injury in mice. PLoS One. 2013;8:e57208.
Helmy A, De Simoni MG, Guilfoyle MR, Carpenter KL, Hutchinson PJ. Cytokines and innate inflammation in the pathogenesis of human traumatic brain injury. Prog Neurobiol. 2011;95:352–72.
Woodcock T, Morganti-Kossmann MC. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98.
Pleines UE, Stover JF, Kossmann T, Trentz O, Morganti-Kossmann MC. Soluble ICAM-1 in CSF coincides with the extent of cerebral damage in patients with severe traumatic brain injury. J Neurotrauma. 1998;15:399–409.
Roberts I, Yates D, Sandercock P, Farrell B, Wasserberg J, Lomas G, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC-CRASH trial): randomized placebo-controlled trial. Lancet. 2004;364:1321–8.
Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, et al. Final results of MRC CRASH, a randomized placebo controlled trial of intravenous corticosteroids in adults with head injury—outcomes at 6 months. Lancet. 2005;365:1957–9.
von Andrian UH, Engelhardt B. α4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348:68–72.
Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348:15–23.
Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910.
Fabene PF, Navarro Mora G, Martinello M, Rossi B, Merigo F, Ottoboni L, et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14:1377–83.
Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353:369–74.
Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–81.
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–80.
Massberg S, von Andrian UH. Fingolimod and sphingosine-1-phosphate—modifiers of lymphocyte migration. N Engl J Med. 2006;355:1088–91.
Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med. 2012;366:339–47.
Helmy A, Guilfoyle MR, Carpenter KL, Pickard JD, Menon DK, Hutchison PJ. Recombinant human interleukin-1 receptor antagonist in severe traumatic brain injury: a phase II trial randomized control trial. J Cereb Blood Flow Metab. 2014;34:845–51. This is an exciting trial in humans demonstrating that subcutaneous administration of IL-1 receptor antagonist after TBI is safe, reaches therapeutic levels in the brain interstitial space, and alters cytokine profiles.
Clausen F, Hånell A, Björk M, Hillered L, Mir AK, Gram H, et al. Neutralization of interleukin-1β modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur J Neurosci. 2009;30:385–96.
Clausen F, Hånell A, Björk M, Hillered L, Mir AK, Gram H, et al. Neutralization of interleukin-1beta reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur J Neurosci. 2011;34:110–23.
Sanchez-Meija RO, Ona VO, Li M, Friedlander RM. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48:1393–9.
Homsi S, Federico F, Croci N, Palmier B, Plotkine M, Marchand-Leroux C, et al. Minocycline effects on cerebral edema: relations with inflammatory and oxidative stress markers following traumatic brain injury in mice. Brain Res. 2009;1291:122–32.
Plane JM, Shen Y, Pleasure DE, Deng W. Prospects for minocycline neuroprotection. Arch Neurol. 2010;67:1442–8.
Lopez-Rodriguez AB, Siopi E, Finn DP, Marchand-Leroux C, Garcia-Segura LM, Jafarian-Tehrani M, et al. CB1 and CB2 cannabinoid receptor antagonists prevent minocycline-induced neuroprotection following traumatic brain injury in mice. Cereb Cortex. 2013. doi:10.1093/cercor/bht202.
Okuma Y, Liu K, Wake H, Zhang J, Maruo T, Date I, et al. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol. 2012;72:373–84. Administering a monoclonal anti-HMGB1 antibody after fluid percussion injury caused a dramatic reduction in lesion volume and cerebral edema, as well as significant functional improvement. This study paves the way for human trials investigating whether inhibiting HMGB1 signaling may improve outcomes after TBI.
Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51.
Katzenberger RJ, Loewen CA, Wassarman DR, Petersen AJ, Ganetzky B, Wassarman DA. A Drosophila model of closed head injury. Proc Natl Acad Sci U S A. 2013. doi:10.1073/pnas.1316895110. The authors developed a simple, reproducible model of TBI in Drosophila, paving the way for forward genetic screens that can identify genes that may protect against secondary brain injury. Susceptibility to TBI in flies was shown to depend on genetic background and components of the innate immune system. Similar approaches have identified numerous important clinical targets in a variety of other diseases.
Günther M, Al Nimer F, Gahm C, Piel F, Mathiesen T. iNOS-mediated secondary inflammatory response differs between rat strains following experimental brain contusion. Acta Neurochir. 2012;154:689–97.
Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505223-8. The authors used in vivo multiphoton microscopy to study the dynamics of immune cell activation after a concussion-type injury in mice. They showed early changes in microglial morphology followed by infiltration of neutrophils. Adding blockers of reactive oxygen species to the surface of the thinned skull remarkably blocked most of the inflammatory response.
Compliance with Ethics Guidelines
Conflict of Interest
Ramani Balu declares he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neurotrauma
Rights and permissions
About this article
Cite this article
Balu, R. Inflammation and Immune System Activation After Traumatic Brain Injury. Curr Neurol Neurosci Rep 14, 484 (2014). https://doi.org/10.1007/s11910-014-0484-2
Published:
DOI: https://doi.org/10.1007/s11910-014-0484-2