Abstract
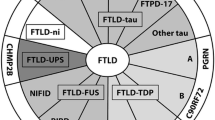
The clinical disorders associated with frontotemporal lobar degeneration (FTLD) are increasingly recognized as an important cause of early-onset dementia. Patients usually present with progressive changes in personality, behavior, or language, progressing to general cognitive impairment and ultimately death. In the past decade, improved clinical and histopathologic characterization uncovered extensive heterogeneity, and multiple clinical and pathologic FTLD subtypes were defined. Simultaneously, the discovery of four causal FTLD genes emphasized the genetic complexity associated with FTLD. More recently, the field of FTLD has gained increased attention as a result of two major findings. First, mutations in the progranulin gene (PGRN) were recognized as a major cause of FTLD with ubiquitin-positive and tau-negative inclusions (FTLDU), and subsequently the TAR DNA-binding protein-43 (TDP-43) was identified as a key protein within the ubiquitinated inclusions in FTLD-U and amyotrophic lateral sclerosis (ALS). In this report, we outline the progress made in the study of the genetic etiologies and neuropathologic substrates in FTLD.
Similar content being viewed by others
References and Recommended Reading
Graff-Radford NR, Woodruff BK: Frontotemporal dementia. Semin Neurol 2007, 27:48–57.
Neary D, Snowden JS, Gustafson L, et al.: Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998, 51:1546–1554.
Lomen-Hoerth C, Anderson, T, Miller B: The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology 2002, 59:1077–1079.
Morris HR, Khan MN, Janssen JC, et al.: The genetic and pathological classification of familial frontotemporal dementia. Arch Neurol 2001, 58:1813–1816.
Josephs KA, Holton JL, Rossor MN, et al.: Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol 2004, 30:369–373.
Mackenzie IR, Feldman HH: Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia, and frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. J Neuropathol Exp Neurol 2005, 64:730–739.
Sampathu DM, Neumann M, Kwong LK, et al.: Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol 2006, 169:1343–1352.
Neumann M, Sampathu DM, Kwong LK, et al.: Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314:130–133.
Baker M, Mackenzie IR, Pickering-Brown SM, et al.: Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006, 442:916–919.
Cruts M, Gijselinck I, van der Zee J, et al.: Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006, 442:920–924.
Hutton M, Lendon CL, Rizzu P, et al.: Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998, 393:702–705.
Skibinski G, Parkinson NJ, Brown JM, et al.: Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet 2005, 37:806–808.
Watts GD, Wymer J, Kovach MJ, et al.: Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 2004, 36:377–381.
Morita M, Al-Chalabi A, Andersen PM, et al.: A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology 2006, 66:839–844.
Vance C, Al-Chalabi, A, Ruddy, D, et al.: Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain 2006, 129:868–876.
Cairns NJ, Neumann, M, Bigio EH, et al.: TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol 2007, 171:227–240.
Wilhelmsen KC, Lynch T, Pavlou E, et al.: Localization of disinhibition-dementia-parkinsonism-amyotrophy complex to 17q21-22. Am J Hum Genet 1994, 55:1159–1165.
Foster NL, Wilhelmsen K, Sima AA, et al.: Frontotemporal dementia and parkinsonism linked to chromosome 17: a consensus conference. Conference Participants. Ann Neurol 1997, 41:706–715.
Rademakers R, Cruts M, van Broeckhoven C: The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum Mutat 2004, 24:277–295.
Gass J, Cannon A, Mackenzie IR, et al.: Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 2006, 15:2988–3001.
Hirokawa N: Microtubule organization and dynamics dependent on microtubule-associated proteins. Curr Opin Cell Biol 1994, 6:74–81.
Sato-Harada R, Okabe S, Umeyama T, et al.: Microtubuleassociated proteins regulate microtubule function as the track for intracellular membrane organelle transports. Cell Struct Funct 1996, 21:283–295.
Goedert M, Spillantini MG, Potier MC, et al.: Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J 1989, 8:393–399.
Kosik KS, Orecchio LD, Bakalis S, Neve RL: Developmentally regulated expression of specific tau sequences. Neuron 1989, 2:1389–1397.
Gustke N, Trinczek B, Biernat J, et al.: Domains of tau protein and interactions with microtubules. Biochemistry 1994, 33:9511–9522.
Goedert M, Jakes R: Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J 1990, 9:4225–4230.
Vershinin M, Carter BC, Razafsky DS, et al.: Multiplemotor based transport and its regulation by Tau. Proc Natl Acad Sci U S A 2007, 104:87–92.
King ME, Gamblin TC, Kuret J, Binder LI: Differential assembly of human tau isoforms in the presence of arachidonic acid. J Neurochem 2000, 74:1749–1757.
Goode BL, Denis PE, Panda D, et al.: Functional interactions between the proline-rich and repeat regions of tau enhance microtubule binding and assembly. Mol Biol Cell 1997, 8:353–365.
Hong M, Zhukareva V, Vogelsberg-Ragaglia V, et al.: Mutation-specific functional impairments in distinct tau isoforms of hereditary FTDP-17. Science 1998, 282:1914–1917.
Goedert M, Jakes R, Crowther RA: Effects of frontotemporal dementia FTDP-17 mutations on heparin-induced assembly of tau filaments. FEBS Lett 1999, 450:306–311.
Hutton M: Missense and splice site mutations in tau associated with FTDP-17: multiple pathogenic mechanisms. Neurology 2001, 56:S21–25.
Ingram EM, Spillantini, MG: Tau gene mutations: dissecting the pathogenesis of FTDP-17. Trends Mol Med 2002, 8:555–562.
Heutink P: Untangling tau-related dementia. Hum Mol Genet 2000, 9:979–986.
Wszolek ZK, Tsuboi Y, Ghetti B, et al.: Frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Orphanet J Rare Dis 2006, 1:30.
Bronner IF, Rizzu P, Seelaar H, et al.: Progranulin mutations in Dutch familial frontotemporal lobar degeneration. Eur J Hum Genet 2007, 15:369–374.
Leverenz JB, Yu CE, Montine TJ, et al.: A novel progranulin mutation associated with variable clinical presentation and tau, TDP43 and alpha-synuclein pathology. Brain 2007, 130:1360–1374.
Mukherjee O, Pastor P, Cairns NJ, et al.: HDDD2 is a familial frontotemporal lobar degeneration with ubiquitin-positive, tau-negative inclusions caused by a missense mutation in the signal peptide of progranulin. Ann Neurol 2006, 60:314–322.
Froelich S, Basun H, Forsell C, et al.: Mapping of a disease locus for familial rapidly progressive frontotemporal dementia to chromosome 17q12-21. Am J Med Genet 1997, 74:380–385.
Le Ber I, van der Zee J, Hannequin D, et al.: Progranulin null mutations in both sporadic and familial frontotemporal dementia. Hum Mutat 2007, In press.
Spina S, Murrell JR, Huey ED, et al.: Clinicopathologic features of frontotemporal dementia with progranulin sequence variation. Neurology 2007, 68:820–827.
van der Zee J, Le Ber I, Maurer-Stroh S, et al.: Mutations other than null mutations producing a pathogenic loss of progranulin in frontotemporal dementia. Hum Mutat 2007, 28:416.
Huey ED, Grafman J, Wassermann EM, et al.: Characteristics of frontotemporal dementia patients with a Progranulin mutation. Ann Neurol 2006, 60:374–380.
Pickering-Brown SM, Baker M, Gass J, et al.: Mutations in progranulin explain atypical phenotypes with variants in MAPT. Brain 2006, 129:3124–3126.
Bateman A, Bennett HP: Granulins: the structure and function of an emerging family of growth factors. J Endocrinol 1998, 158:145–151.
He Z, Bateman A: Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med 2003, 81:600–612.
He Z, Ong CH, Halper J, Bateman A: Progranulin is a mediator of the wound response. Nat Med 2003, 9:225–229.
Zhu J, Nathan C, Jin W, et al.: Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 2002, 111:867–878.
Daniel R, He Z, Carmichael KP, et al.: Cellular localization of gene expression for progranulin. J Histochem Cytochem 2000, 48:999–1009.
Mackenzie IR, Baker M, Pickering-Brown S, et al.: The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain 2006, 129:3081–3090.
Boeve BH: Frontotemporal dementia with parkinsonism linked to chromosome 17 redefined. Arch Neurol 2007, In press.
Brown J, Ashworth A, Gydesen S, et al.: Familial nonspecific dementia maps to chromosome 3. Hum Mol Genet 1995, 4:1625–1628.
Holm I, Englund E: The FReJA Consortium. 16th International Congress on Neuropathology. San Francisco, CA; 2006.
Cannon A, Baker M, Boeve B, et al.: CHMP2B mutations are not a common cause of frontotemporal lobar degeneration. Neurosci Lett 2006, 398:83–84.
Momeni P, Rogaeva E, Van Deerlin V, et al.: Genetic variability in CHMP2B and frontotemporal dementia. Neurodegener Dis 2006, 3:129–133.
Parkinson N, Ince PG, Smith MO, et al.: ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology 2007, In press.
Rizzu P, van Mil SE, Anar B, et al.: CHMP2B mutations are not a cause of dementia in Dutch patients with familial and sporadic frontotemporal dementia. Am J Med Genet B Neuropsychiatr Genet 2006, 141:944–946.
Babst M: A protein’s final ESCRT. Traffic 2005, 6:2–9.
Kovach MJ, Waggoner B, Leal SM, et al.: Clinical delineation and localization to chromosome 9p13.3-p12 of a unique dominant disorder in four families: hereditary inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Mol Genet Metab 2001, 74:458–475.
Neumann M, Mackenzie IR, Cairns NJ, et al.: TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol 2007, 66:152–157.
Forman MS, Mackenzie IR, Cairns NJ, et al.: Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol 2006, 65:571–581.
Guyant-Marechal L, Laquerriere A, Duyckaerts C, et al.: Valosin-containing protein gene mutations: clinical and neuropathologic features. Neurology 2006, 67:644–651.
Haubenberger D, Bittner RE, Rauch-Shorny S, et al.: Inclusion body myopathy and Paget disease is linked to a novel mutation in the VCP gene. Neurology 2005, 65:1304–1305.
Schroder R, Watts GD, Mehta SG, et al.: Mutant valosin-containing protein causes a novel type of frontotemporal dementia. Ann Neurol 2005, 57:457–461.
Woodman PG: p97, a protein coping with multiple identities. J Cell Sci 2003, 116:4283–4290.
Valdmanis PN, Dupre N, Bouchard JP, et al.: Three families with amyotrophic lateral sclerosis and frontotemporal dementia with evidence of linkage to chromosome 9p. Arch Neurol 2007, 64:240–245.
Momeni P, Schymick J, Jain S, et al.: Analysis of IFT74 as a candidate gene for chromosome 9p-linked ALS-FTD. BMC Neurol 2006, 6:44.
Seelaar H, Schelhaas HJ, Azmani A, et al.: TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain 2007, 130:1375–1385.
Neumann MK, Sampathu LK, Truax DM, et al.: 8th International Conference ADPD. Salzburg, Austria, 2007.
Verpillat P, Camuzat A, Hannequin D, et al.: Apolipoprotein E gene in frontotemporal dementia: an association study and meta-analysis. Eur J Hum Genet 2002, 10:399–405.
Cruts M, Rademakers R, Gijselinck I, et al.: Genomic architecture of human 17q21 linked to frontotemporal dementia uncovers a highly homologous family of low-copy repeats in the tau region. Hum Mol Genet 2005, 14:1753–1762.
Caffrey TM, Joachim C, Paracchini S, et al.: Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet 2006, 15:3529–3537.
Myers AJ, Pittman AM, Zhao AS, et al.: The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol Dis 2007, 25:561–570.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rademakers, R., Hutton, M. The genetics of frontotemporal lobar degeneration. Curr Neurol Neurosci Rep 7, 434–442 (2007). https://doi.org/10.1007/s11910-007-0067-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11910-007-0067-6