Abstract
Purpose of Review
While mosquitoes have been primarily responsible for outbreaks of Zika virus worldwide, most prominently in the Americas during 2015 and 2016, there has been increased recognition of the importance of sexual transmission. We review human reports and animal model studies of Zika sexual transmission and summarize potential therapeutic candidates.
Recent Findings
Male-to-female, male-to-male, and female-to-male transmission has been reported, among unprotected sexual contacts of returning travelers. Human studies have shown the potential importance of long-term persistence of Zika virus in semen while animal models have begun to yield important insights into pathogenesis of Zika infection of the genital tract.
Summary
Adherence to federal and global guidelines for prevention of sexual transmission of Zika virus from travelers to their sexual partners represents the best strategy for reducing the risk of transmission outside of endemic areas. Active research on potential treatments may soon yield candidates for clinical trials.
Similar content being viewed by others
Introduction
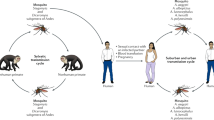
Originally identified in a rhesus monkey at an arboviral surveillance site in the Zika Forest of Uganda [1], Zika virus has gone from an obscure cause of febrile illness in Africa and Southeast Asia to an emerging global pathogen [2]. Since the first recognition of Zika infections in Brazil in 2015, this virus has spread widely throughout Latin America, the Caribbean, Mexico, and, with local vector transmission in Florida and Texas in the USA [3, 4], and recent identification in Southeast Asia and several African countries. Although most Zika infections are mild or asymptomatic [5], recognition of the disastrous effects of infection in pregnant women including fetal demise and the Zika congenital syndrome [6, 7] and Guillain-Barré syndrome [8, 9] has resulted in greatly enhanced surveillance and research on Zika virus.
The primary mode of transmission of the Zika virus has been through mosquitoes of the Aedes genus, especially Ae. aegypti [2, 10]. However, multiple alternative modes of transmission have been demonstrated including transplacentally [6, 11], via transfusion [12, 13], close contact with an infected patient [14], and unprotected sex [15••]. The latter mechanism, sexual transmission of Zika virus, has rapidly generated a wave of interest in understanding the characteristics of this novel mode of flavivirus transmission. This review will therefore focus on this topic including what is known from animal model data about Zika carriage and excretion, recent insight into our understanding of human-to-human sexual transmission, and recommendations for treatment and prevention.
Animal Model Data on Zika Carriage and Excretion
Concurrent with studies in humans, scientists have explored and tested animal models of Zika virus infection, primarily in monkeys and mice. To date, most studies have focused on pathogenicity of the virus. A few animal studies have started to provide potentially relevant results regarding sexual transmission of Zika virus.
Since Zika virus was initially isolated from a monkey [1], and multiple monkey species were seropositive, primates are potential animal models. Dudley and colleagues assessed infection of eight rhesus macaques inoculated subcutaneously with a French Polynesian Zika virus isolate (an Asian strain), including two pregnant monkeys infected on gestational days 31 and 38 [16]. All animals had Zika RNA detected on day 1 post inoculation, and Zika RNA persisted up to 21 days in six animals; Zika RNA in the two pregnant monkeys persisted up to day 57. Zika RNA was also present in saliva, oral swabs, urine, CSF, and vaginal swabs. Peak plasma viremia between days 2 and 6 ranged from 8.2 × 104 to 2.5 × 106 viral RNA copies per mL [16]. Vaginal swabs of three monkeys had viral RNA starting at day 1 and day 7 post infection but RNA was not detected on days 14, 21, and 28. Rechallenge by subcutaneous inoculation with the same Zika virus isolate 10 weeks after initial infection led to no evidence for reinfection.
Researchers have also explored murine models of Zika virus infection. Among mice studied, wild-type or control mice (C57BL/6, 129Sv/Ev) that have functional responses of types I (α/β) and II (γ) interferons (IFN) did not become infected or exhibit clinical symptoms from Zika virus infection, whereas genetically modified mice (A129, AG129, SJL, lfnar1 −/−, and other immune-deficient mice) became infected [17,18,19,20]. Among several types of mice studied, Lazear et al. found that lfnar1 −/− mice (lacking receptors to IFN-α/β), inoculated subcutaneously with strains of Zika virus from French Polynesia, Uganda, and Senegal, developed high viral loads in all tested tissues including the spleen, liver, kidney, serum, testes, brain, and spinal cord [17]. The testes had the highest levels of Zika RNA, at 107 focus-forming units (FFU) equivalents per gram. In mice infected with the French Polynesia isolate, viral RNA was detected in the testes of all surviving mice at 28 days after subcutaneous inoculation [17]. Infectious virus in the testes of this mouse model may provide a useful model for study of sexual transmission.
Rossi et al. tested a low-passage Cambodian isolate of Zika virus in A129 mice and AG129 mice. The A129 mice lack the IFN-α receptor and the AG129 mice lack both type I and II IFN responses; AG129 had more severe disease, especially neurologic features [18]. Viremia in these mice reached high titers in spleen by day 1 after inoculation, and peaked at 107 plaque-forming units/mL by day 2. The brain had viral RNA by day 3, and neurologic signs developed by day 6. The testes were also RNA-positive, therefore potentially useful for study of sexual transmission. The severity of infection was age-dependent, where infection at 3 weeks led to fatality by day 7 after infection, and infection at 11 weeks led to illness, viremia, and weight loss, but recovery started on day 8 after infection [18].
Dowall et al. infected A129 mice and their parent strain (129Sv/Ev) with Uganda strain of Zika virus [19]. They found that the parent mice 129Sv/Ev had no clinical symptoms or histological changes, although viral RNA was present in the blood, spleen, and ovaries. In contrast, A129 mice exhibited pathology in the brain manifested by inflammatory and degenerative changes, and viral load assays found Zika RNA in the blood, brain, ovary, spleen, and liver of all mice; however, the ovaries appeared normal [19]. On the other hand, Ma et al. showed that Zika virus caused inflammation in the testes and epididymis in mice, which raised concern for a possible role in male infertility [21].
To date, the most relevant animal study on sexual transmission used vaginal inoculation to expose pregnant mice to the Cambodian 2010 strain of Zika virus, and found that Zika virus appeared to replicate well in the vaginal mucosa, even in wild-type mice [22•]. The investigators detected viral RNA and infectious virus in the majority of mice from 1 to 4 days after infection with viremia decreasing to undetectable by 7 days after infection in most mice. Measurement of Zika qPCR demonstrated Zika virus replication in the vaginal tract; mice with deficiencies in the IFN pathway had increased Zika replication in the vaginal mucosa compared to wild-type mice. Furthermore, vaginal infection with Zika virus resulted in fetal brain infection in WT mice even though these pregnant mice did not develop detectable viremia. Thus, Yockey et al. hypothesize that the vaginal transmission of Zika virus might directly infect the placenta or the fetus via an ascending route [22•]. They further suggest that humans are more susceptible to Zika virus infection than mice due to the suppression by Zika NS5 protein on the interferon response in human cells.
Although more specific studies on sexual transmission still need to be carried out, murine experiments have already contributed towards understanding of the pathogenesis of Zika virus. Using a Brazil isolate of Zika virus, Cugola et al. found that C57BL/6 mice did not become infected after intravenous injection based on negative PCR, indicating that the virus did not cross placenta, whereas pregnant SJL mice (defect in suppressor T cell and functional B cell responses) had fetuses with growth delay or intrauterine growth restrictions [20]. The fetuses had cortical malformations in their brains, reduced cell number, and cortical layer thickness, which would be consistent with microcephaly in humans. The Brazil Zika isolate induced apoptosis and autophagy in neural tissue, with vacuolar nuclei in neurons in the cortex, thalamus, and hypothalamus [20].
Cugola et al. developed two neural cell culture systems, neurospheres, and brain organoids, for evaluating the impact of the Brazil Zika virus strain (Asian lineage) relative to that of African strain. They also tested these strains on brain organoids from human pluripotent stem cells versus non-human primate (chimpanzee) stem cells. Their series of experiments demonstrated different strain infectivity and kinetics on the human brain organoids versus chimpanzee brain organoids [20], suggesting that microcephaly may be unique to the Zika Asian lineage virus, which originated in the Pacific and has spread widely in the Americas.
The demonstration of infectious Zika in the testes raise questions regarding the duration of viral persistence and cells that support Zika virus survival in immune-privileged sites. Some murine models will aid in elucidating further questions regarding sexual transmission of Zika virus. However, there are differences among the murine models in their susceptibility for infection and disease, and there may be differences among the Zika isolates in their effect on reproductive organs. Finally, the murine model may be good screening for candidate therapeutics or vaccines that can then be tested in nonhuman primates, followed by human clinical trials.
Sexual Transmission of Zika Virus in Humans
The first documented case of probable sexual transmission occurred in 2008 when an entomologist who acquired Zika virus disease in Senegal characterized by rash, arthralgias, prostatitis symptoms, and hematospermia, subsequently infected his wife via unprotected sexual contact [23]. Further suspicion of the potential for Zika virus to be spread through sexual contact arose during the outbreak in French Polynesia when a Tahitian man with hematospermia was found to have Zika virus RNA in his semen and urine [24]. Although there was no evidence of onward sexual transmission in this case, the research team who evaluated this patient was able to culture replicative virus from his semen sample.
Since the advent of the large Zika outbreak in the Americas, there have been several well-documented cases of male-to-female [25,29,30,31,, 26•, 27•, 28–32], male-to-male [33], and female-to-male sexual transmission of Zika virus [34]. Table 1 presents details of reports involving probable sexual transmission, including the timing of transmission, country where transmission occurred, probable mode of infection (e.g., unprotected vaginal intercourse, anal sex, and oral sex), and clinical presentation and mode of diagnosis in the infected sexual partner. Sexual contact occurred during or within 4 days of the end of symptoms in the index patient in all cases (Table 1) [23,29,30,31,32,33,34,35,36,, 25, 26•, 27•, 28–37]. Yakob et al. estimated the median time between first sexual contact with the returned traveler and symptom onset to be 9.5 days [38]. A case series of nine United States patients with sexually transmitted Zika infection found a similar time between first sexual contact with the index case and onset of symptoms in the non-traveler to be 8–21 days [26•]. All infected patients described in Table 1 had symptoms of Zika infection that were similar to previously reported case series in endemic areas and travelers [39, 40].
While most episodes involved male-to-female sexual transmission through unprotected vaginal sex with a symptomatic male partner, there are two cases of transmission that occurred when the male partner was asymptomatic [27•, 31]. Both partners were asymptomatic in one of these two reports with their Zika infection discovered fortuitously during an evaluation for assisted reproduction treatment that was planned due to non-obstructive azoospermia in the man [27•]. Another notable episode of male-to-female transmission involved a vasectomized man [30].
One notable feature of the earliest reported case of probable sexual transmission was the presence of visible hematospermia in the symptomatic transmitting male partner [23]. Hematospermia, which occurred 2 weeks after a symptomatic episode of Zika disease, was described in a Tahitian man whose semen was positive for Zika virus RNA by real-time reverse transcriptase polymerase chain reaction (rRT-PCR), and culture grew replicative virus particles (Table 2) [24]. More recently, a study of three men with Zika virus infection in Venezuela demonstrated the presence of microhematospermia in association with Zika virus PCR-positive semen specimens (Table 2) [52]. These three men had no symptoms of prostatitis, urethritis, or cystitis and had normal urinalyses.
Demonstration of Zika Virus in Genital Secretions
A number of studies have evaluated semen carriage of Zika virus, primarily by rRT-PCR [41,42,43, 45•, 48]. Of those that have evaluated the duration of semen carriage [32, 45•, 46, 47], Zika virus was detected by RT-PCR for as long as 188 days after symptom onset. Notably, only a few studies have been able to culture live virus from semen [24, 30, 32, 43] with the longest duration of Zika culture positivity in semen being 69 days [30].
Recent studies have also identified Zika virus in genital tract secretions from women of childbearing age [49,50,51, 53•]. Zika virus has been detected by RT-PCR in cervical mucus, endocervical swabs, and vaginal mucosal swabs. One prospective study of five women in a fertility preservation program in Guadeloupe evaluated the kinetics of Zika virus in blood, urine, and genital secretions by RT-PCR and found the longest duration of positive samples to be 8–9 days in blood, 21–27 days for urine, and 12–13 days in vaginal secretions and cervical mucus [53•]. By contrast, a more recent study of serial specimens in a non-pregnant woman in the USA who had been infected in Honduras demonstrated persistence of Zika virus in vaginal mucosal specimens until day 14 and in blood until day 81, using quantitative RT-PCR [51]. To date, there has been no report of infective Zika virus having been isolated from the female genital tract.
Concentrations of Virus in Various Fluids
Table 2 displays confirmed cases of Zika virus infections in travelers with their respective tests from various body fluids [41,42,43,47,48,49,50,51,44, 45•, 46–52, 53•]. As illustrated, the findings are consistent with other studies in endemic populations noting higher viral loads (based on RT-PCR) in semen compared to blood. Urine also typically demonstrates higher PCR load compared to blood. Saliva, semen, genital swab, and urine all demonstrate longer persistence of Zika as measured by PCR.
Given evidence of virus shedding in the female and male genital tract, and highly suggestive epidemiological evidence suggestive of transmission through unprotected sexual contact, there appears to be a definite risk of asymptomatic and symptomatic travelers transmitting Zika virus to their partners after travel to endemic areas. Although culturable virus does not appear to persist as long as virus identified by molecular diagnostic methods, developing appropriate messages for travelers and their sexual partners about how to best prevent Zika infection through unprotected sexual contact has proven to be a challenge. It is also uncertain to what extent sexual transmission of Zika has contributed to epidemic spread in the Americas although at least one study in Rio de Janeiro has demonstrated a higher incidence in women than men [54]. However, this may relate to differences in daytime exposure to infected Aedes aegypti mosquitoes among women relative to men in Rio. Two mathematical modeling analyses of the potential for sexually transmitted Zika found that the basic reproduction number (R 0) is likely to be less than one and therefore highly unlikely to sustain transmission in areas without mosquito vector transmission [55, 56].
Treatment—Is There Anything on the Horizon?
Scientists are scrambling to find agents that can prevent Zika virus infection or slow its progression and consequences. They have turned to drugs already approved for human use and are screening other compounds for evidence of anti-Zika virus activity. Investigators have identified polymerases and proteases of flaviviruses as the most promising targets for inhibition. Feranchuk and colleagues constructed NS3 protease and NS5 polymerase structures of the Zika virus and used virtual in silico screening of ligands to identify potential drug candidates for treating infections. They identified the phytochemicals bisabolol and andrographolide as potential candidates for antiviral therapy, and suggest this approach may be used to screen large numbers of agents [57].
Teams of investigators collaborated to conduct a drug repurposing screen of about 6000 compounds to identify those that inhibit Zika virus or suppress Zika infection-induced caspase-3 activity in different neural cells [58]. Using ultra-high-throughput assays, they found that emricasan, a pan-caspase inhibitor, inhibited increases in caspase-3 induced by Zika virus and also protected human cortical neural progenitors. Emricasan, the most potent inhibitor of cell death, is currently being used in phase 2 clinical trials for chronic hepatitis C infection and has been well tolerated by humans. The FDA-approved antihelminthic drug, niclosamide, appeared to inhibit Zika virus, probably by acting on the viral replication step. They have identified other unrelated inhibitors of cyclin-dependent kinases that inhibited Zika virus replication, and also explored combinations of categories of drugs and the effect of sequential treatment of different agents.
Other researchers have assessed the ability of agents to inhibit ZIKV replication in cell culture [59]. Because many antiviral agents commonly used to treat human viral infections, such as AIDS, hepatitis B, CMV, and herpes simplex, are nucleoside analogs, Eyer et al. studied several of these in cell culture. They tested ZIKV strain MR766 (originally isolated from the blood of an experimental forest sentinel rhesus monkey, Uganda, 1947; virus had been passaged >100 times in suckling mice and/or in Vero cells before this study) in a Vero cell system for Zika virus multiplication, antiviral assays, and for plaque assays and included dose–response studies. They assessed 29 nucleoside analogs for their ability to inhibit cytopathic effect as well as their cytotoxicity. 2′-C-methylated nucleosides showed antiviral activity and demonstrated no or negligible cytotoxicity in Vero cells, porcine kidney cells, and human neuroblastoma cells. The identification of these candidates holds promise for further testing [59].
A related viral polymerase inhibitor 7-deaza-2′-C-methyladenosine (7DMA) has been shown to be a potent inhibitor of Zika virus replication [60]. Zmurka and colleagues also tested 7DMA in the AG129 mouse model (IFN-α/β and IFN-ɣ receptor knock-out mice) and observed that this agent reduced viremia and delayed virus-induced morbidity and mortality in treated mice.
Given that hepatitis C virus is a flavivirus, like Zika virus, Brazilian researchers tested the uridine nucleotide analog anti-HCV drug, sofosbuvir, for activity against the Zika virus, using a virus isolated in Brazil during the current outbreak [61]. Sofosbuvir inhibited Zika virus replication in a dose-dependent manner in BHK (baby hamster kidney fibroblasts)-21 cells and in SH-SY5Y, a human-derived cell line. The drug targets Zika virus RNA polymerase activity, affecting amino acid residues that are conserved in many flaviviruses. The authors note that in contrast to many broad-spectrum antiviral agents, such as interferon, ribavirin, and favipiravir, which show harm in pregnant animal models, sofosbuvir has not been associated with teratogenicity. It has been approved in the USA since 2013 for the treatment of chronic hepatitis C.
The antimalarial drug, chloroquine, which also has anti-inflammatory properties and activity against some viruses, was tested in a variety of cells, including Vero, human brain microvascular endothelial, and neural stem cells. In the laboratory, chloroquine reduced the number of Zika-infected cells, virus production, and cell death; chloroquine was effective at levels that were not cytotoxic [62]. Chloroquine inhibited both lineages in vitro, including one virus of African lineage and two Brazilian isolates of Asian lineage. Chloroquine appeared to work in early stages of viral infection whereas when it was added 24-h post-infection, it had no effect on production of viral particles. Delvecchio and colleagues concluded that chloroquine is a promising candidate for clinical trials and note that it can safely be given to pregnant women. Chloroquine apparently also crosses the placenta barrier and the blood brain barrier, another attractive characteristic for a drug candidate. There is extensive experience with the drug for malaria chemoprophylaxis and in treating inflammatory diseases. Other investigators found modest protection against Zika virus-induced cyopathogenic effect from chloroquine at low concentrations but significant toxicity from higher levels of chloroquine [63].
Investigators studying placental and brain cell populations most susceptible to Zika virus infection and mechanisms for viral entry found that azithromycin, the commonly used macrolide antibiotic, protects cultured brain cells by inhibiting viral proliferation [63]. Azithromycin inhibited the virus in a glial cell line and prevented virus-induced cytopathic effects at clinically achievable concentrations. Three strains of Zika virus were used in these studies: one from Cambodia 2010, and 2015 isolates from Brazil and Puerto Rico. Azithromycin had surprising activity against the Zika virus through an unknown mechanism. Because it is safe in pregnancy [64] and may be effective at levels achieved with usual doses, clinical trials in high-risk pregnant women are warranted.
Others have looked for agents with broad-spectrum antimicrobial activity, which would have wider use in acute febrile illness and would avoid the need to make a specific etiologic diagnosis early in infection. Bovine lactoferrin (bLf), an iron-binding glycoprotein, with demonstrated activity against viruses and bacteria, was tested [65]. Using African green monkey kidney (Vero) cells and a Brazilian strain of Zika virus, investigators showed that bLf had a dose-dependent inhibitory effect, reducing infection efficiency by about 80% without causing observable cytotoxicity. The effect was exerted primarily at the pre-entry step (probably binding) but it also appeared to have some effect on replication. The investigators found that bLf also inhibited a Brazilian strain of chikungunya virus.
In summary, these early studies, mostly in vitro, provide data to support continued work using animal models and perhaps limited clinical trials of drugs that have already undergone regulatory approval. An important issue for drugs used for treatment is whether they could reach protected sites, such as the central nervous system, fetus in utero, tissues of the eye, and the testes.
WHO, European CDC, and CDC Recommendations Regarding Prevention of Zika Virus Infection via Sexual Transmission
WHO and CDC currently recommend that sexual partners of pregnant women, living in or returning from areas where local transmission of Zika virus is known to occur, should use condoms or abstain from sexual activity for the duration of the pregnancy [66••, 67]. Testing of pregnant women with possible exposure including sexual contact is recommended. Currently, Zika RT-PCR on serum and urine is recommended if possible exposure occurred within 2 weeks, and IgM is recommended if potential exposure occurred 2 to 12 weeks earlier. International and national health authorities differ in specific recommendations to minimize sexual transmission. Current pre-conception recommendations are as follows [15••, 66••]:
-
All health authorities advise that men who have Zika virus-like symptoms after returning from areas with active Zika virus transmission should wait at least 6 months before attempting conception.
-
Most health authorities advise that asymptomatic men and women returning from areas with active Zika virus transmission should wait at least 8 weeks before attempting conception. In September 2016, WHO updated recommendations and now advises waiting 6 months after returning from areas with active Zika virus transmission before attempting conception [15••].
-
To minimize sexual transmission, WHO also advises that travelers returning from areas with active Zika virus transmission should adopt safer sex practices or consider abstinence for at least 6 months [15••].
-
Testing of pregnant women with possible exposure to Zika virus is recommended; symptomatic returning travelers who are planning conception may be tested [67].
Conclusions
There has been rapid progress into our understanding of the epidemiology and pathogenesis of Zika virus, especially since late 2015, as a result of the widespread outbreak in the Americas. Recognition of the importance of carriage of the virus in the male and, more recently, female genital tracts has highlighted the importance of sexual transmission of Zika virus and the risk for partners of travelers to endemic areas. Nevertheless, there remain many additional research questions (Box). While there is limited evidence of female-to-male spread, the growing evidence base suggests a much greater risk of male-to-female spread as a result of high concentrations and the persistence of Zika virus in semen. Given the devastating impact of Zika virus on the developing fetus in utero and in developing infants, measures to reduce the risk of sexual transmission including consistent international guidelines for reducing the risk in travelers and their sexual partners are critical to preventing potential complications in adults and the development of the congenital Zika syndrome.
Box
Research priorities regarding sexual transmission of Zika virus |
• Duration of excretion in semen; vaginal fluids? • Infectivity of genital fluids? • Pathogenesis? How can Zika virus persist in reproductive organs? Location of persistence? • Role of persistence in saliva? Transmission via deep kissing? Orovaginal sex? • Can antibodies, drugs, and other agents reach sites of viral persistence? • What factors are associated with persistence? Co-infections? Age? Pre-pubertal? Post-pubertal? Severity of infection? Level of viremia? • Are HIV-infected individuals more likely to become chronic carriers? • Besides Ebola and Zika, are there other pathogens that can persist in semen? • Are sperm infected? • Will Zika infection of the testes result in long-term impact on spermatogenesis? • What is anatomic and cellular route of infection in persons infected via semen? Per vagina? Rectal? Pharyngeal? • Can Zika be sexually transmitted in animals? If so, which ones? • Virus has been found in non-human primates in Brazil. Will it be maintained? How broad is the distribution? What is the range of potential spread in animals? |
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dick GWA, Kitchen SF, Haddow A. Zika isolation and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–20.
Chen LH, Hamer DH. Zika virus: rapid spread in the Western hemisphere. Ann Intern Med. 2016;164:613–5. doi:10.7326/M16-0150.
Likos A, Griffin I, Bingham AM, Stanek D, Fischer M, White S, et al. Local mosquito-borne transmission of Zika virus—Miami-Dade and Broward Counties, Florida, June–August 2016. Morb Mortal Wkly Rep. 2016;65:1032–8. doi:10.15585/mmwr.mm6538e1.
CDC. Advice for people living in or traveling to Brownsville, Texas [Internet]. Centers for Disease Control and Prevention. 2016 [cited 2016 Dec 27]. Available from: https://www.cdc.gov/zika/intheus/texas-update.html
Duffy MR, Chen T-H, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43. doi:10.1056/NEJMoa0805715.
Brasil P, Pereira Jr JP, Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report. N Engl J Med. 2016;375:2321–34.
Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374:1981–7.
Cao-Lormeau V, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barré syndrome outbreak caused by Zika virus infection in French Polynesia. Lancet. 2016;387:1531–9. doi:10.1016/S0140-6736(16)00562-6.
Brasil P, Sequeira PC, Freitas AD, Zogbi H, Calvet GA, de Souza RV, et al. Guillain-Barré syndrome associated with Zika virus infection. Lancet. 2016;387:1482. doi:10.1016/S0140-6736(16)30058-7.
Musso D, Gubler DJ. Zika virus. Clin Microbiol Rev. 2016;29:487–524. doi:10.1128/CMR.00072-15.
Besnard M, Lastère S, Teissier A, Cao-Lormeau VM, Musso D. Evidence of perinatal transmission of Zika virus. Fr Polynesia Euro Surveill. 2014;19:20751.
Aubry M, Finke J, Teissier A, Roche C, Broult J, Paulous S, et al. Seroprevalence of arboviruses among blood donors in French Polynesia, 2011–2013. Int J Infect Dis. 2015;41:11–2. doi:10.1016/j.ijid.2015.10.005.
Barjas-Castro ML, Angerami RN, Cunha MS, Suzuki A, Nogueira JS, Rocco IM, et al. Probable transfusion-transmitted Zika virus in Brazil. Transfusion. 2016;56:1684–8. doi:10.1111/trf.13681.
Swaminathan S, Schlaberg R, Lewis J, Hanson KE, Couturier MR. Fatal Zika virus infection with secondary nonsexual transmission. N Engl J Med. 2016;375:1907–9.
•• World Health Organization. Prevention of sexual transmission of Zika virus—interim guidance update 6 September 2016. (WHO/ZIKV/MOC/16.1 Rev.3) Available at http://www.who.int/csr/resources/publications/zika/sexual-transmission-prevention/en/. Accessed Sep 7, 2016. This document presents a succinct review of sexual transmission of Zika virus and provides detailed recommendations for member countries on strategies for its prevention.
Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204. doi:10.1038/ncomms12204.
Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, et al. A mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720–30. doi:10.1016/j.chom.2016.03.010.
Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, et al. Characterization of a novel murine model to study Zika virus. Am J Trop Med Hyg. 2016;94:1362–9. doi:10.4269/ajtmh.16-0111.
Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, et al. A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis. 2016;10, e0004658. doi:10.1371/journal.pntd.0004658.
Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, Guimarães KP, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–71. doi:10.1038/nature18296.
Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, et al. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2016;167:1511–24. doi:10.1016/j.cell.2016.11.016.
• Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, et al. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell. 2016;166:1247–56. doi:10.1016/j.cell.2016.08.004. This mouse model demonstrates how Zika virus can be introduced via the vaginal route, replicate in the vagina, and then spread to infect the fetal brain even in mice with an intact immune system. Notably, Zika virus infection in early pregnancy leads to fetal growth restriction.
Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17:880–2. doi:10.3201/eid1705.101939.
Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau V-M. Potential sexual transmission Zika virus. Emerg Infect Dis. 2015;21:359–62. doi:10.3201/eid2102.141363.
Venturi G, Zammarchi L, Fortuna C, Remoli ME, Benedetti E, Florentini C, et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveillance. 2016;21:1–4. doi:10.2807/1560-7917.ES.2016.21.8.30148.
• Russell K, Hills SL, Oster AM, Porse CC, Danyluk G, Cone M, et al. Zika virus sexual transmission male-to-female sexual transmission of Zika virus—United States, Jan–Apr 2016. Clin Infect Dis. 2016 Oct 19. This is a summary of nine cases of male travelers to Latin America and the Caribbean whose female partners became infected within 10 to 19 days after the travelers’ onset of symptoms. Full clinical and laboratory details of these nine cases are provided.
• Fréour T, Mirallié S, Hubert B, Splingart C, Barrière P, Maquart M, et al. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveillance. 2016;21:8–10. doi:10.2807/1560-7917.ES.2016.21.23.30254. This unusual case report describes an asymptomatic couple with the wife identified with Zika virus infection by PCR during routine screening for assisted reproductive treatment. Based on the timing of her illness and their mutual travel to Martinique, her infection most likely occurred 8 to 23 days after return to France.
Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, et al. Late sexual transmission of Zika virus related to persistence in the semen. Lancet. 2016;387:2501. doi:10.1016/S0140-6736(16)30775-9.
Frank C, Cadar D, Schlaphof A, Neddersen N, Günther S, Schmidt-Chanasit J, et al. Sexual transmission of Zika virus in Germany. Euro Surveillance. 2016;21:2–5. doi:10.2807/1560-7917.ES.2016.21.23.30252.
Arsuaga M, García Bujalance S, Díaz-Menéndez M, Vázquez A, Arribas JR. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect Dis. 2016;16:1107–8. doi:10.1016/S1473-3099(16)30320-6.
Brooks RB, Carlos MP, Myers RA, White MG, Bobo-Lenoci T, Aplan D, et al. Likely sexual transmission of Zika virus from a man with no symptoms of infection—Maryland, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:915–6. doi:10.15585/mmwr.mm6534e2.
D’Ortenzio E, Matheron S, Yazdanpanah Y, de Lamballerie X, Hubert B, Piorkowski G, et al. Evidence of sexual transmission of Zika virus. N Engl J Med. 2016;374:2195–8. doi:10.1056/NEJMc1604449.
Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, et al. Male-to-male sexual transmission of Zika virus—Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:372–4. doi:10.15585/mmwr.mm6514a3.
Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus—New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:716–7. doi:10.15585/mmwr.mm6528e2.
Harrower J, Kiedrzynski T, Baker S, Upton A, Rahnama F, Sherwood J, et al. Sexual transmission of Zika virus and persistence in semen, New Zealand, 2016. Emerg Infect Dis. 2016;22:1855–7. doi:10.3201/eid2210.160951.
Hills SJ, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMMR Morb Mortal Wkly Rep. 2016;65:1–3. doi:10.15585/mmwr.mm6508e2.
WHO Zika virus infection—disease outbreak news (15/04/2016). http://www.who.int/csr/don/15-april-2016-zika-chile/en/Accessed Jan 3, 2017.
Yakob L, Walker T. Zika virus outbreak in the Americas: the need for novel mosquito control methods. Lancet Glob Healt. 2016;4:e148–9. doi:10.1016/S2214-109X(16)00048-6.
Brasil P, Calvet GA, Siqueira AM, Wakimoto M, de Sequeira PC, Nobre A, et al. Zika virus outbreak in Rio de Janeiro, Brazil: clinical characterization, epidemiological and virological aspects. PLoS Negl Trop Dis. 2016;10, e0004636. doi:10.1371/journal.pntd.0004636.
Hamer DH, Barbre KA, Chen LH, Grobusch MP, Schlagenhauf P, Goorhuis A, et al. Travel-associated Zika virus disease acquired in the Americas through February 2016. A GeoSentinel analysis. Ann Intern Med. 2016. doi:10.7326/M16-1842.
Musso D. Zika virus transmission from French Polynesia to Brazil. Emerg Infect Dis. 2015;21:1887. doi:10.3201/eid2110.151125.2016;21:2014-6.
Atkinson B, Hearn P, Afrough B, Lumley S, Carter D, Aarons EJ, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22:940. doi:10.3201/eid2205.160107.
Mansuy JM, Pasquier C, Daudin M, Chapuy-Regaud S, Moinard N, Chevreau C, et al. Zika virus in semen of a patient returning from a non-epidemic area. Lancet Infect Dis. 2016;16(8):894–5. doi:10.1016/S1473-3099(16)30153-0.
Barzon L, Pacenti M, Berto A, Sinigaglia A, Franchin E, Lavezzo E, et al. Isolation of infectious Zika virus from saliva and prolonged viral RNA shedding in a traveller returning from the Dominican Republic to Italy, January 2016. Euro Surveillance. 2016;21(10). doi: 10.2807/1560-7917.
• Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy February 2016. Euro Surveillance. 2016;21:8–11. doi:10.2807/1560-7917.ES.2016.21.32.30314. This study demonstrated persistent shedding of Zika virus by PCR for 188 days, the longest duration of shedding yet reported.
Barzon L, Pacenti M, Franchin E, Lavezzo E, Trevisan M, Sgarabotto D, et al. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveillance. 2016;21:8–11. doi:10.2807/1560-7917.ES.2016.21.32.30316.
Gaskell KM, Houlihan C, Nastouli E, Checkley AM. Persistent Zika virus detection in semen in a traveler returning to the United Kingdom from Brazil, 2016. Emerg Infect Dis. 2017;23:137–9. doi:10.3201/eid2301.161300.
Reusken C, Pas S, Geurtsvankessel C, Mögling R, van Kampen J, Langerak T, et al. Longitudinal follow-up of Zika virus RNA in semen of a traveller returning from Barbados to the Netherlands with Zika virus disease, March 2016. Euro Surveillance. 2016;21:2–5. doi:10.2807/1560-7917.ES.2016.21.23.30251.
Nicastri E, Castilletti C, Balestra P, Galgani S, Ippolito G. Zika virus infection in the central nervous system and female genital tract. Emerg Infect Dis. 2016;22:2228–30. doi:10.3201/eid2212.161280.
Prisant N, Bujan L, Benichou H, Hayot P-H, Pavili L, Lurel S, et al. Zika virus in the female genital tract. Lancet Infect Dis. 2016;16:1000–1. doi:10.1016/S1473-3099(16)30193-1.
Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis. 2017;23:99–101. doi:10.3201/eid2301.161394.
Torres JR, Martínez N, Moros Z. Microhematospermia in acute Zika virus infection. Int J Infect Dis. 2016;51:9712. doi:10.1016/j.ijid.2016.08.025.
• Prisant N, Breurec S, Moriniere C, Bujan L, Joguet G. Zika virus genital tract shedding in infected women of child-bearing age. Clin Infect Dis. 2016;64:107–9. The authors reported five women enrolled in a fertility preservation program in Guadeloupe who presented with Zika-like symptoms, with positive Zika PCR, and demonstrated negative Zika PCR in genital samples by the 3rd week.
Coelho FC, Durovni B, Saraceni V, Lemos C, Codeco CT, Camargo S et al. Higher incidence of Zika in adult women in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int J Infect Dis. 2016;128–132. doi: 10.1016/j.ijid.2016.08.023.
Yakob L, Kucharski A, Hue S, Edmunds WJ. Low risk of a sexually-transmitted Zika virus outbreak. Lancet Infect Dis. 2016;16:1100–2. doi:10.1016/S1473-3099(16)30324-3.
Althaus CL, Low N. How relevant is sexual transmission of Zika virus? PLoS Med. 2016;13, e1002157. doi:10.1371/journal.pmed.1002157.
Feranchuk S, Potapova U, Belikov S. Virtual screening of inhibitors for the Zika virus proteins. bioRxiv. 2016. doi:10.1101/060798.
Xu M, Lee EM, Wen Z, Cheng Y, Huang W-K, Qian X, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22:1101–7. doi:10.1038/nm.4184.
Eyer L, Nencka R, Huvarova I, Palus M, Alves MJ, Gould EA, et al. Nucleoside inhibitors of ZIka virus. J Infect Dis. 2016 Advance ac:1–21. doi:10.1093/infdis/jiw226.
Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJF, Neyts J. The viral polymerase inhibitor 7-deaza-2′-C-methyladenosine Is a potent inhibitor of in vitro Zika virus replication and delays disease progression in a robust mouse infection model. PLoS Negl Trop Dis. 2016;10:1–15. doi:10.1371/journal.pntd.0004695.
Sacramento CQ, de Melo GR, Rocha N, Hoelz LVB, Mesquita M, Al. E. The clinically approved antiviral drug sofosbuvir impairs Brazilian Zika virus replication. bioRxiv. 2016; Available at http://biorxiv.org/content/early/2016/07/06/061671doi: https://doi.org/10.1101/061671.
Delvecchio R, Higa LM, Pezzuto P, Valadao AL, Garcez PP, Monteiro FL, et al. Chloroquine inhibits Zika virus infection in different cellular models. Viruses. 2016;8. (bioRxiv. 2016;51268.)
Retallack H, Di Lullo E, Arias C, Knopp KA, Sandoval-Espinosa C, Laurie MT, et al. Zika virus in the human placenta and developing brain: cell tropism and drug inhibition. bioRxiv. 2016;58883.
Lin KJ, Mitchell AA, Yau WP, Louik C, Hernández-Díaz S. Safety of macrolides during pregnancy. Am J Obstet Gynecol. 2013;208:221. doi:10.1016/j.ajog.2012.12.023. e1-8.
Carvalho CAM, Casseb SMM, Gonçalves RB, Silva EVP, Gomes AMO, Vasconcelos PFC. Bovine lactoferrin activity against chikungunya and Zika viruses. bioRxiv. 2016;71571.
•• Petersen EE, Meaney-Delman D, Neblett-Fanfair R, Havers F, Oduyebo T, Hills SL, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1077–81. doi:10.15585/mmwr.mm6539e1. This is a succinct summary of evidence supporting the link between Zika virus infection during pregnancy and the congenital Zika syndrome. The guidelines provide extensive details on strategies for pre- and post-conception prevention of Zika virus infection, and guidance for screening and counselling pregnant women.
CDC. Infographic: when to test for Zika virus. Available at https://www.cdc.gov/zika/pdfs/when-to-test-zika.pdf. Accessed Dec 19, 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Hamer, Wilson, Jean, and Chen declare no conflicts of interests
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Additional information
This article is part of the Topical Collection on Tropical, Travel and Emerging Infections
Rights and permissions
About this article
Cite this article
Hamer, D.H., Wilson, M.E., Jean, J. et al. Epidemiology, Prevention, and Potential Future Treatments of Sexually Transmitted Zika Virus Infection. Curr Infect Dis Rep 19, 16 (2017). https://doi.org/10.1007/s11908-017-0571-z
Published:
DOI: https://doi.org/10.1007/s11908-017-0571-z