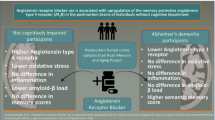
Abstract
Purpose of Review
To provide an overview of the association between angiotensin II receptor blocker (ARB) use and cognitive outcomes.
Recent Findings
ARBs have previously shown greater neuroprotection compared to other anti-hypertensive classes. The benefits are primarily attributed to the ARB’s effect on modulating the renin-angiotensin system via inhibiting the Ang II/AT1R pathway and activating the Ang II/AT2R, Ang IV/AT4R, and Ang-(1–7)/MasR pathways. These interactions are associated with pleiotropic neurocognitive benefits, including reduced β-amyloid accumulation and abnormal hyperphosphorylation of tau, ameliorated brain hypo-fusion, reduced neuroinflammation and synaptic dysfunction, better neurotoxin clearing, and blood–brain barrier function restoration. While ACEis also inhibit AT1R, they simultaneously lower Ang II and block the Ang II/AT2R and Ang IV/AT4R pathways that counterbalance the potential benefits.
Summary
ARBs may be considered an adjunctive approach for neuroprotection. This preliminary evidence, coupled with their underlying mechanistic pathways, emphasizes the need for future long-term randomized trials to yield more definitive results.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Dementia presents a significant and growing public health challenge, imposing substantial burdens on individuals, families, and society. It primarily affects older adults, with the risk of dementia doubling every 10 years after age 65 [1]. There are over 55 million people living with dementia worldwide, and approximately 10 million new cases are recorded every year [2]. Unfortunately, there is no known cure for dementia due to its complex and largely irreversible nature. Consequently, efforts geared toward preventing or delaying the progress of dementia in its early stages remain a primary strategy for reducing the burden of the disease. Identifying currently available medications that are associated with lower risks of dementia and cognitive decline is an important ancillary strategy to address this public health issue.
Up to one-third of dementia cases could be attributable to modifiable risk factors [3]. Hypertension, for example, has been demonstrated to accelerate cognitive decline and increase the risk of both vascular dementia and Alzheimer’s disease (AD). The brain, lacking energy reserves, relies on cerebral blood flow to supply necessary nutrients and oxygen. Adequate vascular perfusion is therefore fundamental for maintaining brain energy, homeostasis, and normal functioning. Hypertension can impair neurocognitive function and disrupt normal brain homeostasis by promoting intracerebral inflammation and oxidative stress, reducing cerebral blood flow, and leading to cerebral hypoperfusion via thickening and hardening of the brain arteriole walls, narrowing the lumen, and causing injuries to small vessels [4,5,6,7]. Hypertension has also been demonstrated to accelerate the deposition of microvascular β-amyloid and neurofibrillary tangles by promoting inward brain vascular remodelling, increasing blood–brain barrier permeability, enhancing the processing of the β-amyloid protein precursor, and aggravating brain hypoperfusion and neuroinflammation [8]. A recent clinical trial (SPRINT-MIND) found that intensive blood pressure (BP) control improved neurocognitive outcomes, though the results were not statistically significant due to the early termination of the trial [9•].
Numerous studies have demonstrated the benefits of anti-hypertensive medications in reducing the risk of vascular dementia and AD, both in individuals with and without initial cognitive impairment [10, 11]. This benefit is partly attributed to the reversal of the detrimental effects of reduced cerebral blood flow caused by hypertension. Considering the increased risk of dementia with aging and the fact that up to half of individuals of age over 70 have hypertension [12], identifying specific anti-hypertensive medication classes that have the potential to protect against dementia and cognitive decline, while effectively controlling BP and cardiovascular disease (CVD) risk, has become a clinical priority.
Epidemiologic studies have yielded mixed findings on the relative neurocognitive effects of different anti-hypertensive medication classes, with some reporting no significant difference and others showing the opposite [13,14,15]. Despite the inconsistency, there has been accumulating evidence supporting a greater neuroprotective association with angiotensin II receptor blockers (ARBs) than other classes [16]. ARBs have been proposed in many studies to exert pleiotropic neuroprotective effects beyond BP regulation, including anti-inflammation, anti-oxidation, and anti-proptosis, maintaining the normal vascular and cellular structure and functions of the brain, preventing the accumulation of β-amyloid and reducing the hyperphosphorylation of tau.
To inform clinical practice, this review is intended to summarize the updated evidence from preclinical studies, clinical randomized trials, and observational studies comparing ARB use, with other classes or with no anti-hypertensive medication use, on cognitive outcomes. A particular focus was placed on comparing ARBs with angiotensin-converting enzyme inhibitors (ACEis), considering their similar mechanisms of action and clinical equipoise in BP and CVD risk reduction. The potential pharmacological and pathophysiological mechanisms underlying ARB’s putative neurocognitive benefits are thoroughly discussed. We also reviewed potential risk modifiers of the association between ARBs and neurocognitive outcomes as identified in previous studies. Finally, we highlight evidence gaps and suggest opportunities for future research examining the association between ARBs, dementia, and cognitive decline.
Postulated Neuroprotective Effects of Renin-Angiotensin System (RAS) Inhibiting Medications
Blockage of the renin-angiotensin system (RAS) lowers BP and is recognized as a useful strategy for preventing incident and recurrent CVD events, including strokes and their sequelae such as vascular dementia (VaD) [17]. ARBs and ACEis are typical RAS-inhibiting anti-hypertensive medications that are widely used in clinical practice. ARBs work by inhibiting the function of type-1 receptors of angiotensin II (AT1Rs), thus diminishing the vessel-constricting effect from the Ang II binding to AT1. ACEis act upstream by blocking the action of angiotensin-converting enzyme 1 (ACE-1), leading to a reduction in downstream Ang II synthesis.
Both ACEi and ARBs have been shown to confer direct and indirect benefits in preventing and delaying cognitive decline and dementia beyond BP regulation [18,19,20,21,22]. ACEi and ARBs have been suggested to enhance neurovascular coupling and induce synaptic plasticity by increasing the release of glutamate to induce the production of growth factors, cytokines, and other intercellular messengers in neurons and glial cells, further enhancing learning and memory processes [23]. Preclinical studies suggested that ACEis may reduce β-amyloid degradation which is catalyzed by the ACE-1 N-terminus, while ARBs catabolize β-amyloid peptides by increasing the levels of proteins involved in its metabolism, including insulin-degrading enzyme, neprilysin, and transthyretin [24]. A handful of studies found a reduction in β-amyloid deposition only in ARB users but not in ACEi users [25,26,27, 28•]. For example, a recent longitudinal study in cognitively normal older adults (n = 142) found that ARB users had slower β-amyloid accumulation in the cortex, specifically in the caudal anterior cingulate and precuneus, and the precentral and postcentral gyri, compared with ACEi users [28•]. However, the strength of the evidence was limited by the small study sample size. A study of 83 individuals with mild cognitive impairment found a lower risk of progression to AD in RAS inhibitor users compared to non-RAS inhibitor users, and brain imaging showed that RAS inhibitor users had fewer neurofibrillary tangles, comprised of hyperphosphorylated microtubule-associated tau protein in multiple brain regions [29].
Although the efficacy of ARBs and ACEis is comparable in BP-lowering and reduction of vascular events, studies have consistently reported better neurocognitive outcomes in ARB users compared with ACEi users [30, 31]. In a large UK case–control study of 48,363 individuals (mean age 82 years) with good adherence to anti-hypertensive therapy, the use of either ARBs or ACEis was associated with a reduced risk of AD compared with the use of other classes, and a stronger inverse association was observed with ARBs than ACEis (odds ratios, 0.47 and 0.76, respectively) [32]. A recent meta-analysis [33••] including 3 million individuals, investigating the association between RAS inhibitor anti-hypertensive medications and dementia risk, found that ARB use was associated with a 22% reduced risk of all-cause dementia and a 26% decreased risk of AD when compared to other classes; while there was no significant difference between ACEIs and other classes, the risk of dementia was 14% lower in ARB users when compared to ACEi. This study, however, did not find any significant benefits from ARBs against vascular dementia, suggesting a greater direct benefit conferred by ARBs on neurodegeneration compared to their systemic vascular benefits. This finding was supported by another case–control study (n = 48,363) observing a stronger association of ARB use with AD than with vascular dementia and other dementia types [32].
Not all studies report consistent findings. Some observational studies and early randomized trials suggest no significant neuroprotection with ARB or the use of other anti-hypertensive classes and no difference in the risk of neurological outcomes between ARBs and other classes. For example, two randomized-controlled trials, ONTARGET (the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial) and TRANSCEND (the parallel Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease), involving 25,620 and 5926 individuals aged 55 years and older with established CVD or diabetes with end-organ damage, respectively, reported neither a significant effect of RAS inhibitor anti-hypertensive agents on cognitive function nor a differential effect between ARBs and ACEis during a median follow-up of 4.7 years [34••]. This neutral effect may be explained by the younger population studied. It must be acknowledged that there remains significant uncertainty regarding the effect of RAS inhibitors on neurological outcomes and the relative effects between different classes, due to the inconsistency of historical evidence. Table 1 shows a summary of key historical studies comparing ARBs with other anti-hypertensive classes or no use of anti-hypertensive medications.
Possible Mechanisms for the Pleiotropic Neuroprotective Benefits of ARBs Independent of Blood-Pressure-Lowering Effect
Angiotensin Hypothesis
Several plausible explanations have been proposed for the putative superiority of ARBs over ACEis and other classes in neurocognitive outcomes. The “Angiotensin hypothesis” has gained significant attention as a plausible explanation, although much of the evidence comes from animal and laboratory studies [51, 52]. The process begins with renin cleaving the N-terminal of angiotensinogen to produce angiotensin I (Ang I), which is then converted to the bioactive peptide, Ang II, by ACE. Ang II and its derivates in the brain are known to play a role in neurodegeneration by modulating neuroinflammation, oxidative stress, and neuronal apoptosis [52]. Ang II can bind to two receptors, AT1R (Ang II type-1 receptors) and AT2R (Ang II type-2 receptors), which are expressed in multiple sites in the brain, including neurons, astrocytes, oligodendrocytes, and microglia [53]. Activation of AT1R can exert deleterious effects on the brain, including disrupting brain functions and metabolism and damaging cerebral vasculature. Specifically, AT1R activation can lead to vasoconstriction, reduced oxygen and nutrient supply to the brain, activation of neuroinflammation, oxidative stress, and impairment of mitochondrial and cholinergic function. It can also contribute to blood–brain barrier (BBB) dysfunction, brain cell injury, and the retention of β-amyloid, collectively contributing to neurodegeneration [54••, 55].
ACEis and ARBs may confer neuroprotection via antagonizing AT1 receptor activation and blocking the ACE/Ang II/AT1R axis, with ARBs selectively and competitively blocking the AT1R, preventing it from binding to Ang II, and ACEi acting upstream in the ACE pathway. Prevention of the synthesis of AT1R by ARB could further lead to substantial upregulation in Ang II levels as a result of negative feedback regulation. In contrast, ACEis inhibit ACE activity, thus downregulating the production of downstream Ang II. The elevation in circulating Ang II levels with ARBs further promotes the upregulation of the unopposed Ang II/AT2R pathway, which is associated with a wide range of purported neurocognitive benefits. This includes, but is not limited to, the improvement of cerebral hypoperfusion and brain metabolism by increasing the oxygen and nutrient supply to the brain and maintaining brain homeostasis by reducing hypoxia, inflammation, oxidative stress, and cell proptosis. In addition, activation of the Ang II/AT2R pathway may further slow the deposition of β-amyloid, promote axonal regeneration, and improve neurogenesis by enhancing neurovascular coupling, neuronal differentiation, neurite outgrowth, and mitigating neural injuries [56, 57•, 58, 59].
Excessive circulating Ang II following ARB use can be cleaved into Ang IV by aminopeptidases A and N (AP-A/AP-N) and bind to AT4R to take action. Similar to Ang II/AT2R, Ang IV/AT4R activation is neuroprotective by improving cerebral blood flow, reducing inflammation and oxidative stress, and increasing the release of dopamine and acetylcholine. Its activation is also associated with enhanced long-term potentiation, improvement of memory consolidation and retrieval, and learning ability [59, 60••, 61].
ARBs also reputedly increase the activity of ACE2, a vasodilator peptide that downregulates Ang II levels by degrading it to a heptapeptide, Ang-(1–7). Preclinical evidence suggests that Ang-(1–7) binding to the Mas receptor exerts anti-inflammatory, anti-ischaemic, vasodilatory, and neuroprotective effects [59]. In particular, activation of Ang-(1–7)/MasR can reduce the deposition of β-amyloid and phosphorylated tau and facilitate the release of intercellular messengers, such as prostanoids and nitric oxide, which are important factors in maintaining learning ability and memory [62, 63]. In humans, plasma Ang-(1–7) level is reduced and associated with cognitive function in AD patients [64]. ACEi has no noticeable effect on the expression of ACE2 and Ang-(1–7) in humans, as seen in previous studies [65,66,67,68]. In addition, a handful of preclinical studies revealed that ACE2 per se can convert β-amyloid 43, an early-depositing β-amyloid species that contributes to AD pathogenesis, into the less neurotoxic β-amyloid 40 with neuroprotective effects [69].
The “Angiotensin hypothesis” has also garnered support from many clinical studies. Thiazides and dihydropyridine CCBs were demonstrated to increase Ang II-mediated activity by increasing renin and have been shown to be neuroprotective [70, 71]. In contrast, β-blockers and most non-dihydropyridine CCBs can downregulate Ang II expression by reducing renin production and could be potentially harmful. In a post hoc analysis of the PreDIVA Trial, Schroevers et al. [71] assessed the association between the use of Ang II-stimulating anti-hypertensive medications (ARBs, dihydropyridine CCBs, and thiazides) versus any other anti-hypertensive classes and the risk of incident dementia among 1907 community-dwelling adults over a middle- and long-term follow-up. They found that the use of Ang II stimulators was associated with a 32% decreased risk of dementia over a 7-year follow-up, which attenuated to 20% with over a decade of extended follow-up. Notably, the use of ARB and dihydropyridine CCBs was associated with a 46% and 48% reduced risk of dementia over 7 years, respectively, but was attenuated to 25% and 27% over a decade. A post hoc analysis of the SPRINT-MIND trial yielded similar results, with Ang II stimulators associated with a 25% lower risk of dementia compared with other anti-hypertensive medications [72••]. A network meta-analysis of 7 randomized trials and 15 observational studies found that ARBs and CCBs were associated with significantly reduced risk of dementia compared to ACEis and β-blockers [15]. More recently, in a cohort of older adults (n = 5047) residing in residential care, Marcum et al. found a significantly lower incidence of cognitive impairment among users of Ang II-stimulators compared to users of Ang II-inhibitors over a median 5.4-month follow-up [73]. Table 2 summarizes recent studies comparing Ang II-stimulating with Ang II-inhibiting anti-hypertensive medications.
Other Possible Mechanisms
Several ARBs, including telmisartan, irbesartan, and candesartan, have been found to modulate the activity of the peroxisome proliferator-activated receptor (PPAR-γ). PPAR-γ is a neuroprotective receptor that can regulate metabolism and reduce inflammation and insulin resistance [54••, 75, 76]. Activation of PPAR-γ reduces inflammation and oxidative stress, improves brain function and metabolism, alters amyloid precursor protein (APP) processing, and reduces β-amyloid accumulation, consequently ameliorating brain cell injury and cognitive function decline. ARBs can also indirectly elevate PPAR-γ expression by blocking AT1R, which is inversely linked to PPAR-γ expression level [77]. Among all ARBs, telmisartan has the strongest agonistic effect on PPAR-γ. By leveraging the National Health Insurance Research Database, a Taiwanese study [78] found that the dementia risk was lower in telmisartan users compared with other ARB users (hazard ratio, 0.72; 95% CI, 0.53 to 0.97; p = 0.030). The authors attributed this to the greater effect of telmisartan on increasing PPAR-γ expression.
ARBs may also directly reduce the expression of pro-inflammatory factors, including LPS, IL-1β, and transforming growth factor β (TGF-β), followed by the activation of pro-inflammatory signals, further ameliorating brain cell injuries [79,80,81]. In contrast, ACEis elevate the level of bradykinin, a pro-inflammatory plasma peptide associated with an increased risk of cognitive impairment, whereas ARBs do not [82]. Studies of animal models found that bradykinin infusion in the brain of mice can lead to significant learning and memory deficits through oxidative stress and synaptic dysfunction, as well as inducing AD-like tau hyperphosphorylation [83]. Conversely, blockade of bradykinin with an antagonist or genetic deletion of the bradykinin B1 receptor can improve cognitive deficits by reducing neuroinflammation and deposition of β-amyloid in AD mice [84, 85]. Elevated bradykinin levels were also seen in the cerebrospinal fluid of mice after cerebral injection of β-amyloid [86].
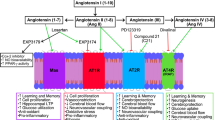
Figure 1A illustrates the major putative mechanisms underlying the proposed association between ARBs and neurocognitive protection. Figure 1B summarizes the putative mechanisms for the proposed effects of ACEi use on neurocognition. In brief, ARBs exert protective effects by directly blocking the Ang II/AT1R pathway and stimulating neuroprotective Ang II/AT2R, Ang IV/AT4R, and Ang-(1–7)/MasR pathways, while ACEis block the Ang II/AT2R and Ang IV/AT4R pathways that counterbalance the neurocognitive benefits of blockage of the Ang II/AT1R pathway. ARB use has also been linked to the activation of PPAR-γ, a biomarker associated with enhanced brain function and metabolism, minimized expression of pro-inflammatory factors, and reduced β-amyloid accumulation.
Putative mechanisms by which the ARB and ACEis affect neurodegeneration. Renin cleaves angiotensinogen to form angiotensin (Ang) I, which is subsequently converted to Ang II by the angiotensin-converting enzyme (ACE). Ang II exerts physiologic effects by binding to AT1 or AT2 receptors (AT1R or AT2R). Ang II can be further cleaved to Ang IV by AP-A and AP-N, which bind to AT4R to take effect. ARBs selectively prevent Ang II from binding to AT1R without affecting ACE activity. Blocking AT1R activation can in turn increase Ang II expression upstream as a result of negative feedback regulation, and this will result in an upregulation of Ang II/AT2R and Ang IV/AT4R signalling to exert pleiotropic neuroprotective benefits such as improvement of episodic memory. Additionally, ARBs may increase the activity of ACE2, which can convert Ang II to Ang-(1–7). Ang-(1–7) can bind to the Mas receptor to exert neuroprotective benefits. Several ARBs, including telmisartan, irbesartan, and candesartan, can also modulate the activity of PPAR-γ to exert benefits. In addition, ARBs were found to ameliorate inflammation by downregulating the expression of pro-inflammatory factors such as LPS, IL-1β, and TGF-β to pro-inflammatory signals and provide neuroprotection. ACE inhibitors (ACEis) directly inhibit ACE activity, thereby inhibiting Ang II production and its derivatives, AT1R, AT2R, and AT4R. Inhibiting AT1R activity is beneficial for cognition, while inhibiting AT2R and AT4R are harmful for cognition. ACEis have no obvious impact on ACE2 expression and thus do not impact the formation of Ang-(1–7). ACEi can however increase the levels of bradykinin by preventing it from degrading to inactive peptides and this will contribute to learning and memory deficits. Abbreviations: ACE, angiotensin-converting enzyme; ACEi, angiotensin-converting enzyme inhibitor; APA, aminopeptidase A; APN, aminopeptidase N; APP, β-amyloid (Aβ) precursor protein; ARB, angiotensin receptor blocker; AT1R, angiotensin II type-1 receptor; AT2R, angiotensin II type-2 receptors; AT4R, angiotensin II type-4 receptors; BBB, blood–brain barrier; IL-1β, interleukin 1β; LPS, lipopolysaccharides; PPAR-γ, peroxisome proliferator-activated receptor; TGF-β, tumor growth factor-β
Potential Modifiers of the Putative Beneficial Effects of ARBs
The mechanisms underlying the role of ARBs in dementia and cognition decline remain inadequately understood. Mechanistic studies are needed to provide deeper insights to support the proposed mechanisms, and more clinical studies are needed to verify the postulated benefits of ARBs in humans. Identifying potential modifiers of these putative benefits may help elucidate the inconsistent results of previous studies. In the following section, we summarize the findings from existing studies conducted in diverse populations and identify possible modifiers of ARB actions.
Blood-Brain Barrier (BBB) permeability
The neuroprotective effects of specific anti-hypertensive classes or agents may be mediated by their ability to penetrate the BBB. Some, but not all, epidemiologic studies found a stronger inverse association between BBB-crossing RAS anti-hypertensive medications and dementia risk among individuals with and without cognitive impairment [87]. In mouse models, hippocampal neurodegeneration induced by β-amyloid accumulation was entirely rescued, and neurons were protected by brain-penetrant ACEi (captopril) and ARB (losartan) [88]. A cohort study [89•] conducted on patients with AD reported the slowest decline in delayed recall performance among users of BBB-crossing ARBs, followed by non-BBB-crossing ARBs and BBB-crossing ACEis when compared to non-BBB-crossing ACEis. The Alzheimer’s Disease Neuroimaging Initiative (ADNI) study of individuals without dementia found that BBB-crossing ARBs were associated with better memory performance and a lower volume of white matter hyperintensities when compared to other anti-hypertensive agents [90]. One study indicated that BBB penetrability only modifies the potentially neuroprotective effect of ARBs, not ACEis, a finding that warrants further investigation [50].
Cognitive Impairment and Genetic Risk Factors
The neurological effects of ARBs may differ in populations with different initial dementia risks. Most previous studies were conducted on individuals initially free of any neurocognitive or cerebrovascular disorders. A network meta-analysis of 19 randomized trials in a total of 18,515 individuals without prior cerebrovascular events found that ARB use was associated with decreased risks of overall cognitive decline and dementia compared with other anti-hypertensive classes while maintaining similar effectiveness in BP control [10].
The question of whether ARBs also provide benefits to individuals with mild cognitive impairment (MCI) in terms of preventing progression to dementia as well as to those diagnosed with AD in terms of improving prognostic outcomes remains inconclusive. Some studies showed positive findings in this regard. Ramipril had no effect on β-amyloid levels in CSF [91], while ARB use compared with no use or other anti-hypertensive class use was associated with significantly reduced tau and phosphorylated tau expression and ameliorated amyloid pathology among MCI patients [92•]. Deng et al. demonstrated that ARB use in MCI individuals was associated with a decreased risk of progression to dementia compared with ACEi use, other class use, and no anti-hypertensive medication use [93••]. Similar results were seen in an AD cohort (n = 1689), where ARB users exhibited better preservation of memory, attention, and psychomotor processing speed than ACEi users [89•]. Subsequent subgroup analyses suggested that the ARB-associated benefits were limited to APOE ε4 non-carriers. Another recent cohort study of a mixed older population, including those with normal cognition, MCI, and dementia [28•], found that in individuals with normal cognition, ARBs were only beneficial in the absence of APOE ε4. Among individuals with AD/MCI and accumulation of β-amyloid, there was no difference between ARBs and ACEis in rates of overall mid/late-stage β-amyloid accumulation, regardless of APOE ε4 carrier status.
CVD as a Risk Factor
CVD is an important risk factor and contributor to the progression of dementia. In addition to its BP-controlling properties, ARBs are commonly utilized for the treatment of various cardiovascular conditions, such as heart failure. ARBs have potential neuroprotective effects irrespective of patients’ history of CVD. The ONTARGET trial studied 25,620 older patients (mean age of 66 years) with coronary heart disease and mildly elevated systolic BP (142 mm Hg) [34••]. The incidence of combined neurological outcome, stroke, and cognitive impairment was 10% lower in the telmisartan group compared to the ramipril group, despite a mere 0.9 mmHg lower systolic BP with telmisartan. In contrast, when examining the same outcome, the TRANSCEND trial (n = 5926; mean age: 67 years), which compared telmisartan to placebo in individuals intolerant to ACE inhibitors, did not report a significant association of cognitive function with telmisartan [34••]. Furthermore, in an older cohort initially free of cognitive problems but with a history of ischemic heart disease, BBB-crossing ARBs were associated with a lower risk of AD when compared with non-users. This relationship appeared to be dependent on dosage and treatment length [50]. These data collectively suggest that ARBs may provide superior protection against AD compared to other anti-hypertensive classes in CVD patients.
While the effects of ARBs and other classes on vascular dementia in individuals with CVD may be similar, as they demonstrate comparable efficacy in BP reduction, the magnitude of the reduction in AD risk with ARBs may surpass the reduction in vascular dementia risk. A decline in visuospatial skills, executive function, and attention is more characteristic of vascular dementia, while a decline in episodic memory and language ability is more pronounced in AD patients [94]. Previous studies have consistently reported the benefits of ARBs on memory and language, suggesting their direct impact on AD pathology [90, 94, 95•]. Patient populations with high CVD risk in whom the ratio of vascular to AD pathology is increased may be less likely to obtain more benefit from ARBs compared with other classes.
Duration of ARB Use
The duration of ARB use is a crucial aspect that must be considered in dementia research. Dementia can develop insidiously over many years and take decades to manifest clinically, such that if ARBs confer any protection, the benefits may become apparent only after long-term use. Short-term studies on anti-hypertensive medications have predominantly focused on assessing cognition, while longer-term studies have explored dementia outcomes. A small-scale trial of adults aged over 55 years with mild cognitive impairment and hypertension found that 1-year treatment with candesartan was associated with a slower decline in executive function and episodic memory compared with lisinopril, despite similar BP control between the two treatment arms [48]. A meta-analysis has revealed the anti-hypertensive medication-associated benefits of dementia may increase with longer follow-up [15]. A prospective cohort study with a decade of follow-up found that anti-hypertensive medication use was associated with a 5% reduced risk of incident dementia per year, with the strongest association observed for ARBs, showing a 15% risk reduction per year [96]. A population-based study in Taiwan (n = 49,062) matching users of ARBs with non-ARB anti-hypertensive medications found that, compared with other anti-hypertensive medications, the HRs of incident dementia with ARB use < 4 years and > 4 years in duration were 0.62 (95% CI 0.57–0.66) and 0.34 (95% CI 0.30–0.39), respectively [42]. An early clinical trial involving 4954 subjects aged 70–89 years old found no significant difference in the change of cognitive function measured by the mini-mental state examination over time and dementia risk between candesartan and placebo [97]. The authors attributed these non-significant findings to the short-term follow-up period (mean 3.7 years) and the modest reduction in BP achieved with candesartan (systolic BP: − 3.2 mm Hg, diastolic BP: − 1.6 mm Hg).
Dosage
The magnitude of ARB-associated neuroprotection has been shown to be dose-dependent. Li et al. followed 819,491 older American veterans with CVD for 4 years and reported a significant association between the use of ARB and lower incidence and progression of AD and dementia when compared with ACEi and other cardiovascular drugs, and the strength of the association increased with a higher ARB dose [39••]. Another large-scale case–control study reported dose-dependent inverse associations between ARB and AD [32]. Thus, a careful balance of countering the risk of using high-dose ARBs with its benefits is important in clinical practice.
Clinical Implications and Remaining Questions
Existing evidence regarding the overall effects of anti-hypertensive medication use on cognitive decline and dementia, as well as determining which specific class or agent provides the greatest neuroprotection, remains inconclusive. A significant body of evidence supports the potential beneficial effects of ARBs compared with other classes. However, most studies supporting ARBs’ ability to lower dementia risk have relied on preclinical evidence and observational data. To inform evidence-based clinical practice and relevant treatment guidelines, it is imperative to have high-quality evidence derived from large-scale, meticulously conducted prospective randomized controlled trials with long-term follow-up. An important clinical question arises regarding whether ARBs should replace ACEis in populations at high risk of neurocognitive disorders, given that both medications have similar indications and clinical uses, often leading to their interchangeable prescribing. While awaiting more robust evidence and consideration of dementia as an indication for ARB use in hypertensive individuals within clinical guidelines, it may be advisable to recommend ARBs over ACEis for those with indications for RAS inhibitors who express concern about or susceptibility to cognitive decline and dementia. Additionally, compared to ACEis, ARBs confer additional safety benefits, including a lower risk of cough, angioedema, pancreatitis, and gastrointestinal bleeding [98].
Future research concerning the neurocognitive effects of ARBs and other anti-hypertensive classes should incorporate additional elements such as blood biomarkers, brain imaging scans, and genetic factors like APOE ε4 carrier status and familial aggregation. These measures can contribute to a deeper understanding of the underlying mechanisms driving the observed associations. Consideration should also be given to the pre-existing risk of cognitive decline and dementia in individuals, the duration and intensity of treatment, and the pharmacological properties of specific ARB agents (e.g., BBB permeability). These factors are important as they can potentially modify the associations of interest. Studies relying solely on baseline exposure data may be susceptible to biases, particularly long-term follow-up studies where the data are less representative of actual medication use. This issue is especially pertinent among older individuals, as their BP can vary, and changes in anti-hypertensive medication usage are common. To ensure the reliability and robustness of the results, careful consideration and elimination of potential biases commonly encountered in epidemiological studies, such as reverse causality, indication bias, biases arising from unmeasured or unobserved factors, time-varying treatment exposure, and uncertainty regarding dosages, are necessary.
Conclusion
ARBs may outperform other anti-hypertensive classes in preventing or delaying cognitive decline and dementia and, therefore, offer a promising therapeutic avenue. However, the inconsistent findings observed in previous studies regarding the neuroprotective effects of ARBs emphasize the need for future long-term follow-up studies involving larger and more diverse populations to yield more robust evidence in this context. Gaining a better understanding of the intricate mechanisms involved in the potential neurocognitive benefits of ARBs, mediated through interconnected RAS pathways and other mechanisms, can offer valuable insights into identifying molecular targets aimed at preventing and treating dementia and cognitive decline.
Data Availability Statement
No data were used for drafting this manuscript.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185–9.
World Health Organization (WHO). Dementia -key fact. March 2023. Accessed from: https://www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Key%20facts,injuries%20that%20affect%20the%20brain. 20 Apr 2023.
Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–94.
Wahidi N, Lerner AJ. Blood pressure control and protection of the aging brain. Neurotherapeutics. 2019;16:569–79.
Ungvari Z, Toth P, Tarantini S, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17:639–54.
Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI, Group SS. Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol. 2012;71:825–33.
Novak V, Chowdhary A, Farrar B, et al. Altered cerebral vasoregulation in hypertension and stroke. Neurology. 2003;60:1657–63.
Eglit GML, Weigand AJ, Nation DA, Bondi MW, Bangen KJ. Hypertension and Alzheimer’s disease: indirect effects through circle of Willis atherosclerosis. Brain Commun. 2020;2:fcaa114.
• Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321:553–61. This randomized clinical trial demonstrated that intensive blood pressure control significantly reduced the risk of mild cognitive impairment and the combined rate of mild cognitive impairment or probable dementia.
Levi Marpillat N, Macquin-Mavier I, Tropeano AI, Bachoud-Levi AC, Maison P. Antihypertensive classes, cognitive decline and incidence of dementia: a network meta-analysis. J Hypertens. 2013;31:1073–82.
Peters R, Xu Y, Fitzgerald O, et al. Blood pressure lowering and prevention of dementia: an individual patient data meta-analysis. Eur Heart J. 2022;43:4980–90.
Skoog I, Gustafson D. Hypertension, hypertension-clustering factors and Alzheimer’s disease. Neurol Res. 2003;25:675–80.
Peters R, Yasar S, Anderson CS, et al. Investigation of antihypertensive class, dementia, and cognitive decline: a meta-analysis. Neurology. 2020;94:e267–81.
Ding J, Davis-Plourde KL, Sedaghat S, et al. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2020;19:61–70.
den Brok M, van Dalen JW, Abdulrahman H, et al. Antihypertensive medication classes and the risk of dementia: a systematic review and network meta-analysis. J Am Med Dir Assoc. 2021;22(1386–1395): e15.
Yasar S, Wharton W. Angiotensin receptor blockers and dementia prevention: do not RAS to a conclusion yet. Hypertension. 2022;79:2170–2.
Ma TK, Kam KK, Yan BP, Lam YY. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br J Pharmacol. 2010;160:1273–92.
Kehoe PG, Wilcock GK. Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer’s disease? Lancet Neurol. 2007;6:373–8.
Jiang T, Zhang YD, Zhou JS, et al. Angiotensin-(1–7) is reduced and inversely correlates with tau hyperphosphorylation in animal models of Alzheimer’s disease. Mol Neurobiol. 2016;53:2489–97.
Schmieder RE, Hilgers KF, Schlaich MP, Schmidt BM. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–19.
Wright JW, Harding JW. The brain RAS and Alzheimer’s disease. Exp Neurol. 2010;223:326–33.
Hajjar I, Hart M, Mack W, Lipsitz LA. Aldosterone, cognitive function, and cerebral hemodynamics in hypertension and antihypertensive therapy. Am J Hypertens. 2015;28:319–25.
Fournier A, Oprisiu-Fournier R, Serot JM, et al. Prevention of dementia by antihypertensive drugs: how AT1-receptor-blockers and dihydropyridines better prevent dementia in hypertensive patients than thiazides and ACE-inhibitors. Expert Rev Neurother. 2009;9:1413–31.
Drews HJ, Yenkoyan K, Lourhmati A, et al. Intranasal losartan decreases perivascular beta amyloid, inflammation, and the decline of neurogenesis in hypertensive rats. Neurotherapeutics. 2019;16:725–40.
Garcia-Lluch G, Pena-Bautista C, Royo LM, Baquero M, Canada-Martinez AJ, Chafer-Pericas C. Angiotensin II receptor blockers reduce tau/Ass42 ratio: a cerebrospinal fluid biomarkers’ case-control study. Pharmaceutics. 2023;15.
Glodzik L, Rusinek H, Kamer A, et al. Effects of vascular risk factors, statins, and antihypertensive drugs on PiB deposition in cognitively normal subjects. Alzheimers Dement (Amst). 2016;2:95–104.
Hajjar I, Brown L, Mack WJ, Chui H. Impact of Angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Arch Neurol. 2012;69:1632–8.
• Ouk M, Wu CY, Rabin JS et al. Associations between brain amyloid accumulation and the use of angiotensin-converting enzyme inhibitors versus angiotensin receptor blockers. Neurobiol Aging. 2021;100:22–31. This cohort study of 142 cognitively normal older adults found that ARB versus ACE-I use was associated with slower β-amyloid accumulation in the cortex.
Wharton W, Zhao L, Steenland K, et al. Neurofibrillary tangles and conversion to mild cognitive impairment with certain antihypertensives. J Alzheimers Dis. 2019;70:153–61.
ONTARGET Investigators, Yusuf S, Teo KK et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59.
Moran C, Xie K, Poh S, et al. Observational study of brain atrophy and cognitive decline comparing a sample of community-dwelling people taking angiotensin converting enzyme inhibitors and angiotensin receptor blockers over time. J Alzheimers Dis. 2019;68:1479–88.
Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM. Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis. 2011;26:699–708.
•• Scotti L, Bassi L, Soranna D, et al. Association between renin-angiotensin-aldosterone system inhibitors and risk of dementia: a meta-analysis. Pharmacol Res. 2021;166: 105515. This recent meta-analysis found that ARB was superior to ACEi and other antihypertensives in reducing the risk of dementia.
•• Anderson C, Teo K, Gao P, et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10:43–53. Two early randomized trials of people aged over 55 years old found no significant effect of RAS inhibitor antihypertensives on cognitive function and no differential effect between ARBs and ACEis over 4.7 years.
Lithell H, Hansson L, Skoog I, et al. The Study on COgnition and Prognosis in the Elderly (SCOPE); outcomes in patients not receiving add-on therapy after randomization. J Hypertens. 2004;22:1605–12.
Skoog I, Lithell H, Hansson L, et al. Effect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on COgnition and Prognosis in the Elderly (SCOPE). Am J Hypertens. 2005;18:1052–9.
Diener HC, Sacco RL, Yusuf S, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7:875–84.
Hanon O, Berrou JP, Negre-Pages L, et al. Effects of hypertension therapy based on eprosartan on systolic arterial blood pressure and cognitive function: primary results of the observational study on cognitive function and systolic blood pressure reduction open-label study. J Hypertens. 2008;26:1642–50.
•• Li NC, Lee A, Whitmer RA et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340:b5465. This large-scale population-based cohort study using the administrative database of the US Veteran Affairs suggests a significant association between ARB use and reduced incidence and progression of AD and dementia compared with ACEis and other cardiovascular drugs.
Hsu CY, Huang CC, Chan WL, et al. Angiotensin-receptor blockers and risk of Alzheimer’s disease in hypertension population–a nationwide cohort study. Circ J. 2013;77:405–10.
Yasar S, Xia J, Yao W, et al. Antihypertensive drugs decrease risk of Alzheimer disease: ginkgo evaluation of memory study. Neurology. 2013;81:896–903.
Chiu WC, Ho WC, Lin MH, et al. Angiotension receptor blockers reduce the risk of dementia. J Hypertens. 2014;32:938–47.
Goh KL, Bhaskaran K, Minassian C, Evans SJ, Smeeth L, Douglas IJ. Angiotensin receptor blockers and risk of dementia: cohort study in UK Clinical Practice Research Datalink. Br J Clin Pharmacol. 2015;79:337–50.
Kuan YC, Huang KW, Yen DJ, Hu CJ, Lin CL, Kao CH. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers reduced dementia risk in patients with diabetes mellitus and hypertension. Int J Cardiol. 2016;220:462–6.
van Middelaar T, van Vught LA, Moll van Charante EP et al. Lower dementia risk with different classes of antihypertensive medication in older patients. J Hypertens. 2017;35:2095–2101.
Barthold D, Joyce G, Wharton W, Kehoe P, Zissimopoulos J. The association of multiple anti-hypertensive medication classes with Alzheimer’s disease incidence across sex, race, and ethnicity. PLoS ONE. 2018;13: e0206705.
Bohlken J, Jacob L, Kostev K. The relationship between the use of antihypertensive drugs and the incidence of dementia in general practices in Germany. J Alzheimers Dis. 2019;70:91–7.
Hajjar I, Okafor M, McDaniel D, et al. Effects of candesartan vs lisinopril on neurocognitive function in older adults with executive mild cognitive impairment: a randomized clinical trial. JAMA Netw Open. 2020;3: e2012252.
Cohen JB, Marcum ZA, Zhang C, et al. Risk of mild cognitive impairment or probable dementia in new users of angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors: a secondary analysis of data from the Systolic Blood Pressure Intervention Trial (SPRINT). JAMA Netw Open. 2022;5: e2220680.
Lee HW, Kim S, Jo Y, Kim Y, Ye BS, Yu YM. Neuroprotective effect of angiotensin II receptor blockers on the risk of incident Alzheimer’s disease: a nationwide population-based cohort study. Front Aging Neurosci. 2023;15:1137197.
Kehoe PG. The coming of age of the angiotensin hypothesis in Alzheimer’s disease: progress toward disease prevention and treatment? J Alzheimers Dis. 2018;62:1443–66.
Abiodun OA, Ola MS. Role of brain renin angiotensin system in neurodegeneration: an update. Saudi J Biol Sci. 2020;27:905–12.
Balogh M, Aguilar C, Nguyen NT, Shepherd AJ. Angiotensin receptors and neuropathic pain. Pain Rep. 2021;6: e869.
•• Saavedra JM. Evidence to consider angiotensin II receptor blockers for the treatment of early Alzheimer’s disease. Cell Mol Neurobiol. 2016;36:259–79. This is a comprehensive review paper illustrating the potential benefits of ARB use on preventing early Alzheimer’s disease.
Wu H, Sun Q, Yuan S, et al. AT1 receptors: their actions from hypertension to cognitive impairment. Cardiovasc Toxicol. 2022;22:311–25.
Lucius R, Gallinat S, Rosenstiel P, Herdegen T, Sievers J, Unger T. The angiotensin II type 2 (AT2) receptor promotes axonal regeneration in the optic nerve of adult rats. J Exp Med. 1998;188:661–70.
• Ahmed HA, Ishrat T. The brain AT2R-a potential target for therapy in Alzheimer’s disease and vascular cognitive impairment: a comprehensive review of clinical and experimental therapeutics. Mol Neurobiol. 2020;57:3458–3484. This is a comprehensive literature review summarizing the major causes, pathophysiology, and treatments of dementia as well as the potential impact of modulating the Ang II/AT2R pathway via the RAS signalling on neurocognitive outcomes.
Horiuchi M, Mogi M. Role of angiotensin II receptor subtype activation in cognitive function and ischaemic brain damage. Br J Pharmacol. 2011;163:1122–30.
Ho JK, Nation DA. Cognitive benefits of angiotensin IV and angiotensin-(1–7): a systematic review of experimental studies. Neurosci Biobehav Rev. 2018;92:209–25.
•• Wright JW, Harding JW. Contributions by the brain renin-angiotensin system to memory, cognition, and Alzheimer’s disease. J Alzheimers Dis. 2019;67:469–480. This review paper summarized the existing evidence that supports the brain renin-angiotensin system as an effective drug target for improving neurocognitive outcomes.
Royea J, Zhang L, Tong XK, Hamel E. Angiotensin IV receptors mediate the cognitive and cerebrovascular benefits of losartan in a mouse model of Alzheimer’s disease. J Neurosci. 2017;37:5562–73.
Evans CE, Miners JS, Piva G, et al. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathol. 2020;139:485–502.
Kehoe PG, Wong S, Al Mulhim N, Palmer LE, Miners JS. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-beta and tau pathology. Alzheimers Res Ther. 2016;8:50.
Jiang T, Tan L, Gao Q, et al. Plasma angiotensin-(1–7) is a potential biomarker for Alzheimer’s disease. Curr Neurovasc Res. 2016;13:96–9.
Campbell DJ, Zeitz CJ, Esler MD, Horowitz JD. Evidence against a major role for angiotensin converting enzyme-related carboxypeptidase (ACE2) in angiotensin peptide metabolism in the human coronary circulation. J Hypertens. 2004;22:1971–6.
Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1–7) in essential hypertension. J Hypertens. 1996;14:799–805.
Furuhashi M, Moniwa N, Mita T, et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28:15–21.
Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-Angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–9.
Liu S, Liu J, Miura Y, et al. Conversion of Abeta43 to Abeta40 by the successive action of angiotensin-converting enzyme 2 and angiotensin-converting enzyme. J Neurosci Res. 2014;92:1178–86.
Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002;162:2046–52.
Schroevers JL, Eggink E, Hoevenaar-Blom MP, et al. Antihypertensive medication classes and the risk of dementia over a decade of follow-up. J Hypertens. 2023;41:262–70.
•• Marcum ZA, Cohen JB, Zhang C et al. Association of antihypertensives that stimulate vs inhibit types 2 and 4 angiotensin II receptors with cognitive impairment. JAMA Netw Open. 2022;5:e2145319. A post hoc analysis of the SPRINT randomized trial reported a significantly lower risk of cognitive impairment in participants taking AT2R/AT4R stimulator anti-hypertensive medications compared with those taking AT2R/AT4R inhibitor anti-hypertensive medications over a 5-year follow-up.
Marcum ZA, Li Y, Lee SJ, et al. Association of antihypertensives and cognitive impairment in long-term care residents. J Alzheimers Dis. 2022;86:1149–58.
•• Marcum ZA, Gabriel N, Bress AP, Hernandez I. Association of new use of antihypertensives that stimulate vs inhibit type 2 and 4 angiotensin II receptors with dementia among Medicare beneficiaries. JAMA Netw Open. 2023;6:e2249370. A large cohort study of Medicare beneficiaries reported lower risks of AD and related dementia with the use of AT2R/AT4R stimulator anti-hypertensive medications compared with AT2R/AT4R inhibitor anti-hypertensive medications over a 7-year follow-up.
Kaundal RK, Sharma SS. Peroxisome proliferator-activated receptor gamma agonists as neuroprotective agents. Drug News Perspect. 2010;23:241–56.
Heneka MT, Reyes-Irisarri E, Hull M, Kummer MP. Impact and therapeutic potential of PPARs in Alzheimer’s disease. Curr Neuropharmacol. 2011;9:643–50.
Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–7.
Liu CH, Sung PS, Li YR, et al. Telmisartan use and risk of dementia in type 2 diabetes patients with hypertension: a population-based cohort study. PLoS Med. 2021;18: e1003707.
Benicky J, Sanchez-Lemus E, Honda M, et al. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36:857–70.
Saavedra JM, Angiotensin II. AT(1) receptor blockers ameliorate inflammatory stress: a beneficial effect for the treatment of brain disorders. Cell Mol Neurobiol. 2012;32:667–81.
Lanz TV, Ding Z, Ho PP, et al. Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest. 2010;120:2782–94.
Singh PK, Chen ZL, Ghosh D, Strickland S, Norris EH. Increased plasma bradykinin level is associated with cognitive impairment in Alzheimer’s patients. Neurobiol Dis. 2020;139: 104833.
Wang Q, Wang J. Injection of bradykinin or cyclosporine A to hippocampus induces Alzheimer-like phosphorylation of tau and abnormal behavior in rats. Chin Med J (Engl). 2002;115:884–7.
Prediger RD, Medeiros R, Pandolfo P, et al. Genetic deletion or antagonism of kinin B(1) and B(2) receptors improves cognitive deficits in a mouse model of Alzheimer’s disease. Neuroscience. 2008;151:631–43.
Passos GF, Medeiros R, Cheng D, Vasilevko V, Laferla FM, Cribbs DH. The bradykinin B1 receptor regulates Abeta deposition and neuroinflammation in Tg-SwDI mice. Am J Pathol. 2013;182:1740–9.
Iores-Marcal LM, Viel TA, Buck HS, et al. Bradykinin release and inactivation in brain of rats submitted to an experimental model of Alzheimer’s disease. Peptides. 2006;27:3363–9.
Wharton W, Goldstein FC, Zhao L, Steenland K, Levey AI, Hajjar I. Modulation of renin-angiotensin system may slow conversion from mild cognitive impairment to Alzheimer’s disease. J Am Geriatr Soc. 2015;63:1749–56.
Cuddy LK, Prokopenko D, Cunningham EP et al. Abeta-accelerated neurodegeneration caused by Alzheimer’s-associated ACE variant R1279Q is rescued by angiotensin system inhibition in mice. Sci Transl Med. 2020;12.
• Ouk M, Wu CY, Rabin JS et al. The use of angiotensin-converting enzyme inhibitors vs. angiotensin receptor blockers and cognitive decline in Alzheimer’s disease: the importance of blood-brain barrier penetration and APOE epsilon4 carrier status. Alzheimers Res Ther. 2021;13:43. This study suggests that the neuroprotective effects of ARBs may be mediated by their blood-brain barrier permeability and patients’ APOE epsilon4 carrier status.
Ho JK, Nation DA, Alzheimer’s disease neuroimaging I. Memory is preserved in older adults taking AT1 receptor blockers. Alzheimers Res Ther. 2017;9:33.
Wharton W, Stein JH, Korcarz C, et al. The effects of ramipril in individuals at risk for Alzheimer’s disease: results of a pilot clinical trial. J Alzheimers Dis. 2012;32:147–56.
•Nation DA, Ho J, Yew B, Alzheimer’s disease neuroimaging I. Older adults taking AT1-receptor blockers exhibit reduced cerebral amyloid retention. J Alzheimers Dis. 2016;50:779–89. A human study demonstrated an attenuation of age-related increases in cerebral β-amyloid retention and P-tau in ARB users.
•• Deng Z, Jiang J, Wang J, et al. Angiotensin receptor blockers are associated with a lower risk of progression from mild cognitive impairment to dementia. Hypertension. 2022;79:2159–69. This study using data of the Alzheimer’s Disease Neuroimaging Initiative cohort reported that ARB users had a significantly lower annual risk of developing dementia compared with users of ACE-I, other- or no-antihypertensive users over a 3-year follow-up.
Liampas I, Hatzimanolis A, Siokas V, et al. Antihypertensive medication class and the risk of dementia and cognitive decline in older adults: a secondary analysis of the prospective HELIAD cohort. J Alzheimers Dis. 2022;89:709–19.
• Stuhec M, Keuschler J, Serra-Mestres J, Isetta M. Effects of different antihypertensive medication groups on cognitive function in older patients: a systematic review. Eur Psychiatry. 2017;46:1–15. This meta-analysis of 15 randomized trials in older adults without initial dementia demonstrated a superiority of ARBs in improving cognitive function over other antihypertensive classes, despite their similar efficacy in lowering blood pressure as ARBs.
Haag MD, Hofman A, Koudstaal PJ, Breteler MM, Stricker BH. Duration of antihypertensive drug use and risk of dementia: a prospective cohort study. Neurology. 2009;72:1727–34.
Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–86.
Chen R, Suchard MA, Krumholz HM, et al. Comparative first-line effectiveness and safety of ACE (angiotensin-converting enzyme) inhibitors and angiotensin receptor blockers: a multinational cohort study. Hypertension. 2021;78:591–603.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Michael E. Ernst and Suzanne G. Orchard had the idea for the article. Zhen Zhou performed the literature search and drafted the manuscript. Michael E. Ernst, Suzanne G. Orchard, Mark R. Nelson, and Michelle A. Fravel critically revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, Z., Orchard, S.G., Nelson, M.R. et al. Angiotensin Receptor Blockers and Cognition: a Scoping Review. Curr Hypertens Rep 26, 1–19 (2024). https://doi.org/10.1007/s11906-023-01266-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-023-01266-0