Abstract
Purpose of the Review
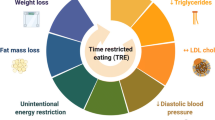
Time-restricted eating (TRE) is a promising dietary intervention for weight loss and improvement of cardiometabolic risk factors. We aim to provide a critical review of blood pressure outcomes reported in clinical TRE studies in adults with metabolic syndrome, in the context of the proposed mechanisms that underlie the relationship between timing of eating and blood pressure.
Recent Findings
Clinical TRE studies report mixed results pertaining to blood pressure outcomes, likely due to significant heterogeneity in study design and TRE protocols. Mechanistically, TRE’s metabolic benefits have been speculated to be mediated by alignment of meal timing with circadian regulation of metabolic processes and/or enhancement of catabolism as a result of prolonging the fasting period. TRE protocols that start and end earlier appear to have more pronounced blood pressure lowering effects. Blood pressure also tends to be lower with narrower eating windows. Concurrent weight loss is not consistently linked to blood pressure reduction, while lower insulin levels may be an important factor for blood pressure reduction. Notably, no published studies have reported 24-h blood pressure profiles or data on blood pressure variability.
Summary
Blood pressure has only been examined in limited TRE studies, measured at a single time point. Given the clinical relevance of blood pressure’s diurnal variability and the mechanistic evidence underlying timing of eating and blood pressure effects, more studies are needed to investigate TRE’s effects on the diurnal variability of blood pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic syndrome (MS) describes a constellation of co-occurring clinical traits that place an individual at high risk for developing cardiometabolic diseases and is associated with increased all-cause mortality [1]. The National Cholesterol Education Program (NCEP) Adult Treatment Panel (ATP) III defines MS as the presence of any 3 of the following: (1) abdominal obesity, defined as a waist circumference ≥ 102 cm in men and ≥ 88 cm in women; (2) serum triglycerides ≥ 150 mg/dL or on medications for elevated triglycerides; (3) serum high-density lipoprotein (HDL) cholesterol < 40 mg/dL in men and < 50 mg/dL in women or on medications for low HDL; (4) blood pressure (BP) ≥ 130/85 mmHg or on medications for elevated BP; and (5) fasting plasma glucose ≥ 100 mg/dL or on medications for elevated glucose [2]. The prevalence of MS (using NCEP ATP III criteria) increased from 22% in 1994–1998 to 35% in 2011–2016 using NHANES data [3], with high likelihood of further increase in the near future given the rising prevalence of obesity among US adults. While the usefulness of the MS construct has been debated, as the treatment of the syndrome entails treatment of the individual components of MS [4], it remains critical that any patients who exhibit one MS component be evaluated for other risk factors and referred to an aggressive lifestyle intervention to prevent the development of cardiometabolic diseases. Large randomized controlled trials have consistently demonstrated that behavioral weight loss of 2.5–5.5 kg in 2 years can reduce the risk of progression from prediabetes to type 2 diabetes by 30–60%, and behavioral weight loss of 5% can lower systolic and diastolic BP by 3 and 2 mmHg, respectively [5]. Even after initiation of medications for prediabetes/type 2 diabetes, hypertension, and hyperlipidemia, lifestyle modification continues to be a vital treatment component.
Studies from recent years have evaluated time-restricted eating (TRE) as a promising dietary intervention for weight loss and improvement of cardiometabolic risk factors. TRE involves consuming food only within a daily eating window without intentional caloric restriction. One important concept is the distinction between TRE and intermittent fasting (IF). IF involves complete or partial caloric restriction to a limited number of days per week, and the window of fasting or eating is not consistent on a daily basis. TRE can be categorized as a subtype of IF. The timing of eating, as opposed to the caloric content or quantity, is the key principle of TRE. Additionally, the lack of intentional caloric restriction with TRE has the potential to improve adherence. Rodent studies have consistently demonstrated that restricting feeding to the animals’ biologically active hours can prevent and reverse diet-induced obesity and its metabolic complications, even when the animals are fed a high fat or high-fructose diet [6, 7].
Pilot human studies over the past several years have attempted to translate preclinical TRE findings into a clinical intervention to prevent or treat metabolic diseases, with significant heterogeneity in TRE protocols, yielding mixed results [8••]. It remains unclear whether TRE provides any additional metabolic benefits independent of the inherent, albeit unintentional, caloric reduction associated with a restricted eating window. In fact, recent randomized controlled trials have called into question the clinical impact of TRE independent of caloric restriction [9, 10•]. However, the role of TRE in BP outcomes remains inadequately explored, as no TRE studies have examined BP as a primary outcome. The diurnal rhythm of BP is a well-established phenomenon and altered diurnal variability in BP is closely associated with cardiovascular morbidity and mortality [11–13]. As such, manipulation of meal timing could potentially affect BP rhythm and impact cardiovascular outcomes. In this article, we will first discuss the mechanisms and clinical significance of the diurnal rhythm of BP and then provide a critical review of the BP outcomes reported in clinical studies on TRE in adults with metabolic syndrome, in the context of the proposed mechanisms that underlie the relationship between timing of eating and BP.
Diurnal Rhythm of Blood Pressure
-
A.
Mechanisms Underlying the Diurnal Rhythm of Blood Pressure
BP exhibits a diurnal rhythm, which means a characteristic daily rhythm that is higher during the daytime than during the night. Specifically, BP is lower in sleep than in wakefulness by 10–20% and has a characteristic morning surge after awakening [14, 15]. However, the observed diurnal rhythm is not necessarily the same as the endogenous circadian rhythm, which is an internally driven, approximately 24-h rhythm that self-sustains in the absence of any environmental cues. This is because behavioral and physiological factors are greater contributors to the observed diurnal rhythm in BP than endogenous circadian rhythmicity. In fact, the endogenous circadian rhythm of BP exhibits peaks in both systolic and diastolic BP at ~ 21:00 with a peak-to-trough amplitude of approximately 3–6 mmHg for SBP and 2–3 mmHg for DBP. This has been demonstrated in two studies of healthy, normotensive adults following inpatient protocols of either continuous wakefulness with constant posture and isocaloric snacks every 2 h in dim light (“constant routine protocol”) or recurring sleep/wake cycles where sleep, food intake, and activities were evenly distributed across a non-24-h cycle in dim light (“forced desynchrony protocol”) [16, 17]. These two validated protocols are able to isolate the endogenous circadian rhythm from environmental (i.e., light exposure) and behavioral (i.e., physical activity, sleep, food intake) influences.
Behavioral and physiologic factors largely dictate the observed diurnal rhythm. Physiologic regulators of BP, such as sympathetic activity, cortisol levels, cardiac vagal tone, vascular tone, and renal sodium retention, exert their own endogenous circadian influence. Sympathetic activity, measured by circulating epinephrine and norepinephrine levels, exhibits a circadian trough at night and peaks during the day, with highest levels around mid-day. Plasma cortisol has a circadian peak in the morning and trough at night [17]. Cardiac vagal tone, derived from heart rate variability, peaks in the morning and has a trough around mid-day. Urinary sodium excretion is higher during the day and lower at night, which is likely the result of both circadian and sleep effects [18,19,20]. Furthermore, murine models show that the canonical clock proteins Per1, Per2, Clock, and Cry1/2 are involved in regulating renal sodium retention and consequently BP rhythm [21,22,23,24,25]. Animal studies also demonstrate that genes involved in maintaining the integrity and function of vascular smooth muscle cells exhibit circadian oscillations [26] and Bmal1 deletion in murine vascular smooth muscle cells tempers the amplitude of BP oscillations, shifts the timing of BP peak, and abolishes circadian variation of pulse pressure [27].
Behavioral factors, such as sleep/wake and fast/feed patterns, play important roles in regulating BP rhythms. Nocturnal sleep is associated with lower BP while arousal from sleep is associated with higher BP. Sleep onset is characterized by a reduction in sympathetic drive, recumbent body position, and circadian-mediated decline in core body temperature. These three components facilitate vasodilatation resulting in arterial BP reduction [28, 29]. In fact, an increase in peripheral blood flow and a decrease in peripheral vascular resistance were observed during nocturnal sleep in a study that measured blood flow rate to the subcutaneous adipose tissue of the lower leg in 15 normotensive lean individuals [30]. Additionally, BP varies across the night in association with sleep stages, with the lowest BP observed during deep sleep and highest BP observed during lighter sleep stages [31]. As such, the morning BP surge upon waking is likely the result of the higher prevalence of lighter sleep stages, redistribution of blood volume from the skin to central arterial circulation due to the circadian rise in core body temperature, and increased sympathetic activity [29].
Fasting/feeding pattern is another important behavioral factor that can regulate BP rhythms. Both short-term (48 h) and long-term (4–41 days) medically supervised fasting reduce systolic and diastolic BP [32, 33]. Fasting has been associated with natriuresis, which can be reversed by glucose administration, in both normotensive and hypertensive individuals [34, 35]. The exact mechanisms underlying fasting-induced natriuresis remain unknown but have been postulated to be due to insulin’s effects on renal sodium retention and the decline of insulin during fasting [36]. In support of this hypothesis, an euglycemic-hyperinsulinemic clamp study demonstrated that insulin administration induced a reduction in urinary sodium excretion in the absence of changes in filtered load of glucose, glomerular filtration rate, renal blood flow, and plasma aldosterone concentration [37]. Insulin has been postulated to directly increase BP via several mechanisms, including stimulation of sodium reabsorption in the kidney [36], trophic effect on vascular smooth muscle cell migration and proliferation [38], upregulation of angiotensin system [39], and tissue-specific modulation of the sympathetic nervous system [40, 41]. However, the causal relationship between hyperinsulinemia and hypertension remains controversial, with insulin administration leading to lower BP in patients with obesity and hypertension without diabetes and no changes in BP after correction of hyperinsulinemia after tumor removal in patients with insulinomas [42, 43]. Alternatively, alterations in the levels of aldosterone, glucagon, natriuretic peptides, ketonuria, and sympathetic activity have also been implicated as potential mediators for fasting-induced natriuresis [36].
-
B.
Clinical Significance of the Diurnal Rhythm of Blood Pressure
Altered diurnal variability of BP has been found in patients with cardiometabolic diseases and may be a marker or mediator of increased mortality and morbidity. Specifically, the lack of nocturnal BP decline (termed “non-dipping BP”) and increased nocturnal BP relative to daytime BP (termed “reverse dipping”) are associated with increased cardiovascular disease and mortality risk, even after adjusting for mean 24-h BP [11–13]. In addition, patients with chronic kidney disease (CKD) often do not exhibit normal night-time BP dipping, even compared to those with essential hypertension without CKD [44]. Interestingly, among patients with hypertension, those with MS had a higher prevalence of non-dipping nocturnal BP than those without MS [45], suggesting dysregulation of renal water and sodium excretion [46]. Similarly, in patients with diabetes, a reversed dipping BP rhythm was associated with higher cardiovascular events and mortality [47]. Changing the timing of behaviors such as sleep or food intake can alter BP. In an experimental setting, healthy volunteers who ate and slept out of sync with their habitual times for 8 days increased their mean arterial pressure by 3% [48]. Thus, understanding the chronobiology of BP rhythm may reveal novel ways to control BP and mitigate cardiometabolic morbidity and mortality risk, particularly in those with MS.
Proposed Mechanisms Behind Time Restricted Eating
The mechanisms behind TRE’s purported effectiveness on weight loss and improvement of cardiometabolic risk factors are much debated and are likely multi-factorial. One hypothesized mechanism is the alignment of meal timing with the endogenous circadian rhythm to optimize metabolic health (“circadian alignment” hypothesis). Animal models have shown the critical role of circadian rhythm in the regulation of metabolism, as mice with whole-body or tissue-specific loss of function alleles of circadian genes develop dysfunction in glucose homeostasis, lipid metabolism, and weight regulation [49]. Similarly, high prevalence of obesity and MS are found in night shift workers [50]. These observations suggest that circadian misalignment, described as a mismatch between our endogenous circadian rhythm and the timing of our behaviors such as eating, sleeping, and physical activity, may play a role in the development of obesity, metabolic dysfunction, and cardiovascular disease. Based on this “circadian alignment” hypothesis, timing of the eating window should be shifted to earlier in the day, when human metabolism is designed to be more active. Theoretically this may benefit BP, since earlier salt intake coincides with the diurnal upregulation of urinary sodium excretion by the kidneys [18, 19]. On the other hand, TRE’s metabolic benefits may be derived from prolonging the habitual fasting period and consequently enhancing catabolism. Based on this “fasting” hypothesis, the eating window should be narrowed based on the individual’s baseline eating window, but the timing of the eating window can be earlier or later in the day. Under this conceptual model, any salient effects of TRE on BP may be due to fasting-induced natriuresis and reduction in insulin levels.
Studies of time-restricted feeding (TRF) in animal models have examined BP outcomes and have explored potential mechanisms behind these findings. Mice, the most commonly studied animal model in TRF, are nocturnal animals with their metabolically active phase occurring in the dark (i.e., night-time). Zhang et al. showed that timing of feeding can dictate BP rhythms in a study that telemetrically recorded BP in mice [51]. In mice with ad libitum 24-h feeding, where higher food and water intake naturally occurs during the active/dark phase, the highest mean arterial pressure (MAP) is found in the active/dark phase and lowest MAP is found in the inactive/light phase. Conversely, when mice are fed solely during their inactive/light phase, the day/night pattern in MAP becomes reversed such that MAP is now highest in the inactive/light phase. Furthermore, the ability for food timing to entrain BP rhythms, such that MAP is highest during the feeding period, persists even in the absence of the light/dark cycle (i.e., when mice were fed in constant darkness) and in Bmal1 (a key transcription factor in cellular circadian regulation) knockout mice. Hou et al. conducted a study that continuously monitored feeding and BP rhythms in diabetic db/db mice. Consistent with findings of a prior study [52], db/db mice exhibit dampened rhythms in MAP, which was largely the result of higher MAP during the inactive/light phase (i.e., “non-dipping”) coinciding with increased food intake during the inactive/light phase. This study found that 8-h or 10-h TRF during the active/dark phase protected against the development of non-dipping BP in diabetic mice and restored BP dipping in non-dipping diabetic mice [53•]. Mechanistically, the study revealed that TRF selectively suppressed sympathetic activity during the inactive/fasting phase in diabetic mice, as evidenced by decreased heart rate, increased heart rate variability, increased spontaneous baroreflex sensitivity, and decreased urinary norepinephrine and normetanephrine levels. The authors postulated that the beneficial effects of TRF on BP in diabetes may be mediated through decreased sympathetic vascular tone during the inactive/light phase via fasting. In summary, these TRF studies in murine models show that timing of food can entrain BP rhythm independent of other circadian cues and that restricting feeding to the active/dark phase lowers BP in the inactive/light phase, consistent with the “fasting” hypothesis.
Blood Pressure Outcomes in Time Restricted Eating Studies
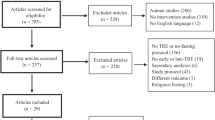
Over the past several years, TRE has been studied in clinical trials in adults with overweight/obesity and/or metabolic syndrome (Tables 1 and 2; Fig. 1). No TRE studies have evaluated BP as a primary outcome and only a few TRE studies have reported BP changes as a secondary outcome, with mixed findings. BP reduction was found after TRE in one randomized crossover trial in men with prediabetes [54••], a non-randomized study in adults with metabolic syndrome [55•], and two other non-randomized studies in adults with overweight/obesity [56, 57]. In contrast, no significant changes in BP were found in several randomized TRE trials and other non-randomized TRE studies in adults with overweight/obesity [10•, 58–66]. A unifying conclusion based on these findings is difficult to attain due to substantial differences in study cohorts, TRE protocols (timing and duration of eating windows), and timing of outcome measurement. Furthermore, concurrent weight loss was reported in almost all TRE studies, thus limiting our ability to investigate whether BP reduction is mediated by TRE per se. Finally, unintentional caloric reduction is prevalent in TRE studies, which posits that the BP lowering effects of TRE may be solely due to caloric restriction.
Summary of time-restricted eating (TRE) studies with blood pressure (BP) outcomes. White bars, randomized studies; Gray bars, non-randomized studies; superscript letter “a” self-selected window but 10:40–18:40 was the average eating window; superscript letter “b” self-selected window starts between 8:00–10:00 and ends by 18:00–20:00; black down-pointing triangle (▼) means significant weight loss with TRE; teardrop-spoked asterisk (✻) means lower fasting insulin with TRE
The most robust evidence for BP reduction with TRE is a study by Sutton et al., which demonstrated that TRE lowered morning BP in the absence of weight loss in a randomized, cross-over, eucaloric (i.e., prescribed caloric intake calculated for weight maintenance), and isocaloric (i.e., caloric intake remained the same for each individual throughout the study) controlled feeding study [54••]. The study evaluated a 5-week TRE protocol consisting of 6-h eating window with dinner no later than 15:00 compared to 12-h control condition in 8 men with prediabetes. The primary outcome was glucose and insulin dynamics assessed by 3-h oral glucose tolerance test. TRE decreased fasting and post-prandial insulin levels without significant changes in glucose levels, which was interpreted as improved insulin sensitivity and β-cell responsiveness. Participants at baseline had normal morning systolic and diastolic BPs and were not on any anti-hypertensive medications. TRE lowered morning systolic and diastolic BP by 11 ± 4 mmHg and 10 ± 4 mmHg (mean ± SD), respectively. This dramatic reduction in BP was surprising, particularly in the setting of minimal weight loss (~ 1 kg for both conditions). One possible explanation is that the longer fasting period for TRE (18-h overnight fast) may have induced lower BP when measured the following morning, compared to the 12-h overnight fast for the control condition. This hypothesis could be further investigated in a future study by comparing BP during the eating window to the fasting window to see if 24-h BP rhythms were altered. The authors additionally speculate that reduction in insulin levels may have contributed to BP reduction, based on the mechanisms discussed in the previous section.
Similarly, two non-randomized TRE studies reported BP improvement along with a trend towards lower fasting insulin levels (Fig. 1) [55•, 56]. Notably, both studies also found significant unintentional caloric reduction and weight loss. Wilkinson et al. conducted a non-randomized study (i.e., pre/post design) of a 12-week, self-selected 10-h TRE protocol in 19 participants with metabolic syndrome and baseline daily eating window of ≥ 14 h [55•]. For the self-selected eating window, participants had to start eating between 8:00 and 10:00 and finish eating between 18:00 and 20:00. TRE resulted in significant reductions in systolic and diastolic BP (− 5.12 ± 9.51 mmHg, − 6.47 ± 7.94 mmHg, respectively) compared to pre-intervention measurements. Participants also experienced 3% weight loss, but mixed linear model analysis did not reveal a significant association between weight and BP outcomes. Similar to the study by Sutton et al. [54••], fasting insulin levels trended downwards after TRE (− 3.6 ± 8.01 uIU/mL, p = 0.064). Notably, BP-lowering medications were not altered during the study. The authors speculate that TRE may be an effective adjunctive therapy to pharmacotherapy for BP control. Despite no recommendations for calorie reduction, daily caloric intake by self-report during TRE decreased by 8.6% (~ 198.6 kcal/day). Gabel et al. evaluated a 12-week, 8-h ad libitum eating window (10:00–18:00) TRE regimen in 23 adults with obesity compared to an age-, sex-, and BMI-matched historic control group from a different weight loss study [56]. The study found that systolic BP decreased by 7 ± 2 mmHg after TRE, along with a 2.6% weight reduction. Similar to Wilkinson et al. [55•], TRE led to an unintentional caloric reduction of 341 ± 53 kcal/day. Fasting insulin levels were lower in the TRE group (from 8.3 at baseline to 5.7 uIU/mL after TRE) while insulin levels remained the same for the control group, though this was not significant (p = 0.16). Taken together, these two non-randomized studies support the findings of Sutton et al. that TRE reduced BP concurrently with lowering insulin levels. However, the lack of controlled feeding in these non-randomized studies resulted in caloric reduction and weight loss, which may have contributed meaningfully to BP reduction.
Several randomized controlled trials have reported no significant impact of TRE on BP outcomes in adults with overweight/obesity (without metabolic syndrome), both in the presence and absence of concurrent weight loss (Fig. 1) [10•, 58–62]. However, these findings must be interpreted in the context of different TRE protocols, specifically in the length and timing of the eating window. Notably, the study that implemented the narrowest eating window showed a trend towards lower SBP with TRE. Cienfuegos et al. conducted an 8-week randomized, parallel arm study with 3 ad libitum eating conditions (4-h TRE vs 6-h TRE vs control) in adults with obesity [58]. The investigators chose later eating windows to improve adherence as these eating windows allowed participants to engage in social eating and family meals. There was a 3% reduction in body weight, which is likely explained by the unintentional caloric reduction of 550 kcal/day in both TRE groups, and a trend towards lower systolic BP with both TRE eating windows (− 5.0 ± 2.2 mmHg in 4-h TRE; − 4.4 ± 2.3 mmHg in 6-h TRE; + 3.7 ± 2.8 mmHg in control; p = 0.06). Notably, TRE groups also had significant reductions in fasting insulin levels. Thus similar to the mechanisms speculated by Sutton et al. [54••], the prolonged fasting period and lower insulin levels may have contributed to lower BP in the TRE groups.
Two other randomized controlled trials evaluated 8-h TRE and found no reductions in BP with TRE. Chow et al. conducted a 12-week randomized, parallel arm study with 8-h self-selected TRE vs unrestricted eating (control) group in 20 adults with overweight/obesity and baseline eating window ≥ 14 h [60]. There were no guidelines for the start and finish times of the eating window. Although more weight loss was seen in the TRE group, changes in systolic and diastolic BP were not different between the two groups. Notably, TRE did not alter fasting insulin and glucose levels. Lowe et al. conducted a 12-week randomized, controlled trial that compared consistent meal timing (control) vs 8-h TRE in 116 adults with overweight/obesity [59]. The TRE eating window was also later in the day, from 12:00 to 20:00. The study found no significant between-group differences in changes in systolic or diastolic BP, weight, or fasting glucose and insulin levels.
Most recently, Liu et al. conducted the largest and longest randomized controlled trial evaluating TRE in adults with obesity [10•]. This study uniquely compared 8-h TRE (8:00–16:00) with caloric restriction (~ 25% caloric reduction from baseline daily caloric intake) to caloric restriction without TRE (eating window of ~ 11 h) for 12 months in 139 participants. TRE and caloric restriction groups achieved similar weight loss and BP reduction. As expected, given the degree of weight loss, fasting and post-prandial glucose, HOMA-IR, and insulin disposition index were also significantly reduced in both arms, without between-group differences. One caveat for this study was that in contrast to a baseline eating window of ≥ 14 h often reported in US-based studies, both groups had a baseline eating window of ~ 10.5 h, which suggests that an eating window difference of only 2–3 h between the groups may not have been large enough to produce additional weight and cardiometabolic benefits in the context of a 25% caloric reduction. Additionally, this study included only Chinese adults, thus limiting the generalizability of the findings.
In summary, clinical TRE studies report mixed results pertaining to BP outcomes, likely due to significant heterogeneity in study design and TRE protocols. TRE protocols that start and end earlier appear to have more pronounced BP lowering effects [54••, 56], compared to TRE protocols with similar eating durations but with later start and end times [58, 59]. BP also tends to be lower with narrower eating windows [54••, 58]. Notably, concurrent weight loss is not consistently linked to BP reduction [58, 60], while lower insulin levels may be an important factor for BP reduction but this relationship warrants further investigation [54••, 55•, 56, 58]. Lastly, no published studies have reported 24-h BP profiles or data on BP variability.
Conclusions and Future Directions
Time-restricted eating is an emerging dietary intervention for weight reduction and mitigation of cardiometabolic risk factors with mixed results in pilot clinical trials. Blood pressure has only been assessed in a few TRE studies, with most randomized controlled trials reporting no improvement in BP measured at a single time point. Additionally, only a few studies examined TRE in patients with cardiometabolic diseases such as diabetes and hypertension. More studies are needed in these patient populations as they may already have derangements in their diurnal BP rhythm (i.e., non-dipping BP) and thus could derive benefits from TRE, as seen in preclinical studies in diabetic mice. Mechanistically, TRE’s metabolic benefits may be mediated by alignment of meal timing with circadian regulation of metabolic processes and/or enhancement of catabolism as a result of prolonging the fasting period. More mechanistic studies in both animals and humans are needed to better understand the potential benefits of TRE on BP outcomes. For example, 24-h ambulatory BP monitoring can be used to investigate the differential and dynamic effects of TRE during the eating and fasting periods. Lastly, a practical consideration is to incorporate TRE into other lifestyle modifications that could also impact BP rhythms such as timing of sleep and/or physical activity.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–78. https://doi.org/10.2337/DIACARE.28.7.1769.
Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome. Circulation. 2009;120(16):1640–5. https://doi.org/10.1161/CIRCULATIONAHA.109.192644.
Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011–2016. JAMA. 2020;323(24):2526–8. https://doi.org/10.1056/nejmsr2005760.
Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28(9):2289–304. https://doi.org/10.2337/DIACARE.28.9.2289.
American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel 2013. Expert panel report: Guidelines (2013) for the management of overweight and obesity in adults. Obesity. 2014;22(S2):S41-S410. https://doi.org/10.1002/OBY.20660.
Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005.
Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–60.
•• Manoogian ENC, Chow LS, Taub PR, Laferrère B, Panda S. Time-restricted eating for the prevention and management of metabolic diseases. Endoc Rev. 2021;XX(Xx):1–32. https://doi.org/10.1210/endrev/bnab027 .Comprehensive review of recent preclinical and human studies of time-restricted eating on cardiometabolic diseases.
Thomas EA, Zaman A, Sloggett KJ, et al. Early time-restricted eating compared with daily caloric restriction: a randomized trial in adults with obesity. Obesity. 2022;30(5):1027–38. https://doi.org/10.1002/OBY.23420.
• Liu D, Huang Y, Huang C, et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. 2022;386(16):1495–504. https://doi.org/10.1056/NEJMOA2114833. This was the largest and longest randomized controlled trial of time-restricted eating in adults with obesity to date. This study found that there was no additional benefit of time-restricted eating in the absence of caloric restriction on weight loss and other metabolic risk factors including blood pressure.
Ohkubo T, Imai Y, Tsuji I, et al. Relation between nocturnal decline in blood pressure and mortality: The Ohasama study. Am J Hypertens. 1997;10(11):1201–7. https://doi.org/10.1016/S0895-7061(97)00274-4/2/AJH.1201.F3.JPEG.
Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night–day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Human Hyperten. 2009;23(10):645–653. https://doi.org/10.1038/jhh.2009.9.
Taylor KS, Heneghan CJ, Stevens RJ, Adams EC, Nunan D, Ward A. Heterogeneity of prognostic studies of 24-hour blood pressure variability: systematic review and meta-analysis. PLoS ONE. 2015;10(5): e0126375. https://doi.org/10.1371/JOURNAL.PONE.0126375.
Weber MA, Drayer JIM, Nakamura DK, Wyle FA. The circadian blood pressure pattern in ambulatory normal subjects. Am J Cardiol. 1984;54(1):115–9. https://doi.org/10.1016/0002-9149(84)90314-X.
Degaute JP, Van De Borne P, Linkowski P, Van Cauter E. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension. 1991;18(2):199–210. https://doi.org/10.1161/01.HYP.18.2.199.
Shea SA, Hilton MF, Hu K, Scheer FAJL. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108(8):980–4. https://doi.org/10.1161/CIRCRESAHA.110.233668.
Scheer FAJL, Hu K, Evoniuk H, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci USA. 2010;107(47):20541–6. https://doi.org/10.1073/PNAS.1006749107/-/DCSUPPLEMENTAL.
Mills JN, Stanbury SW. Persistent 24-hour renal excretory rhythm on a 12-hour cycle of activity. J Physiol. 1952;117(1):22–37. https://doi.org/10.1113/JPHYSIOL.1952.SP004730.
Moore-Ede MC, Kass DA, Herd JA. Transient circadian internal desynchronization after light-dark phase shift in monkeys. Am J Physiol. 1977;232(1):R31–7.
Kamperis K, Hagstroem S, Radvanska E, Rittig S, Djurhuus JC. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am Physiol Renal Physiol. 2010;299(2):F404–11. https://doi.org/10.1152/ajprenal.00126.2010.
Richards J, Cheng KY, All S, et al. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am Physiol Renal Physiol. 2013;305(12):1697–704. https://doi.org/10.1152/AJPRENAL.00472.2013/ASSET/IMAGES/LARGE/ZH20011471490008.JPEG.
Nikolaeva S, Pradervand S, Centeno G, et al. The circadian clock modulates renal sodium handling. J Am Soc Nephrol. 2012;23(6):1019–26. https://doi.org/10.1681/ASN.2011080842.
Zubera AM, Centenoa G, Pradervandb S, et al. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci USA. 2009;106(38):16523–8. https://doi.org/10.1073/PNAS.0904890106.
Gumz ML, Stow LR, Lynch IJ, et al. The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Investig. 2009;119(8):2423–34. https://doi.org/10.1172/JCI36908.
Doi M, Takahashi Y, Komatsu R, et al. Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med. 2010;16(1):67–74. https://doi.org/10.1038/NM.2061
Chalmers JA, Martino TA, Tata N, Ralph MR, Sole MJ, Belsham DD. Vascular circadian rhythms in a mouse vascular smooth muscle cell line (Movas-1). Am J Physiol Renal Physiol. 2008;295(5):1529–38. https://doi.org/10.1152/AJPREGU.90572.2008/SUPPL_FILE/FIGS3.PDF.
Xie Z, Su W, Liu S, et al. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Investig. 2015;125(1):324–36. https://doi.org/10.1172/JCI76881.
Talan MI, Engel BT, Kawate R. Overnight increases in haematocrit: additional evidence for a nocturnal fall in plasma volume. Acta Physiol Scand. 1992;144(4):473–6. https://doi.org/10.1111/J.1748-1716.1992.TB09323.X.
Van Someren EJW. More than a marker: Interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiol Int. 2000;17(3):313–54. https://doi.org/10.1081/CBI-100101050.
Sindrup JH, Kastrup J, Christensen H, Jorgensen B. Nocturnal variations in peripheral blood flow, systemic blood pressure, and heart rate in humans. Am J Physiol. 1991;261(4 30–4). https://doi.org/10.1152/AJPHEART.1991.261.4.H982.
Coccagna G, Mantovani M, Brignani F, Manzini A, Lugaresi E. Arterial pressure changes during spontaneous sleep in man. Electroencephalogr Clin Neurophysiol. 1971;31(3):277–81. https://doi.org/10.1016/0013-4694(71)90098-8.
Andersson B, Wallin G, Hedner T, Ahlberg AC, Andersson OK. Acute Effects of Short-term Fasting on Blood Pressure, Circulating Noradrenaline and Efferent Sympathetic Nerve Activity. Acta Med Scand. 1988;223(6):485–90. https://doi.org/10.1111/J.0954-6820.1988.TB17685.X.
Grundler F, Mesnage R, Michalsen A, de Toledo FW. Blood pressure changes in 1610 subjects with and without antihypertensive medication during long-term fasting. J Am Heart Assoc. 2020;9(23):18649. https://doi.org/10.1161/JAHA.120.018649.
Schloeder FX, Stinebaugh BJ. Renal tubular sites of natriuresis of fasting and glucose-induced sodium conservation. Metabolism. 1970;19(12).
Kraikitpanitch S, Chrysant SG, Lindeman RD. Natriuresis and carbohydrate-induced antinatriuresis in fasted, hydrated hypertensives: Proceedings of the Society of Experimental Biology and Medicine. 1975;149:319–24. https://doi.org/10.3181/00379727-149-38798.
DeFronzo RA. The effect of insulin on renal sodium metabolism. Diabetologia. 1981;21(3):165–71. https://doi.org/10.1007/bf00252649.
DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Investig. 1975;55(4):845–55. https://doi.org/10.1172/JCI107996.
Arnqvist HJ, Bornfeldt KE, Chen Y, Lindström T. The insulin-like growth factor system in vascular smooth muscle: Interaction with insulin and growth factors. Metabolism. 1995;44(SUPPL. 4):58–66. https://doi.org/10.1016/0026-0495(95)90222-8.
Nickenig G, Röling J, Strehlow K, Schnabel P, Böhm M. Insulin induces upregulation of vascular receptor gene expression by posttranscriptional mechanisms. Circulation. 1998;98(22):2453–60. https://doi.org/10.1161/01.CIR.98.22.2453.
Bhanot S, McNeill JH. Insulin and hypertension: a causal relationship? Cardiovasc Res. 1996;31(2):212–21. https://doi.org/10.1016/0008-6363(95)00218-9.
Ohishi M. Hypertension with diabetes mellitus: physiology and pathology review-article. Hypertens Res. 2018;41(6):389–93. https://doi.org/10.1038/s41440-018-0034-4.
Heise T, Magnusson K, Heinemann L, Sawicki PT. Insulin resistance and the effect of insulin on blood pressure in essential hypertension. Hypertension (Dallas, Tex : 1979). 1998;32(2):243–248. https://doi.org/10.1161/01.HYP.32.2.243
Sawicki PT, Heinemann L, Starke A, Berger M. Hyperinsulinaemia is not linked with blood pressure elevation in patients with insulinoma. Diabetologia. 1992;35(7):649–52. https://doi.org/10.1007/BF00400257.
Portaluppi F, Montanari L, Massari M, Chiara VD, Capanna M. Loss of nocturnal decline of blood pressure in hypertension due to chronic renal failure. American Journal of Hypertension. 1991;4(1_Pt_1):20–26. https://doi.org/10.1093/AJH/4.1.20
Ayala DE, Hermida RC, Chayán L, Mojón A, Fontao MJ, Fernández JR. Circadian pattern of ambulatory blood pressure in untreated hypertensive patients with and without metabolic syndrome. Chronobiol Int. 2009;26(6):1189–205. https://doi.org/10.3109/07420520903206294.
Fezeu L, Bankir L, Hansel B, Guerrot D. Differential circadian pattern of water and Na excretion rates in the metabolic syndrome. Chronobiol Int. 2014;31(7):861–7. https://doi.org/10.3109/07420528.2014.917090.
Nakano S, Fukuda M, Hotta F, et al. Reversed circadian blood pressure rhythm is associated with occurrences of both fatal and nonfatal vascular events in NIDDM subjects. Diabetes. 1998;47(9):1501–6. https://doi.org/10.2337/DIABETES.47.9.1501.
Scheer FAJL, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106:4453–8.
Zarrinpar A, Chaix A, Panda S. Daily eating patterns and their impact on health and disease. Trends Endocrinol Metab. 2015;27(2):69–83. https://doi.org/10.1016/j.tem.2015.11.007.
Wang J, Patterson R, Ang A, Emond J, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2014;27(Suppl 2):255–62.
Zhang D, Colson JC, Jin C, et al. Timing of food intake drives the circadian rhythm of blood pressure. Function. 2020;2(1):1–15. https://doi.org/10.1093/function/zqaa034.
Su W, Guo Z, Randall DC, Cassis L, Brown DR, Gong MC. Hypertension and disrupted blood pressure circadian rhythm in type 2 diabetic db/db mice. Am J Physiol Heart Circul Physiol. 2008;295(4). https://doi.org/10.1152/AJPHEART.00257.2008
• Hou T, Su W, Duncan MJ, Olga VA, Guo Z, Gong MC. Time-restricted feeding protects the blood pressure circadian rhythm in diabetic mice. PNAS. 2021;118(25): e2015873118. https://doi.org/10.1073/PNAS.2015873118. A preclinical study that showed that 8-hour time-restricted feeding prevented diabetic mice from developing non-dipping blood pressure and restored disrupted BP rhythms in diabetic mice.
•• Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212-1221.e3. A landmark, controlled, feeding study that showed that 6-hour early time-restricted eating improved blood pressure and insulin sensitivity in men with prediabetes, even in the absence of weight loss.
• Wilkinson MJ, Manoogian ENC, Zadourian A, Navlakha S, Panda S, Taub PR. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2019;31:92–104. A non-randomized study that demonstrated improved cardiometabolic profiles including lower blood pressure in patients with metabolic syndrome who adopted 10-hour time-restricted eating for 12 weeks.
Gabel K, Hoddy KK, Haggerty N, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4:345–53.
Prasad M, Fine K, Gee A, et al. A smartphone intervention to promote time restricted eating reduces body weight and blood pressure in adults with overweight and obesity: a pilot study. Nutrients. 2021;13(7). https://doi.org/10.3390/NU13072148.
Cienfuegos S, Gabel K, Kalam F, et al. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32:1–13.
Lowe DA, Wu N, Rohdin-Bibby L, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity. JAMA Intern Med. 2020;180:1491–9.
Chow LS, Manoogian ENC, Alvear A, et al. Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity. 2020;28:860–9.
Phillips NE, Mareschal J, Schwab N, et al. The effects of time-restricted eating versus standard dietary advice on weight, metabolic health and the consumption of processed food: a pragmatic randomised controlled trial in community-based adults. Nutrients. 2021;13(3). https://doi.org/10.3390/NU13031042.
Pureza IROM, Melo ISV, Macena ML, et al. Acute effects of time-restricted feeding in low-income women with obesity placed on hypoenergetic diets: randomized trial. Nutrition (Burbank, Los Angeles County, Calif). 2020;77. https://doi.org/10.1016/J.NUT.2020.110796.
Anton SD, Lee SA, Donahoo WT, et al. The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. 2019;11(7):1–9. https://doi.org/10.3390/nu11071500.
Parr EB, Devlin BL, Lim KHC, et al. Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: a feasibility study. Nutrients. 2020;12(11):1–22. https://doi.org/10.3390/NU12113228.
Przulj D, Ladmore D, Smith KM, Phillips-Waller A, Hajek P. Time restricted eating as a weight loss intervention in adults with obesity. PloS one. 2021;16(1). https://doi.org/10.1371/JOURNAL.PONE.0246186.
Schroder JD, Falqueto H, Mânica A, et al. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women.J Translat Med. 2021;19(1). https://doi.org/10.1186/S12967-020-02687-0.
Funding
D.D. is funded by the National Institutes of Health 5T32HL110952 and K23DK133690.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension and Metabolic Syndrome
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duan, D., Bhat, S., Jun, J.C. et al. Time-Restricted Eating in Metabolic Syndrome–Focus on Blood Pressure Outcomes. Curr Hypertens Rep 24, 485–497 (2022). https://doi.org/10.1007/s11906-022-01219-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-022-01219-z