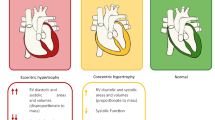
Abstract
Previously considered a disease isolated to the pulmonary circulation, pulmonary arterial hypertension is now being recognized as a systemic disorder that is associated with significant metabolic dysfunction. Numerous animal models have demonstrated the development of pulmonary arterial hypertension following the onset of insulin resistance, indicating that insulin resistance may be causal. Recent publications highlighting alterations in aerobic glycolysis, fatty acid oxidation, and the tricarboxylic acid cycle in the pulmonary circulation and right ventricle have expanded our understanding of the complex pathobiology of this disease. By targeting these derangements in metabolism, numerous researchers are investigating noninvasive techniques to monitor disease activity and therapeutics that address the underlying metabolic condition. In the following review, we will explore pre-clinical and clinical studies investigating the metabolic dysfunction seen in pulmonary arterial hypertension.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–41. doi:10.1016/j.jacc.2013.10.029.
Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2):164–72. doi:10.1161/CIRCULATIONAHA.109.898122.
Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351(16):1655–65. doi:10.1056/NEJMra035488.
Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43(12):25S–32S. doi:10.1016/j.jacc.2004.02.033.
Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circ Res. 2014;115(1):189–202. doi:10.1161/circresaha.115.303404.
Hansmann G, Wagner RA, Schellong S, Perez VA, Urashima T, Wang L, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation. 2007;115(10):1275–84. doi:10.1161/CIRCULATIONAHA.106.663120.
Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, et al. Insulin resistance in pulmonary arterial hypertension. Eur Res J. 2009;33(2):318–24. doi:10.1183/09031936.00000508.
Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant : Off Pub Int Soc Heart Transplant. 2011;30(8):904–11. doi:10.1016/j.healun.2011.02.016. This was one of the first clinical studies that demonstrated glucose intolerance was very prevalent in patients with PAH.
Belly MJ, Tiede H, Morty RE, Schulz R, Voswinckel R, Tanislav C, et al. HbA1c in pulmonary arterial hypertension: a marker of prognostic relevance? J Heart Lung Transplant : Off Pub Int Soc Heart Transplant. 2012;31(10):1109–14. doi:10.1016/j.healun.2012.08.014.
Fessel JP, Hamid R, Wittmann BM, Robinson LJ, Blackwell T, Tada Y, et al. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm Circ. 2012;2(2):201–13. doi:10.4103/2045-8932.97606. This paper highlighted the extent and diversity of metabolic dysfunction seen in patients with PAH, notably an increase in aerobic glycolysis, pentose phosphate pathway activation, and peptide catabolism, along with a decrease in fatty acid oxidation, carnitine metabolism, and TCA enzymatic activity distal to citrate.
Hemnes AR, Brittain EL, Trammell AW, Fessel JP, Austin ED, Penner N, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189(3):325–34. doi:10.1164/rccm.201306-1086OC. This paper demonstrated that BMPR2 mutations, in a mouse model of PAH and in humans with HPAH, leads to lipid deposition in peripheral muscles and the right ventricle. This paper also demonstrated that a high-fat diet in a BMPR2 mouse model amplifies the degree of PAH seen.
Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493–537. doi:10.1093/eurheartj/ehp297.
West J. Cross talk between Smad, MAPK, and actin in the etiology of pulmonary arterial hypertension. Adv Exp Med Biol. 2010;661:265–78. doi:10.1007/978-1-60761-500-2_17.
Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor–II gene. Am J Hum Genet. 2000;67(3):737–44. doi:10.1086/303059.
International PPHC, Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips 3rd JA, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26(1):81–4. doi:10.1038/79226.
Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, et al. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res. 2006;98(2):209–17. doi:10.1161/01.RES.0000200180.01710.e6.
Yang X, Long L, Reynolds PN, Morrell NW. Expression of mutant BMPR-II in pulmonary endothelial cells promotes apoptosis and a release of factors that stimulate proliferation of pulmonary arterial smooth muscle cells. Pulm Circ. 2011;1(1):103–10. doi:10.4103/2045-8932.78100.
Nichols WC, Koller DL, Slovis B, Foroud T, Terry VH, Arnold ND, et al. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31–32. Nat Genet. 1997;15(3):277–80. doi:10.1038/ng0397-277.
Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet. 2000;37(10):741–5.
Humbert M, Deng Z, Simonneau G, Barst RJ, Sitbon O, Wolf M, et al. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Res J. 2002;20(3):518–23.
Runo JR, Vnencak-Jones CL, Prince M, Loyd JE, Wheeler L, Robbins IM, et al. Pulmonary veno-occlusive disease caused by an inherited mutation in bone morphogenetic protein receptor II. Am J Respir Crit Care Med. 2003;167(6):889–94. doi:10.1164/rccm.200208-861OC.
Dewachter L, Adnot S, Guignabert C, Tu L, Marcos E, Fadel E, et al. Bone morphogenetic protein signalling in heritable versus idiopathic pulmonary hypertension. Eur Res J. 2009;34(5):1100–10. doi:10.1183/09031936.00183008.
Austin ED, Menon S, Hemnes AR, Robinson LR, Talati M, Fox KL, et al. Idiopathic and heritable PAH perturb common molecular pathways, correlated with increased MSX1 expression. Pulm Circ. 2011;1(3):389–98. doi:10.4103/2045-8932.87308.
Hansmann G, Zamanian RT. PPARgamma activation: a potential treatment for pulmonary hypertension. Sci Transl Med. 2009;1(12):12ps4. doi:10.1126/scitranslmed.3000267.
Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, et al. Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux. Mol Cell Biol. 2002;22(8):2607–19.
Yang WS, Jeng CY, Wu TJ, Tanaka S, Funahashi T, Matsuzawa Y, et al. Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care. 2002;25(2):376–80.
Galetto R, Albajar M, Polanco JI, Zakin MM, Rodriguez-Rey JC. Identification of a peroxisome-proliferator-activated-receptor response element in the apolipoprotein E gene control region. Biochem J. 2001;357(Pt 2):521–7.
Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100(25):2473–6.
Swertfeger DK, Bu G, Hui DY. Low density lipoprotein receptor-related protein mediates apolipoprotein E inhibition of smooth muscle cell migration. J Biol Chem. 2002;277(6):4141–6. doi:10.1074/jbc.M109124200.
Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105(24):2893–8.
Heldin CH, Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990;1(8):555–66.
Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, et al. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92(10):1162–9. doi:10.1161/01.RES.0000073585.50092.14.
Geraci MW, Moore M, Gesell T, Yeager ME, Alger L, Golpon H, et al. Gene expression patterns in the lungs of patients with primary pulmonary hypertension: a gene microarray analysis. Circ Res. 2001;88(6):555–62.
Park KS, Ciaraldi TP, Abrams-Carter L, Mudaliar S, Nikoulina SE, Henry RR. PPAR-gamma gene expression is elevated in skeletal muscle of obese and type II diabetic subjects. Diabetes. 1997;46(7):1230–4.
Eto M, Watanabe K, Ishii K. Apolipoprotein E polymorphism and hyperlipoproteinemia in obesity. Int J Obes. 1989;13(4):433–40.
Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83.
Summer R, Fiack CA, Ikeda Y, Sato K, Dwyer D, Ouchi N, et al. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease. Am J Physiol Lung Cell Mol Physiol. 2009;297(3):L432–8. doi:10.1152/ajplung.90599.2008.
Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, et al. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41(4):397–406. doi:10.1165/rcmb.2008-0415OC.
West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res. 2004;94(8):1109–14. doi:10.1161/01.res.0000126047.82846.20.
West J, Niswender KD, Johnson JA, Pugh ME, Gleaves L, Fessel JP, et al. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Res J. 2013;41(4):861–71. doi:10.1183/09031936.00030312.
Rehman J, Archer SL. A proposed mitochondrial-metabolic mechanism for initiation and maintenance of pulmonary arterial hypertension in fawn-hooded rats: the Warburg model of pulmonary arterial hypertension. Adv Exp Med Biol. 2010;661:171–85. doi:10.1007/978-1-60761-500-2_11.
Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294(2):H570-8. doi:10.1152/ajpheart.01324.2007.
Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, et al. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res : Off J Am Assoc Cancer Res. 2005;11(8):2785–808. doi:10.1158/1078-0432.ccr-04-2626.
Vazquez A, Liu J, Zhou Y, Oltvai ZN. Catabolic efficiency of aerobic glycolysis: the Warburg effect revisited. BMC Syst Biol. 2010;4:58. doi:10.1186/1752-0509-4-58.
Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, et al. Lung (1) (8)F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Res Crit Care Med. 2012;185(6):670–9. doi:10.1164/rccm.201108-1562OC. This study demonstrated that PET-CT scans can detect an increase in aerobic glycosis soon after the development of PAH in a mouse model of PAH. They determined that this was largely due to normoxic HIF-1α activation leading to increased glucose transporter 1 expression.
Das M, Fessel J, Tang H, West J. A process-based review of mouse models of pulmonary hypertension. Pulm Circ. 2012;2(4):415–33. doi:10.4103/2045-8932.105030.
Archer SL, Fang YH, Ryan JJ, Piao L. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm Circ. 2013;3(1):144–52. doi:10.4103/2045-8932.109960.
Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassell B, Natarajan R, dos Remedios CG, et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail. 2013;6(1):136–44. doi:10.1161/CIRCHEARTFAILURE.111.966127.
Fang YH, Piao L, Hong Z, Toth PT, Marsboom G, Bache-Wiig P, et al. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle’s cycle. J Mol Med. 2012;90(1):31–43. doi:10.1007/s00109-011-0804-9.
Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33(2):243–57.
Randle PJ, Priestman DA, Mistry SC, Halsall A. Glucose fatty acid interactions and the regulation of glucose disposal. J Cell Biochem. 1994;55(Suppl):1–11.
Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1(7285):785–9.
Megha T, Niki P, Mitch F, Aaron WT, Joshua PF, James DW et al. BMPR2 Mutation and Western diet are associated with altered lipid transport and lipotoxicity in the right ventricle. A106. Molecular mechanisms of right ventricular dysfunction. American Thoracic Society International Conference Abstracts: American Thoracic Society; 2014. p. A2338-A.
Holloway GP, Jain SS, Bezaire V, Han XX, Glatz JF, Luiken JJ, et al. FAT/CD36-null mice reveal that mitochondrial FAT/CD36 is required to upregulate mitochondrial fatty acid oxidation in contracting muscle. Am J Physiol Reg, Integr Comp Physiol. 2009;297(4):R960–7. doi:10.1152/ajpregu.91021.2008.
Moller DE, Flier JS. Insulin resistance—mechanisms, syndromes, and implications. N Engl J Med. 1991;325(13):938–48. doi:10.1056/NEJM199109263251307.
American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(1):S62–9. doi:10.2337/dc11-S062.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. doi:10.1161/circulationaha.109.192644.
Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160(8):517–25. doi:10.7326/m13-2411.
Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. National Health Statistics Reports. 2009(13):1–7
DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14(3):173–94.
McLaughlin T, Abbasi F, Cheal K, Chu J, Lamendola C, Reaven G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med. 2003;139(10):802–9.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi:10.1007/BF00280883.
Hill NR, Levy JC, Matthews DR. Expansion of the homeostasis model assessment of beta-cell function and insulin resistance to enable clinical trial outcome modeling through the interactive adjustment of physiology and treatment effects: iHOMA2. Diabetes Care. 2013;36(8):2324–30. doi:10.2337/dc12-0607.
Gelaye B, Revilla L, Lopez T, Suarez L, Sanchez SE, Hevner K, et al. Association between insulin resistance and c-reactive protein among Peruvian adults. Diabetol Metab Syndr. 2010;2(1):30. doi:10.1186/1758-5996-2-30.
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA : J Am Med Assoc. 2001;286(3):327–34.
Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–8.
Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50(10):2384–9.
Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106(4):453–8. doi:10.1172/JCI10762.
Steinberger J, Daniels SR. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation. 2003;107(10):1448–53.
Robbins IM, Newman JH, Johnson RF, Hemnes AR, Fremont RD, Piana RN, et al. Association of the metabolic syndrome with pulmonary venous hypertension. Chest. 2009;136(1):31–6. doi:10.1378/chest. 08-2008.
Heresi GA, Aytekin M, Newman J, DiDonato J, Dweik RA. Plasma levels of high-density lipoprotein cholesterol and outcomes in pulmonary arterial hypertension. Am J Res Crit Care Med. 2010;182(5):661–8. doi:10.1164/rccm.201001-0007OC.
Pugh ME, Newman JH, Williams DB, Brittain E, Robbins IM, Hemnes AR. Hemodynamic improvement of pulmonary arterial hypertension after bariatric surgery: potential role for metabolic regulation. Diabetes Care. 2013;36(3):e32–3. doi:10.2337/dc12-1650.
Zhao Y, Peng J, Lu C, Hsin M, Mura M, Wu L, et al. Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS One. 2014;9(2):e88727. doi:10.1371/journal.pone.0088727.
Santos M, Reis A, Goncalves F, Ferreira-Pinto MJ, Cabral S, Torres S, et al. Adiponectin levels are elevated in patients with pulmonary arterial hypertension. Clin Cardiol. 2014;37(1):21–5. doi:10.1002/clc.22210.
Hansmann G, Rabinovitch M. The protective role of adiponectin in pulmonary vascular disease. Am J Physiol Lung Cell Mol Physiol. 2010;298(1):L1–2. doi:10.1152/ajplung.00367.2009.
Summer R, Walsh K, Medoff BD. Obesity and pulmonary arterial hypertension: is adiponectin the molecular link between these conditions? Pulm Circ. 2011;1(4):440–7. doi:10.4103/2045-8932.93542.
Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112(12):1756–62. doi:10.1161/CIRCULATIONAHA.104.530972.
Huertas A, Tu L, Gambaryan N, Girerd B, Perros F, Montani D, et al. Leptin and regulatory T-lymphocytes in idiopathic pulmonary arterial hypertension. Eur Res J. 2012;40(4):895–904. doi:10.1183/09031936.00159911.
Li ZF, Zhou DX, Pan WZ, Zhang L, Ge JB. Circulating ghrelin was negatively correlated with pulmonary arterial pressure in atrial septal defect patients. Chin Med J. 2013;126(20):3936–9.
Benson L, Brittain EL, Pugh ME, Austin ED, Fox K, Wheeler L, et al. Impact of diabetes on survival and right ventricular compensation in pulmonary arterial hypertension. Pulm Circ. 2014;4(2):311–8. doi:10.1086/675994.
Brunner NW, Skhiri M, Fortenko O, Hsi A, Haddad F, Khazeni N, et al. Impact of insulin resistance on ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant : Off Publ Int Soc Heart Transplant. 2014. doi:10.1016/j.healun.2014.02.016.
Lundgrin EL, Park MM, Sharp J, Tang WH, Thomas JD, Asosingh K, et al. Fasting 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year. Ann Am Thorac Soc. 2013;10(1):1–9. This report explored the use of fasting PET-CT scans in humans with PAH, and they determined that pathologic glycolysis was detected in the right ventricles of patients with PAH compared to health controls that appeared to be HIF-1-α-mediated. doi:10.1513/AnnalsATS.201206-029OC.
Kluge R, Barthel H, Pankau H, Seese A, Schauer J, Wirtz H, et al. Different mechanisms for changes in glucose uptake of the right and left ventricular myocardium in pulmonary hypertension. J Nucl Med : Off Publ, Soc Nucl Med. 2005;46(1):25–31.
Kawut SM, Bagiella E, Lederer DJ, Shimbo D, Horn EM, Roberts KE, et al. Randomized clinical trial of aspirin and simvastatin for pulmonary arterial hypertension: ASA-STAT. Circulation. 2011;123(25):2985–93. doi:10.1161/circulationaha.110.015693.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Tufik R. Assad and Anna R. Hemnes declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pulmonary Hypertension
Rights and permissions
About this article
Cite this article
Assad, T.R., Hemnes, A.R. Metabolic Dysfunction in Pulmonary Arterial Hypertension. Curr Hypertens Rep 17, 20 (2015). https://doi.org/10.1007/s11906-014-0524-y
Published:
DOI: https://doi.org/10.1007/s11906-014-0524-y