Abstract
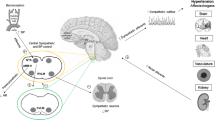
Hypertension is a leading risk factor for the development of several cardiovascular diseases. As the global prevalence of hypertension increases, so too has the recognition of resistant hypertension. Whilst figures vary, the proportion of hypertensive patients that are resistant to multiple drug therapies have been reported to be as high as 16.4 %. Resistant hypertension is typically associated with elevated sympathetic activity and abnormal homeostatic reflex control and is termed neurogenic hypertension because of its presumed central autonomic nervous system origin. This resistance to conventional pharmacological treatment has stimulated a plethora of medical devices to be investigated for use in hypertension, with varying degrees of success. In this review, we discuss a new therapy for drug-resistant hypertension, deep brain stimulation. The utility of deep brain stimulation in resistant hypertension was first discovered in patients with concurrent neuropathic pain, where it lowered blood pressure and improved baroreflex sensitivity. The most promising central target for stimulation is the ventrolateral periaqueductal gray, which has been well characterised in animal studies as a control centre for autonomic outflow. In this review, we will discuss the promise and potential mechanisms of deep brain stimulation in the treatment of severe, resistant hypertension.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fisher JP, Paton JF. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens. 2012;26(8):463–75.
Sobotka PA, Osborn JW, Paton JF. Restoring autonomic balance: future therapeutic targets. EuroInterv. 2013;9 Suppl R:R140–8.
Paton JF et al. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61(1):5–13. This review provides a comprehensive account of most studies in which carotid body ablation was performed. It considers how safe this procedure is and how effective it might be as an interventional approach to treat hypertension.
Esler M. The sympathetic system and hypertension. Am J Hypertens. 2000;13(6 Pt 2):99S–105.
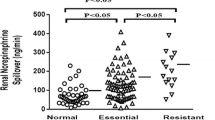
Smith PA et al. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens. 2004;17(3):217–22.
Calhoun DA et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403–19.
Esler M et al. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand. 2003;177(3):275–84.
Hart EC et al. Translational examination of changes in baroreflex function after renal denervation in hypertensive rats and humans. Hypertension. 2013;62(3):533–41. One of the first publications reporting the efficacy of renal denervation in hypertensive patients indicating an approximate 50 % success rate. Interestingly, mean falls in systolic pressure after renal denervation were not dissimilar to those in humans.
Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57(6):1076–80.
de la Sierra A et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898–902. This large study assesses the prevalence of resistant hypertensive patients and addresses the importance of using ambulatory blood pressure recording in this assessment.
Judd E, Calhoun DA. Apparent and true resistant hypertension: definition, prevalence and outcomes. J Hum Hypertens. 2014;28:463–8.
Kearney PM et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23. An important paper providing evidence of the pandemic prevalence of hypertension and its rate of growth.
Tomaszewski M et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100(11):855–61.
Laurent S, Schlaich M, Esler M. New drugs, procedures, and devices for hypertension. Lancet. 2012;380(9841):591–600.
Grassi G. Sympathetic and baroreflex function in hypertension: implications for current and new drugs. Curr Pharm Des. 2004;10(29):3579–89.
Grassi G. Counteracting the sympathetic nervous system in essential hypertension. Curr Opin Nephrol Hypertens. 2004;13(5):513–9.
Grassi G, Mancia G. New therapeutic approaches for resistant hypertension. J Nephrol. 2012;25(3):276–81.
Symplicity HTNI et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–9. An important early paper demonstrating the potential efficacy of renal denervation for the treatment of hypertension.
Krum H et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383(9917):622–9.
Esler MD et al. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976–82.
Brinkmann J et al. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension. 2012;60(6):1485–90.
Vase H et al. Catheter-based renal denervation for treatment of resistant hypertension. Dan Med J. 2012;59(6):A4439.
Kaltenbach B et al. Renal sympathetic denervation as second-line therapy in mild resistant hypertension: a pilot study. Catheter Cardiovasc Interv. 2013;81(2):335–9.
Bakris GL et al. Baroreflex activation therapy provides durable benefit in patients with resistant hypertension: results of long-term follow-up in the Rheos Pivotal Trial. J Am Soc Hypertens. 2012;6(2):152–8.
Schroeder C et al. Truly refractory hypertension. Hypertension. 2013;62(2):231–5.
Simms AE, Paton JF, Pickering AE. Hierarchical recruitment of the sympathetic and parasympathetic limbs of the baroreflex in normotensive and spontaneously hypertensive rats. J Physiol. 2007;579(Pt 2):473–86.
Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans: individualized patterns of regulation and their implications. Hypertension. 2010;56(1):10–6.
Lohmeier TE, Iliescu R. Chronic lowering of blood pressure by carotid baroreflex activation: mechanisms and potential for hypertension therapy. Hypertension. 2011;57(5):880–6.
Heusser K et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55(3):619–26. Land mark paper demonstrating the potential for carotid baroreceptor stimulation as a novel interventional therapeutic approach for the treatment of drug-resistant hypertension in humans.
Benabid AL et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337(8738):403–6.
Pereira EA et al. Deep brain stimulation: indications and evidence. Expert Rev Med Devices. 2007;4(5):591–603.
Pouratian N et al. Deep brain stimulation for the treatment of Parkinson’s disease: efficacy and safety. Degener Neurol Neuromuscul Dis. 2012;2012(2):107–17.
Zrinzo L et al. Reducing hemorrhagic complications in functional neurosurgery: a large case series and systematic literature review. J Neurosurg. 2012;116(1):84–94.
Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58(1–2):27–47. Thorough review on the organisation and role of the periaqueductal gray in blood pressure regulation and affective behaviour.
Netzer F et al. Brain circuits mediating baroreflex bradycardia inhibition in rats: an anatomical and functional link between the cuneiform nucleus and the periaqueductal grey. J Physiol. 2011;589(Pt 8):2079–91.
Hayward LF. Midbrain modulation of the cardiac baroreflex involves excitation of lateral parabrachial neurons in the rat. Brain Res. 2007;1145:117–27.
Bandler R et al. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53(1):95–104.
Carrive P, Bandler R, Dampney RA. Anatomical evidence that hypertension associated with the defence reaction in the cat is mediated by a direct projection from a restricted portion of the midbrain periaqueductal grey to the subretrofacial nucleus of the medulla. Brain Res. 1988;460(2):339–45.
ter Horst GJ, Luiten PG, Kuipers F. Descending pathways from hypothalamus to dorsal motor vagus and ambiguus nuclei in the rat. J Auton Nerv Syst. 1984;11(1):59–75.
Uschakov A et al. Functional correlates of activity in neurons projecting from the lamina terminalis to the ventrolateral periaqueductal gray. Eur J Neurosci. 2009;30(12):2347–55.
Hurley KM et al. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308(2):249–76.
An X et al. Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol. 1998;401(4):455–79.
Fisk GD, Wyss JM. Descending projections of infralimbic cortex that mediate stimulation-evoked changes in arterial pressure. Brain Res. 2000;859(1):83–95.
Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46(6):575–605.
Holstege G. Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: an HRP and autoradiographic tracing study in the cat. J Comp Neurol. 1987;260(1):98–126.
Jansen ASP et al. Local connections between the columns of the periaqueductal gray matter: a case for intrinsic neuromodulation. Brain Res. 1998;784:329–36. Erratum in Brain Res 1998 Jun 29;797(2):368.
Lovick TA. Inhibitory modulation of the cardiovascular defence response by the ventrolateral periaqueductal grey matter in rats. Exp Brain Res. 1992;89(1):133–9.
Farkas E, Jansen AS, Loewy AD. Periaqueductal gray matter input to cardiac-related sympathetic premotor neurons. Brain Res. 1998;792(2):179–92.
Bowman BR et al. Brain sources of inhibitory input to the rat rostral ventrolateral medulla. J Comp Neurol. 2013;521(1):213–32.
Verberne AJ, Guyenet PG. Midbrain central gray: influence on medullary sympathoexcitatory neurons and the baroreflex in rats. Am J Physiol. 1992;263(1 Pt 2):R24–33.
Wang WH, Lovick TA. The inhibitory effect of the ventrolateral periaqueductal grey matter on neurones in the rostral ventrolateral medulla involves a relay in the medullary raphe nuclei. Exp Brain Res. 1993;94(2):295–300.
Snowball RK, Dampney RA, Lumb BM. Responses of neurones in the medullary raphe nuclei to inputs from visceral nociceptors and the ventrolateral periaqueductal grey in the rat. Exp Physiol. 1997;82(3):485–500.
Morgan MM, Carrive P. Activation of the ventrolateral periaqueductal gray reduces locomotion but not mean arterial pressure in awake, freely moving rats. Neuroscience. 2001;102(4):905–10.
Redfern WS, Yardley CP. Contribution of the periaqueductal grey matter to the development of hypertension in the spontaneously hypertensive rat. J Auton Nerv Syst. 1990;31(3):231–9. Land mark paper describing potential contribution of the periaqueductal gray in the development of neurogenic hypertension.
Schenberg LC, Brandao CA, Vasquez EC. Role of periaqueductal gray matter in hypertension in spontaneously hypertensive rats. Hypertension. 1995;26(6 Pt 2):1125–8.
Pelosi GG, Resstel LB, Correa FM. Dorsal periaqueductal gray area synapses modulate baroreflex in unanesthetized rats. Auton Neurosci. 2007;131(1–2):70–6.
Inui K, Murase S, Nosaka S. Facilitation of the arterial baroreflex by the ventrolateral part of the midbrain periaqueductal grey matter in rats. J Physiol. 1994;477(Pt 1):89–101.
Boscan P, Paton JF. Excitatory convergence of periaqueductal gray and somatic afferents in the solitary tract nucleus: role for neurokinin 1 receptors. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R262–9.
Inui K, Nosaka S. Target site of inhibition mediated by midbrain periaqueductal gray matter of baroreflex vagal bradycardia. J Neurophysiol. 1993;70(6):2205–14.
Nosaka S et al. Arterial baroreflex inhibition by midbrain periaqueductal grey in anaesthetized rats. Pflugers Arch. 1993;424(3–4):266–75.
Green AL et al. Deep brain stimulation can regulate arterial blood pressure in awake humans. Neuroreport. 2005;16(16):1741–5. Original study showing unequivocally the powerful influence of deep brain stimulation in distinct regions of the periaqueductal gray on blood pressure regulation in humans.
Green AL et al. Deep brain stimulation: a new treatment for hypertension? J Clin Neurosci. 2007;14(6):592–5.
Pereira EA et al. Sustained reduction of hypertension by deep brain stimulation. J Clin Neurosci. 2010;17(1):124–7.
Patel NK et al. Deep brain stimulation relieves refractory hypertension. Neurology. 2011;76(4):405–7.
Bandler R, Carrive P, Zhang SP. Integration of somatic and autonomic reactions within the midbrain periaqueductal grey: viscerotopic, somatotopic and functional organization. Prog Brain Res. 1991;87:269–305.
Carrive P, Bandler R. Viscerotopic organization of neurons subserving hypotensive reactions within the midbrain periaqueductal grey: a correlative functional and anatomical study. Brain Res. 1991;541(2):206–15. Land mark study describing the classical columnar organisation of the periaqueductal gray in the rat.
Pereira EA et al. Ventral periaqueductal grey stimulation alters heart rate variability in humans with chronic pain. Exp Neurol. 2010;223(2):574–81.
Sverrisdottir YB et al. Differentiated Baroreflex Modulation of Sympathetic Nerve Activity During Deep Brain Stimulation in Humans. Hypertension. 2014;63:1000–10. An important paper demonstrating the influence of deep brain stimulation of the periaqueductal gray on sympathetic tone and baroreceptor reflex in humans.
Patel NK, Plaha P, Gill SS. Magnetic resonance imaging-directed method for functional neurosurgery using implantable guide tubes. Neurosurgery. 2007;61(5 Suppl 2):358–65. Discussion 365–6. First demonstration of blood pressure lowering in a drug-resistant hypertensive patient that was not associated with analgesia during deep brain stimulation of the ventrolateral periaqueductal gray. This study allowed delineation of the anti-hypertensive effect from pain relief.
Morrell MJ, R.N.S.S.i.E.S. Group. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–304.
Chang SY et al. Wireless fast-scan cyclic voltammetry to monitor adenosine in patients with essential tremor during deep brain stimulation. Mayo Clin Proc. 2012;87(8):760–5.
Van Gompel JJ et al. Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia. 2014;55(2):233–44.
Cogiamanian F et al. Non-invasive brain stimulation for the management of arterial hypertension. Med Hypotheses. 2010;74(2):332–6.
Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8(7):559–67.
Jansen AS et al. Local connections between the columns of the periaqueductal gray matter: a case for intrinsic neuromodulation. Brain Res. 1998;784(1–2):329–36.
Farkas E, Jansen AS, Loewy AD. Periaqueductal gray matter projection to vagal preganglionic neurons and the nucleus tractus solitarius. Brain Res. 1997;764(1–2):257–61.
Herbert H, Saper CB. Organization of medullary adrenergic and noradrenergic projections to the periaqueductal gray matter in the rat. J Comp Neurol. 1992;315(1):34–52.
Krout KE, Jansen AS, Loewy AD. Periaqueductal gray matter projection to the parabrachial nucleus in rat. J Comp Neurol. 1998;401(4):437–54.
Ennis M, Xu SJ, Rizvi TA. Discrete subregions of the rat midbrain periaqueductal gray project to nucleus ambiguus and the periambigual region. Neuroscience. 1997;80(3):829–45.
Compliance with Ethics Guidelines
Conflict of Interest
Erin L. O’Callaghan, Fiona D. McBryde, Amy E. Burchell, Laura E. K. Ratcliffe, Liviu Nicolae, Derek Carr, Emma C. Hart and Angus K. Nightingale declare no conflict of interest.
Ivor Gillbe is on the board of Bioinduction Ltd., a company that has worked with Bristol University in connection with this work to validate certain aspects of its neuromodulation device under development and plans to supply devices free of charge to facilitate future research.
Nikunj K. Patel and Julian F. R. Paton have received grants from the British Heart Foundation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Device-Based Approaches for Hypertension
Rights and permissions
About this article
Cite this article
O’Callaghan, E.L., McBryde, F.D., Burchell, A.E. et al. Deep Brain Stimulation for the Treatment of Resistant Hypertension. Curr Hypertens Rep 16, 493 (2014). https://doi.org/10.1007/s11906-014-0493-1
Published:
DOI: https://doi.org/10.1007/s11906-014-0493-1