Abstract
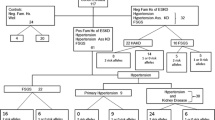
Apolipoprotein L1 (APOL1) gene association studies and results of the African American Study of Kidney Disease and Hypertension are disproving the longstanding concept that mild to moderate essential hypertension contributes substantially to end-stage renal disease susceptibility in African Americans. APOL1 coding variants underlie a spectrum of kidney diseases, including that attributed to hypertension (labeled arteriolar or hypertensive nephrosclerosis), focal segmental glomerulosclerosis, and HIV-associated nephropathy. APOL1 nephropathy risk variants persist because of protection afforded from the parasite that causes African sleeping sickness. This breakthrough will lead to novel treatments for hypertensive African Americans with low-level proteinuria, for whom effective therapies are lacking. Furthermore, APOL1 nephropathy risk variants contribute to racially variable allograft survival rates after kidney transplantation and assist in detecting nondiabetic forms of nephropathy in African Americans with diabetes. Discovery of APOL1-associated nephropathy was a major success of the genetics revolution, demonstrating that secondary hypertension is typically present in nondiabetic African Americans with nephropathy.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
U.S. Renal Data System. USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease, Bethesda, MD. 121–132. 2010.
Kiberd BA, Clase CM. Cumulative risk for developing end-stage renal disease in the US population. J Am Soc Nephrol. 2002;13:1635–44.
McClellan WM, Newsome BB, McClure LA, et al. Poverty and racial disparities in kidney disease: the REGARDS study. Am J Nephrol. 2010;32:38–46.
Evans K, Coresh J, Bash LD, et al. Race differences in access to health care and disparities in incident chronic kidney disease in the US. Nephrol Dial Transplant. 2011;26:899–908.
•• Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010, 329:841–845. This landmark report revealed that two coding variants in the apolipoprotein L1 gene (APOL1), termed G1 and G2, were strongly associated with FSGS and nondiabetic forms of ESRD. These diseases were previously attributed to variation in the adjacent non-muscle myosin heavy chain 9 gene (MYH9). The protein product of nephropathy-associated APOL1 variants lysed Trypanosoma brucei rhodesiense, a cause of African sleeping sickness.
• Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 2010, 128:345–350. This report also revealed that variation in the APOL1 gene contributed to nondiabetic ESRD susceptibility in African Americans. The G1 nephropathy risk variant was detected.
Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21:1422–6.
Howie AJ. Problems with ‘focal segmental glomerulosclerosis’. Pediatr Nephrol. 2011;26:1197–205.
Zarif L, Covic A, Iyengar S, et al. Inaccuracy of clinical phenotyping parameters for hypertensive nephrosclerosis. Nephrol Dial Transplant. 2000;15:1801–7.
Freedman BI, Iskandar SS, Appel RG. The link between hypertension and nephrosclerosis. Am J Kidney Dis. 1995;25:207–21.
Freedman BI, Sedor JR. Hypertension-associated kidney disease: perhaps no more. J Am Soc Nephrol. 2008;19:2047–51.
Schlessinger SD, Tankersley MR, Curtis JJ. Clinical documentation of end-stage renal disease due to hypertension. Am J Kidney Dis. 1994;23:655–60.
Perneger TV, Whelton PK, Klag MJ, Rossiter KA. Diagnosis of hypertensive end-stage renal disease: effect of patient’s race. Am J Epidemiol. 1995;141:10–5.
Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–8.
Kestenbaum B, Rudser KD, de Boer I. Differences in kidney function and incident hypertension: the multi-ethnic study of atherosclerosis. Ann Intern Med. 2008;148:501–8.
Fogo A, Breyer JA, Smith MC, et al. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. AASK Pilot Study Investigators. Kidney Int. 1997;51:244–52.
Marcantoni C, Ma LJ, Federspiel C, Fogo AB. Hypertensive nephrosclerosis in African Americans versus Caucasians. Kidney Int. 2002;62:172–80.
Shulman NB, Ford CE, Hall WD, et al. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The Hypertension Detection and Follow-up Program Cooperative Group. Hypertension. 1989;13:I80–93.
Walker WG, Neaton JD, Cutler JA, et al. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial. Racial and treatment effects. The MRFIT Research Group. JAMA. 1992;268:3085–91.
Freedman BI, Soucie JM, McClellan WM. Family history of end-stage renal disease among incident dialysis patients. J Am Soc Nephrol. 1997;8:1942–5.
Freedman BI, Volkova NV, Satko SG, et al. Population-based screening for family history of end-stage renal disease among incident dialysis patients. Am J Nephrol. 2005;25:529–35.
Spray BJ, Atassi NG, Tuttle AB, Freedman BI. Familial risk, age at onset, and cause of end-stage renal disease in white Americans. J Am Soc Nephrol. 1995;5(10):1806–10.
Freedman BI. Susceptibility genes for hypertension and renal failure. J Am Soc Nephrol. 2003;14(7 Suppl 2):S192–4.
Freedman BI, Spray BJ, Tuttle AB, Buckalew Jr VM. The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis. 1993;21:387–93.
Freedman BI. End-stage renal failure in African Americans: insights in kidney disease susceptibility. Nephrol Dial Transplant. 2002;17(2):198–200.
Freedman BI. Renal microvascular susceptibility in African American pedigrees. Transplant Proc. 1993;25:2423–5.
Cooper RS, Kaufman JS, Ward R. Race and genomics. N Engl J Med. 2003;348:1166–70.
Appel LJ, Middleton J, Miller III ER, et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14:S166–72.
Wright Jr JT, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–31.
•• Appel LJ, Wright JT Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 2010, 363:918–929. This report contained the final AASK Cohort Study results. Strict blood pressure control using ACE inhibition failed to slow or halt nephropathy progression in African Americans thought to have hypertensive kidney disease and lacking proteinuria. Some benefit was seen in those participants with elevated baseline urinary protein excretion.
Korbet SM. Angiotensin antagonists and steroids in the treatment of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:219–28.
Freedman BI, Sedor JR. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:2565–6.
Astor B, Lipkowitz MS, Kao WHL, Parekh R, Choi MH, Kopp JB, et al. MYH9 variations are associated with chronic kidney disease progression in the African American Study of Hypertension and Kidney Diseases [abstract]. J Am Soc Nephrol. 2010;21:81A.
Smith MW, Patterson N, Lautenberger JA, et al. A high-density admixture map for disease gene discovery in African Americans. Am J Hum Genet. 2004;74:1001–13.
Divers J, Moossavi S, Langefeld CD, Freedman BI. Genetic admixture: a tool to identify diabetic nephropathy genes in African Americans. Ethn Dis. 2008;18:384–8.
Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–84.
Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–92.
Reeves-Daniel AM, Iskandar SS, Bowden DW, et al. Is collapsing C1q nephropathy another MYH9-associated kidney disease? A case report. Am J Kidney Dis. 2010;55:e21–4.
Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009;75:736–45.
Freedman BI, Hicks PJ, Bostrom MA, et al. Non-muscle myosin heavy chain 9 gene MYH9 associations in African Americans with clinically diagnosed type 2 diabetes mellitus-associated ESRD. Nephrol Dial Transplant. 2009;11:3366–71.
Gopalakrishnan I, Iskandar SS, Daeihagh P, et al. Coincident idiopathic focal segmental glomerulosclerosis collapsing variant and diabetic nephropathy in an African American homozygous for MYH9 risk variants. Hum Pathol. 2011;42:291–4.
• Freedman BI, Langefeld CD, Lu L, et al. Differential effects of MYH9 and APOL1 risk variants on FRMD3 association with diabetic ESRD in African Americans. PLoS Genet 2011, 7:e1002150. This gene-gene interaction analysis assessed APOL1/MYH9 SNPs and nearly one million other genetic markers across the genome in African Americans with clinically diagnosed type 2 diabetic ESRD. Those with two MYH9 (or APOL1) risk variants did not demonstrate association with FRMD3, a diabetic nephropathy gene. Markers in FRMD3 were robustly associated in those lacking two chromosome 22q risk variants, revealing the potential to “genetically dissect” nondiabetic from diabetic ESRD in African Americans based on the presence of two MYH9 or APOL1 risk variants.
Freedman BI, Kopp JB, Winkler CA, et al. Polymorphisms in the nonmuscle myosin heavy chain 9 gene (MYH9) are associated with albuminuria in hypertensive African Americans: the HyperGEN study. Am J Nephrol. 2009;29:626–32.
Nelson GW, Freedman BI, Bowden DW, et al. Dense mapping of MYH9 localizes the strongest kidney disease associations to the region of introns 13 to 15. Hum Mol Genet. 2010;19:1805–15.
• Oleksyk TK, Nelson GW, An P, et al. Worldwide distribution of the MYH9 kidney disease susceptibility alleles and haplotypes: evidence of historical selection in Africa. PLoS One 2010, 5:e11474. This study assessed SNPs in the MYH9 E haplotype block in a worldwide population survey of major continental populations using International HapMap Project and the Human Genome Diversity Panel data. The worldwide distribution and frequencies of risk/protective haplotypes were presented, relevant to public health implications, including in regions of the world with a high prevalence of HIV infection.
Vanhamme L, Paturiaux-Hanocq F, Poelvoorde P, et al. Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature. 2003;422:83–7.
Pays E, Vanhollebeke B. Human innate immunity against African trypanosomes. Curr Opin Immunol. 2009;21:493–8.
Pattaro C, Aulchenko YS, Isaacs A, et al. Genome-wide linkage analysis of serum creatinine in three isolated European populations. Kidney Int. 2009;76:297–306.
• O’Seaghdha CM, Parekh RS, Hwang SJ, et al. The MYH9/APOL1 region and chronic kidney disease in European-Americans. Hum Mol Genet 2011, 20:2450–2456. MYH9 E1 haplotype SNP rs4821480 and 282 SNPs in the MYH9/APOL1 region were tested for association with subclinical kidney disease in 13,133 European-derived participants from the Framingham Heart and Atherosclerosis Risk in Communities Studies. In a meta-analysis, only rs4821480 was associated with nondiabetic kidney disease. APOL1 G1 and G2 risk variants did not account for the observed MYH9 signal, as they were exceedingly rare in these European-derived populations. This suggests that MYH9 SNP rs4821480 is associated with risk of nondiabetic nephropathy in individuals of European ancestry.
Cooke JN, Bostrom MA, Hicks PJ, Ng MC, Comeau ME, Divers J, Langefeld CD, Freedman BI, Bowden DW. Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrol Dial Transplant 2011 Oct 3 (Epub ahead of print). doi: 10.1093/ndt/gfr522.
Steiner RW. ‘Normal for now’ or ‘at future risk’: a double standard for selecting young and older living kidney donors. Am J Transplant. 2010;10:737–41.
Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int. 2000;58:1311–7.
Swanson SJ, Hypolite IO, Agodoa LY, et al. Effect of donor factors on early graft survival in adult cadaveric renal transplantation. Am J Transplant. 2002;2:68–75.
Chakkera HA, O’Hare AM, Johansen KL, et al. Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol. 2005;16:269–77.
Callender CO, Cherikh WS, Traverso P, et al. Effect of donor ethnicity on kidney survival in different recipient pairs: an analysis of the OPTN/UNOS database. Transplant Proc. 2009;41:4125–30.
Cherikh WS, Young CJ, Kramer BF, et al. Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant. 2011;11:1650–5.
•• Reeves-Daniel AM, Depalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant 2011, 11:1025–1030. Allograft survival was significantly impacted by the presence of APOL1 variants in African American deceased kidney donors. Kidneys donated by African Americans with two APOL1 risk variants functioned for shorter durations than those donated by individuals with fewer than two risk variants, and African American donor kidneys without two APOL1 risk variants functioned as long as those donated by European Americans. Hence, donor APOL1 genotype, not donor race, explained racial differences in renal allograft survival. If replicated, this study may alter practice patterns, including selection of (a) African American live kidney donors at lower risk for subsequent nephropathy, and (b) deceased kidneys with higher likelihood for long-term function.
Davis CL, Cooper M. The state of U.S. living kidney donors. Clin J Am Soc Nephrol. 2010;5:1873–80.
Srinivas TR. Defining living kidney donor ESRD risk-looking beyond race and gender. Am J Transplant. 2011;11:1556–8.
Kopp JB, Winkler CA, Nelson GW. MYH9 genetic variants associated with glomerular disease: What is the role for genetic testing? Semin Nephrol. 2010;30:409–17.
Bostrom MA, Freedman BI. The spectrum of MYH9-associated nephropathy. Clin J Am Soc Nephrol. 2010;5:1107–13.
Freedman BI, Edberg JC, Comeau ME, et al. The non-muscle myosin heavy chain 9 gene (MYH9) is not associated with lupus nephritis in African Americans. Am J Nephrol. 2010;32:66–72.
McDonough CW, Palmer ND, Hicks PJ, et al. A genome-wide association study for diabetic nephropathy genes in African Americans. Kidney Int. 2011;79:563–72.
Pezzolesi MG, Poznik GD, Mychaleckyj JC, et al. Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes. 2009;58(6):1403–10.
Gordon SM, Hofmann S, Askew DS, Davidson WS. High density lipoprotein: It’s not just about lipid transport anymore. Trends Endocrinol Metab. 2011;22:9–15.
Freedman BI, Langefeld CD, Murea M, Ma L, Otvos J, Turner JJ, Antinozzi PA, Rocco M, Parks JS. Apolipoprotein L1 nephropathy risk variants associate with HDL subfraction concentration in African Americans. Nephrol Dial Transplant 2011 Sep 19. (Epub ahead of print). doi: 10.1093/ndt/gfr542.
Kuller LH, Grandits G, Cohen JD, et al. Lipoprotein particles, insulin, adiponectin, C-reactive protein and risk of coronary heart disease among men with metabolic syndrome. Atherosclerosis. 2007;195:122–8.
Sharma M, Sharma R, McCarthy ET, Savin VJ. “The FSGS factor:” enrichment and in vivo effect of activity from focal segmental glomerulosclerosis plasma. J Am Soc Nephrol. 1999;10:552–61.
Gohh RY, Yango AF, Morrissey PE, et al. Preemptive plasmapheresis and recurrence of FSGS in high-risk renal transplant recipients. Am J Transplant. 2005;5:2907–12.
Candiano G, Musante L, Zennaro C, et al. Inhibition of renal permeability towards albumin: a new function of apolipoproteins with possible pathogenetic relevance in focal glomerulosclerosis. Electrophoresis. 2001;22:1819–25.
Candiano G, Musante L, Carraro M, et al. Apolipoproteins prevent glomerular albumin permeability induced in vitro by serum from patients with focal segmental glomerulosclerosis. J Am Soc Nephrol. 2001;12:143–50.
Wan G, Zhaorigetu S, Liu Z, et al. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem. 2008;283:21540–9.
Salem RM, Cadman PE, Chen Y, et al. Chromogranin A polymorphisms are associated with hypertensive renal disease. J Am Soc Nephrol. 2008;19:600–14.
Fung MM, Salem RM, Lipkowitz MS, et al. Methylenetetrahydrofolate reductase (MTHFR) polymorphism A1298C (Glu429Ala) predicts decline in renal function over time in the African-American Study of Kidney Disease and Hypertension (AASK) Trial and Veterans Affairs Hypertension Cohort (VAHC). Nephrol Dial Transplant 2011 May 25 (Epub ahead of print). PMID: 21613384.
Dusel JA, Burdon KP, Hicks PJ, et al. Identification of podocin (NPHS2) gene mutations in African Americans with nondiabetic end-stage renal disease. Kidney Int. 2005;68:256–62.
Acknowledgments
This work was supported by NIH grants R01 HL56266, RO1 DK070941, and RO1 DK084149.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freedman, B.I., Murea, M. Target Organ Damage in African American Hypertension: Role of APOL1 . Curr Hypertens Rep 14, 21–28 (2012). https://doi.org/10.1007/s11906-011-0237-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-011-0237-4
Keywords
- African American
- African sleeping sickness
- Arteriolar nephrosclerosis
- APOL1
- Chronic kidney disease
- Dialysis
- End-stage renal disease
- ESRD: Focal segmental glomerulosclerosis
- Genetics
- Glomerulosclerosis
- Hypertension
- Hypertensive nephrosclerosis
- Kidney disease
- Kidney donors
- MYH9
- Nondiabetic nephropathy
- Racial differences
- Trypanosoma brucei rhodesiense
- Transplantation