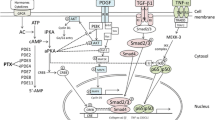
Abstract
There is no question about the contributory risk of hypertension in morbidity and mortality from cardiovascular (CV) disease and chronic kidney disease (CKD). Another independent risk factor for CV disease and CKD is proteinuria, which is most commonly caused by dysfunction of the kidney glomerular filter, in particular of the podocyte. Podocytes are highly differentiated pericyte-like cells that are essential to normal kidney function. Moreover, loss of podocytes is a hallmark of diabetic and nondiabetic progressive CKD. Recent data point to an important role for the renin-angiotensin system (RAS) and calcium signaling in the structural and functional integrity of podocytes. Given this scenario, it is desirable to treat hypertension with agents targeting the RAS, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II (Ang II) type 1-receptor blockers (ARB). These agents have proven effects on lowering blood pressure (BP) and can reduce podocyte injury. Here we review the dual effects of RAS blockade on BP and on podocyte function and emphasize BP-dependent and BP-independent effects of this regimen.
Similar content being viewed by others
References and Recommended Reading
Amann K, Irzyniec T, Mall G, Ritz E: The effect of enalapril on glomerular growth and glomerular lesions after subtotal nephrectomy in the rat: a stereological analysis. J Hypertens 1993, 11:969–975.
von Lutterotti N, Camargo MJ, Campbell WG Jr, et al.: Angiotensin II receptor antagonist delays renal damage and stroke in salt-loaded Dahl salt-sensitive rats. J Hypertens 1992, 10:949–957.
Crary GS, Swan SK, O’Donnell MP, et al.: The angiotensin II receptor antagonist losartan reduces blood pressure but not renal injury in obese Zucker rats. J Am Soc Nephrol 1995, 6:1295–1299.
Kshirsagar AV, Joy MS, Hogan SL, et al.: Effect of ACE inhibitors in diabetic and nondiabetic chronic renal disease: a systematic overview of randomized placebo-controlled trials. Am J Kidney Dis 2000, 35:695–707.
Tarif N, Bakris GL: Angiotensin II receptor blockade and progression of nondiabetic-mediated renal disease. Kidney Int Suppl 1997, 63:S67–70.
Kurokawa K: Effects of candesartan on the proteinuria of chronic glomerulonephritis. J Hum Hypertens 1999, 13(Suppl 1):S57–60; discussion S61.
Brenner BM, Cooper ME, de Zeeuw D, et al.: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001, 345:861–869.
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993, 329:1456–1462.
Somlo S, Mundel P: Getting a foothold in nephrotic syndrome. Nat Genet 2000, 24:333–335.
Endlich N, Kress KR, Reiser J, et al.: Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 2001, 12:413–422.
Petermann AT, Pippin J, Durvasula R, et al.: Mechanical stretch induces podocyte hypertrophy in vitro. Kidney Int 2005, 67:157–166.
Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: is the podocyte the culprit? Kidney Int 1998, 54:687–697.
Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 2005, 67:404–419.
Kriz W, Hosser H, Hahnel B, et al.: From segmental glomerulosclerosis to total nephron degeneration and interstitial fibrosis: a histopathological study in rat models and human glomerulopathies. Nephrol Dial Transplant 1998, 13:2781–2798.
Mitchell JA, Ventura HO, Mehra MR: Early recognition and treatment of hypertensive heart disease. Curr Opin Cardiol 2005, 20:282–289.
Fogo AB: Renal fibrosis and the renin-angiotensin system. Adv Nephrol Necker Hosp 2001, 31:69–87.
Turnbull F, Neal B, Algert C, et al.: Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 2005, 165:1410–1419.
Casas JP, Chua W, Loukogeorgakis S, et al.: Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 2005, 366:2026–2033.
Estacio RO, Jeffers BW, Gifford N, Schrier RW: Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 2000, 23(Suppl 2):B54–64.
Chugh A, Bakris GL: Microalbuminuria: what is it? Why is it important? What should be done about it? An update. J Clin Hypertens (Greenwich) 2007, 9:196–200.
Gaede P, Vedel P, Larsen N, et al.: Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003, 348:383–393.
Mundel P, Kriz W: Structure and function of podocytes: an update. Anat Embryol (Berl) 1995, 192:385–397.
Drenckhahn D, Franke RP: Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest 1988, 59:673–682.
Kerjaschki D: Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 2001, 108:1583–1587.
Asanuma K, Mundel P: The role of podocytes in glomerular pathobiology. Clin Exp Nephrol 2003, 7:255–259.
Takeda T, McQuistan T, Orlando RA, Farquhar MG: Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest 2001, 108:289–301.
Deferrari G, Ravera M, Deferrari L, et al.: Renal and cardiovascular protection in type 2 diabetes mellitus: angiotensin II receptor blockers. J Am Soc Nephrol 2002, 139(Suppl 3):S224–229.
Tryggvason K, Patrakka J, Wartiovaara J: Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med 2006, 354:1387–1401.
Durvasula RV, Petermann AT, Hiromura K, et al.: Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int 2004, 65:30–39.
Weir MR, Dzau VJ: The renin-angiotensin-aldosterone system: a specific target for hypertension management. Am J Hypertens 1999, 12(12 Pt 3):205S–213S.
Sadoshima J, Xu Y, Slayter HS, Izumo S: Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 1993, 75:977–984.
Huber TB, Gloy J, Henger A, et al.: Catecholamines modulate podocyte function. J Am Soc Nephrol 1998, 9:335–345.
Pavenstadt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 2003, 83:253–307.
Durvasula RV, Shankland SJ: The renin-angiotensin system in glomerular podocytes: mediator of glomerulosclerosis and link to hypertensive nephropathy. Curr Hypertens Rep 2006, 8:132–138.
Wright JT Jr, Bakris G, Greene T, et al.: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 2002, 288:2421–2431.
Gloy J, Henger A, Fischer KG, et al.: Angiotensin II depolarizes podocytes in the intact glomerulus of the rat. J Clin Invest 1997, 99:2772–2781.
Henger A, Huber T, Fischer KG, et al.: Angiotensin II increases the cytosolic calcium activity in rat podocytes in culture. Kidney Int 1997, 52:687–693.
Liebau MC, Lang D, Bohm J, et al.: Functional expression of the renin-angiotensin system in human podocytes. Am J Physiol Renal Physiol 2006, 290:F710–719.
Clapham DE: TRP channels as cellular sensors. Nature 2003, 426:517–524.
Möller CC, Wei C, Altintas MM, et al.: Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 2007, 18:29–36.
Goel M, Sinkins W, Keightley A, et al.: Proteomic analysis of TRPC5-and TRPC6-binding partners reveals interaction with the plasmalemmal Na(+)/K(+)-ATPase. Pflugers Arch 2005, 451:87–98.
Reiser J, Polu KR, Möller CC, et al.: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 2005, 37:739–744.
Huber TB, Schermer B, Muller RU, et al.: Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A 2006, 103:17079–17086.
Huber TB, Schermer B, Benzing T: Podocin organizes ion channel-lipid supercomplexes: implications for mechanosensation at the slit diaphragm. Nephron Exp Nephrol 2007, 106:e27–31.
Spassova MA, Hewavitharana T, Xu W, et al.: A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci U S A 2006, 103:16586–16591.
Kuwahara K, Wang Y, McAnally J, et al.: TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 2006, 116:3114–3126.
Winn MP, Conlon PJ, Lynn KL, et al.: A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 2005, 308:1801–1804.
Hoffmann S, Podlich D, Hahnel B, et al.: Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 2004, 15:1475–1487.
Chamorro V, Wangensteen R, Sainz J, et al.: Protective effects of the angiotensin II type 1 (AT1) receptor blockade in low-renin deoxycorticosterone acetate (DOCA)-treated spontaneously hypertensive rats. Clin Sci (Lond) 2004, 106:251–259.
Wolf G, Chen S, Ziyadeh FN: From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 2005, 54:1626–1634.
Bonnet F, Cooper ME, Kawachi H, et al.: Irbesartan normalises the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia 2001, 44:874–877.
Benigni A, Tomasoni S, Gagliardini E, et al.: Blocking angiotensin II synthesis/activity preserves glomerular nephrin in rats with severe nephrosis. J Am Soc Nephrol 2001, 12:941–948.
Macconi D, Ghilardi M, Bonassi ME, et al.: Effect of angiotensin-converting enzyme inhibition on glomerular basement membrane permeability and distribution of zonula occludens-1 in MWF rats. J Am Soc Nephrol 2000, 11:477–489.
Wapstra FH, Navis G, de Jong PE, de Zeeuw D: Chronic angiotensin II infusion but not bradykinin blockade abolishes the antiproteinuric response to angiotensin-converting enzyme inhibition in established adriamycin nephrosis. J Am Soc Nephrol 2000, 11:490–496.
Lenz O, Elliot SJ, Stetler-Stevenson WG: Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol 2000, 11:574–581.
Morano S, Cipriani R, Cerrito MG, et al.: Angiotensin-converting enzyme inhibition modulates high-glucose-induced extracellular matrix changes in mouse glomerular epithelial cells. Nephron Exp Nephrol 2003, 95:e30–35.
Ruiz-Ortega M, Ruperez M, Lorenzo O, et al.: Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 2002:12–22.
Blanco S, Vaquero M, Gomez-Guerrero C, et al.: Potential role of angiotensin-converting enzyme inhibitors and statins on early podocyte damage in a model of type 2 diabetes mellitus, obesity, and mild hypertension. Am J Hypertens 2005, 18(4 Pt 1):557–565.
Griendling KK, Ushio-Fukai M: Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept 2000, 91:21–27.
Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW: Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994, 74:1141–1148.
Laursen JB, Rajagopalan S, Galis Z, et al.: Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation 1997, 95:588–593.
Neale TJ, Ullrich R, Ojha P, et al.: Reactive oxygen species and neutrophil respiratory burst cytochrome b558 are produced by kidney glomerular cells in passive Heymann nephritis. Proc Natl Acad Sci U S A 1993, 90:3645–3649.
Gwinner W, Plasger J, Brandes RP, et al.: Role of xanthine oxidase in passive Heymann nephritis in rats. J Am Soc Nephrol 1999, 10:538–544.
Rohrmoser MM, Mayer G: Reactive oxygen species and glomerular injury. Kidney Blood Press Res 1996, 19:263–269.
Zalba G, Fortuno A, Diez J: Oxidative stress and atherosclerosis in early chronic kidney disease. Nephrol Dial Transplant 2006, 21:2686–2690.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reiser, J., Mundel, P. Dual effects of RAS blockade on blood pressure and podocyte function. Current Science Inc 9, 403–408 (2007). https://doi.org/10.1007/s11906-007-0074-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11906-007-0074-7