Abstract
Purpose of Review
In the gastro-intestinal tract, the complex network of multiple innate cell populations play critical roles not only as a first line of defense against invading pathogens and in driving adaptive immune responses but also in maintaining intestinal homeostasis. Here, we describe the roles of various innate immune cell populations in gut immunity and detail studies investigating the impact of acute and chronic HIV infection on these cell populations.
Recent Findings
Alterations in frequencies, phenotype and/or function of innate lymphoid cells, dendritic cells, macrophages, neutrophils, and innate-like T cells have been reported in people with HIV (PWH), with many of these features persisting despite anti-retroviral therapy and virological suppression.
Summary
Dysregulated gut innate immunity in PWH is a feature of gut pathogenesis. A greater understanding of the mechanisms driving impairment in the multiple different gut innate immune cell populations and the downstream consequences of an altered innate immune response on host defense and gut homeostasis in PWH is needed to develop more effective HIV treatments and cure strategies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Gasteiger G, D’Osualdo A, Schubert DA, Weber A, Bruscia EM, Hartl D. Cellular innate immunity: an old game with new players. J Innate Immun. 2017;9:111–25.
Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–53.
Bennett MS, Round JL, Leung DT. Innate-like lymphocytes in intestinal infections. Curr Opin Infect Dis. 2015;28:457–63.
Chou C, Li MO. Tissue-resident lymphocytes across innate and adaptive lineages. Front Immunol. 2018;9:2104.
Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506.
Castellanos JG, Longman RS. The balance of power: innate lymphoid cells in tissue inflammation and repair. J Clin Invest. 2019;129:2640–50.
Herbert DR, Douglas B, Zullo K. Group 2 innate lymphoid cells (ILC2): type 2 immunity and helminth immunity. Int J Mol Sci. 2019;20:2276.
Pantazi E, Powell N. Group 3 ILCs: peacekeepers or troublemakers? What's your gut telling you?! Front Immunol. 2019;10:676.
Poggi A, Benelli R, Vene R, Costa D, Ferrari N, Tosetti F, et al. Human gut-associated natural killer cells in health and disease. Front Immunol. 2019;10:961.
Seo GY, Giles DA, Kronenberg M. The role of innate lymphoid cells in response to microbes at mucosal surfaces. Mucosal Immunol. 2020;13:399–412.
Colonna M. Innate lymphoid cells: diversity, plasticity, and unique functions in immunity. Immunity. 2018;48:1104–17.
Mela CM, Steel A, Lindsay J, Gazzard BG, Gotch FM, Goodier MR. Depletion of natural killer cells in the colonic lamina propria of viraemic HIV-1-infected individuals. AIDS. 2007;21:2177–82.
Sips M, Sciaranghella G, Diefenbach T, Dugast AS, Berger CT, Liu Q, et al. Altered distribution of mucosal NK cells during HIV infection. Mucosal Immunol. 2012;5:30–40.
Taborda NA, Gonzalez SM, Alvarez CM, Correa LA, Montoya CJ, Rugeles MT. Higher frequency of NK and CD4+ T-cells in mucosa and potent cytotoxic response in HIV controllers. PLoS One. 2015;10:e0136292.
Taborda NA, Gonzalez SM, Correa LA, Montoya CJ, Rugeles MT. Spontaneous HIV controllers exhibit preserved immune parameters in peripheral blood and gastrointestinal mucosa. J Acquir Immune Defic Syndr. 2015;70:115–21.
Kramer B, Goeser F, Lutz P, Glassner A, Boesecke C, Schwarze-Zander C, et al. Compartment-specific distribution of human intestinal innate lymphoid cells is altered in HIV patients under effective therapy. PLoS Pathog. 2017;13:e1006373.
Wang Y, Lifshitz L, Gellatly K, Vinton CL, Busman-Sahay K, McCauley S, et al. HIV-1-induced cytokines deplete homeostatic innate lymphoid cells and expand TCF7-dependent memory NK cells. Nat Immunol. 2020;21:274–86 This study elegantly demonstrates that depletion of gut ILC3s persists in people with HIV (PWH) despite effective virologic suppression by anti-retroviral therapy and lower frequencies of gut ILC3s are associated with neutrophil accumulation and with Type I Interferons.
Utay NS, Vigil KJ, Somasunderam A, Aulicino PC, Smulevitz B, Chiadika S, et al. Timing of antiretroviral therapy initiation determines rectal natural killer cell populations. AIDS Res Hum Retroviruses. 2020;36:314–23.
Zhao J, Cheng L, Wang H, Yu H, Tu B, Fu Q, et al. Infection and depletion of CD4+ group-1 innate lymphoid cells by HIV-1 via type-I interferon pathway. PLoS Pathog. 2018;14:e1006819.
Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–5.
Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, Huibner S, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012;5:670–80.
Zhang Z, Cheng L, Zhao J, Li G, Zhang L, Chen W, et al. Plasmacytoid dendritic cells promote HIV-1-induced group 3 innate lymphoid cell depletion. J Clin Invest. 2015;125:3692–703.
Fernandes SM, Pires AR, Ferreira C, Foxall RB, Rino J, Santos C, et al. Enteric mucosa integrity in the presence of a preserved innate interleukin 22 compartment in HIV type 1-treated individuals. J Infect Dis. 2014;210:630–40.
Dillon SM, Castleman MJ, Frank DN, Austin GL, Gianella S, Cogswell AC, et al. Brief Report: Inflammatory colonic innate lymphoid cells are increased during untreated HIV-1 infection and associated with markers of gut dysbiosis and mucosal immune activation. J Acquir Immune Defic Syndr. 2017;76:431–7.
Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983–94.
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9.
Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–66.
Kloverpris HN, Kazer SW, Mjosberg J, Mabuka JM, Wellmann A, Ndhlovu Z, et al. Innate lymphoid cells are depleted irreversibly during acute HIV-1 infection in the absence of viral suppression. Immunity. 2016;44:391–405.
Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571–8.
Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356:283.
Bain CC, Schridde A. Origin, differentiation, and function of intestinal macrophages. Front Immunol. 2018;9:2733.
Muller PA, Matheis F, Mucida D. Gut macrophages: key players in intestinal immunity and tissue physiology. Curr Opin Immunol. 2020;62:54–61.
Sun T, Nguyen A, Gommerman JL. Dendritic cell subsets in intestinal immunity and inflammation. J Immunol. 2020;204:1075–83.
Viola MF, Boeckxstaens G. Intestinal resident macrophages: multitaskers of the gut. Neurogastroenterol Motil. 2020;32:e13843.
Castleman MJ, Dillon SM, Purba CM, Cogswell AC, Kibbie JJ, McCarter MD, et al. Commensal and pathogenic bacteria indirectly induce IL-22 but not IFNgamma production from human colonic ILC3s via multiple mechanisms. Front Immunol. 2019;10:649.
Castleman MJ, Dillon SM, Purba C, Cogswell AC, McCarter M, Barker E, et al. Enteric bacteria induce IFN gamma and granzyme B from human colonic group 1 innate lymphoid cells. Gut Microbes. 2020;e1667723:12.
Dillon SM, Manuzak JA, Leone AK, Lee EJ, Rogers LM, McCarter MD, et al. HIV-1 infection of human intestinal lamina propria CD4+ T cells in vitro is enhanced by exposure to commensal Escherichia coli. J Immunol. 2012;189:885–96.
Dillon SM, Lee EJ, Kotter CV, Austin GL, Gianella S, Siewe B, et al. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T cell activation in untreated HIV-1 infection. Mucosal Immunol. 2016;9:24–37.
Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32–6.
Murray SM, Zhang Y, Douek DC, Sekaly RP. Myeloid cells enriched for a dendritic cell population from people living with HIV have altered gene expression not restored by antiretroviral therapy. Front Immunol. 2020;11:261.
Allers K, Fehr M, Conrad K, Epple HJ, Schurmann D, Geelhaar-Karsch A, et al. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J Infect Dis. 2014;209:739–48.
Cassol E, Rossouw T, Malfeld S, Mahasha P, Slavik T, Seebregts C, et al. CD14(+) macrophages that accumulate in the colon of African AIDS patients express pro-inflammatory cytokines and are responsive to lipopolysaccharide. BMC Infect Dis. 2015;15:430.
Bracq L, Xie M, Benichou S, Bouchet J. Mechanisms for cell-to-cell transmission of HIV-1. Front Immunol. 2018;9:260.
Coleman CM, Gelais CS, Wu L. Cellular and viral mechanisms of HIV-1 transmission mediated by dendritic cells. Adv Exp Med Biol. 2013;762:109–30.
Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49.
Akiyama H, Ramirez NP, Gibson G, Kline C, Watkins S, Ambrose Z, et al. Interferon-inducible CD169/Siglec1 attenuates anti-HIV-1 Effects of alpha interferon. J Virol. 2017;91:e00972–17.
Izquierdo-Useros N, Lorizate M, Puertas MC, Rodriguez-Plata MT, Zangger N, Erikson E, et al. Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides. PLoS Biol. 2012;e1001448:10.
Jobe O, Trinh HV, Kim J, Alsalmi W, Tovanabutra S, Ehrenberg PK, et al. Effect of cytokines on Siglec-1 and HIV-1 entry in monocyte-derived macrophages: the importance of HIV-1 envelope V1V2 region. J Leukoc Biol. 2016;99:1089–106.
Pino M, Erkizia I, Benet S, Erikson E, Fernandez-Figueras MT, Guerrero D, et al. HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology. 2015;12:37.
Puryear WB, Akiyama H, Geer SD, Ramirez NP, Yu X, Reinhard BM, et al. Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 2013;9:e1003291.
Puryear WB, Yu X, Ramirez NP, Reinhard BM, Gummuluru S. HIV-1 incorporation of host-cell-derived glycosphingolipid GM3 allows for capture by mature dendritic cells. Proc Natl Acad Sci U S A. 2012;109:7475–80.
Rempel H, Calosing C, Sun B, Pulliam L. Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS One. 2008;3:e1967.
Akiyama H, Ramirez NG, Gudheti MV, Gummuluru S. CD169-mediated trafficking of HIV to plasma membrane invaginations in dendritic cells attenuates efficacy of anti-gp120 broadly neutralizing antibodies. PLoS Pathog. 2015;11:e1004751.
Hammonds JE, Beeman N, Ding L, Takushi S, Francis AC, Wang JJ, et al. Siglec-1 initiates formation of the virus-containing compartment and enhances macrophage-to-T cell transmission of HIV-1. PLoS Pathog. 2017;13:e1006181.
Sewald X, Ladinsky MS, Uchil PD, Beloor J, Pi R, Herrmann C, et al. Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science. 2015;350:563–7.
Perez-Zsolt D, Cantero-Perez J, Erkizia I, Benet S, Pino M, Serra-Peinado C, et al. Dendritic cells from the cervical mucosa capture and transfer HIV-1 via Siglec-1. Front Immunol. 2019;10:825.
Dillon SM, Guo K, Austin GL, Gianella S, Engen PA, Mutlu EA, et al. A compartmentalized type I interferon response in the gut during chronic HIV-1 infection is associated with immunopathogenesis. AIDS. 2018;32:1599–611.
Dillon SM, Guo K, Castleman MJ, Santiago ML, Wilson CC. Quantifying HIV-1-mediated gut CD4+ T cell death in the Lamina Propria Aggregate Culture (LPAC) Model. Bio-protocol. 2020;10:e3486.
Bujko A, Atlasy N, Landsverk OJB, Richter L, Yaqub S, Horneland R, et al. Transcriptional and functional profiling defines human small intestinal macrophage subsets. J Exp Med. 2018;215:441–58.
Mann ER, Bernardo D, English NR, Landy J, Al-Hassi HO, Peake ST, et al. Compartment-specific immunity in the human gut: properties and functions of dendritic cells in the colon versus the ileum. Gut. 2016;65:256–70.
Dillon SM, Lee EJ, Donovan AM, Guo K, Harper MS, Frank DN, et al. Enhancement of HIV-1 infection and intestinal CD4+ T cell depletion ex vivo by gut microbes altered during chronic HIV-1 infection. Retrovirology. 2016;13:5.
Scagnolari C, Antonelli G. Type I interferon and HIV: subtle balance between antiviral activity, immunopathogenesis and the microbiome. Cytokine Growth Factor Rev. 2018;40:19–31.
Lombardi VC, Khaiboullina SF. Plasmacytoid dendritic cells of the gut: relevance to immunity and pathology. Clin Immunol. 2014;153:165–77.
Won HY, Lee JY, Ryu D, Kim HT, Chang SY. The role of plasmacytoid dendritic cells in gut health. Immune Netw. 2019;19:e6.
Boichuk SV, Khaiboullina SF, Ramazanov BR, Khasanova GR, Ivanovskaya KA, Nizamutdinov EZ, et al. Gut-associated plasmacytoid dendritic cells display an immature phenotype and upregulated granzyme B in subjects with HIV/AIDS. Front Immunol. 2015;6:485.
Lehmann C, Jung N, Forster K, Koch N, Leifeld L, Fischer J, et al. Longitudinal analysis of distribution and function of plasmacytoid dendritic cells in peripheral blood and gut mucosa of HIV infected patients. J Infect Dis. 2014;209:940–9.
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89.
Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–66.
Deleage C, Schuetz A, Alvord WG, Johnston L, Hao XP, Morcock DR, et al. Impact of early cART in the gut during acute HIV infection. JCI Insight. 2016;e87065:1.
Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, et al. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS. 2015;29:43–51.
Hensley-McBain T, Wu MC, Manuzak JA, Cheu RK, Gustin A, Driscoll CB, et al. Increased mucosal neutrophil survival is associated with altered microbiota in HIV infection. PLoS Pathog. 2019;15:e1007672 This study highlights that the accumulation of neutrophils in the gut of chronically infected, anti-retroviral therapy-treated people with HIV (PWH) is linked to alterations in the enteric microbiome.
Ioannidis M, Cerundolo V, Salio M. The immune modulating properties of mucosal-associated invariant T cells. Front Immunol. 2020;11:1556.
Leeansyah E, Ganesh A, Quigley MF, Sonnerborg A, Andersson J, Hunt PW, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood. 2013;121:1124–35.
Greathead L, Metcalf R, Gazzard B, Gotch F, Steel A, Kelleher P. CD8+/CD161++ mucosal-associated invariant T-cell levels in the colon are restored on long-term antiretroviral therapy and correlate with CD8+ T-cell immune activation. AIDS. 2014;28:1690–2.
Cosgrove C, Ussher JE, Rauch A, Gartner K, Kurioka A, Huhn MH, et al. Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood. 2013;121:951–61.
Kok A, Hocqueloux L, Hocini H, Carriere M, Lefrou L, Guguin A, et al. Early initiation of combined antiretroviral therapy preserves immune function in the gut of HIV-infected patients. Mucosal Immunol. 2015;8:127–40.
Lawand M, Dechanet-Merville J, Dieu-Nosjean MC. Key features of gamma-delta T-cell subsets in human diseases and their immunotherapeutic implications. Front Immunol. 2017;8:761.
McCarthy NE, Eberl M. Human gammadelta T-cell control of mucosal immunity and inflammation. Front Immunol. 2018;9:985.
Nilssen DE, Brandtzaeg P. Intraepithelial gammadelta T cells remain increased in the duodenum of AIDS patients despite antiretroviral treatment. PLoS One. 2012;7:e29066.
Nilssen DE, Muller F, Oktedalen O, Froland SS, Fausa O, Halstensen TS, et al. Intraepithelial gamma/delta T cells in duodenal mucosa are related to the immune state and survival time in AIDS. J Virol. 1996;70:3545–50.
Poles MA, Barsoum S, Yu W, Yu J, Sun P, Daly J, et al. Human immunodeficiency virus type 1 induces persistent changes in mucosal and blood gammadelta T cells despite suppressive therapy. J Virol. 2003;77:10456–67.
Cimini E, Agrati C, D’Offizi G, Vlassi C, Casetti R, Sacchi A, et al. Primary and chronic HIV infection differently modulates mucosal Vdelta1 and Vdelta2 T-cells differentiation profile and effector functions. PLoS One. 2015;e0129771:10.
Olson GS, Moore SW, Richter JM, Garber JJ, Bowman BA, Rawlings CA, et al. Increased frequency of systemic pro-inflammatory Vdelta1(+) gammadelta T cells in HIV elite controllers correlates with gut viral load. Sci Rep. 2018;8:16471.
Kinjo Y, Kitano N, Kronenberg M. The role of invariant natural killer T cells in microbial immunity. J Infect Chemother. 2013;19:560–70.
Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, et al. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology. 2007;122:1–14.
Zhou L, Adrianto I, Wang J, Wu X, Datta I, Mi QS. Single-cell RNA-Seq analysis uncovers distinct functional human NKT cell sub-populations in peripheral blood. Front Cell Dev Biol. 2020;8:384.
Ibarrondo FJ, Wilson SB, Hultin LE, Shih R, Hausner MA, Hultin PM, et al. Preferential depletion of gut CD4-expressing iNKT cells contributes to systemic immune activation in HIV-1 infection. Mucosal Immunol. 2013;6:591–600.
Paquin-Proulx D, Ching C, Vujkovic-Cvijin I, Fadrosh D, Loh L, Huang Y, et al. Bacteroides are associated with GALT iNKT cell function and reduction of microbial translocation in HIV-1 infection. Mucosal Immunol. 2017;10:69–78.
Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32:513–45.
Guo K, Shen G, Kibbie J, Gonzalez T, Dillon SM, Smith HA, et al. Qualitative differences between the IFNalpha subtypes and IFNbeta influence chronic mucosal HIV-1 pathogenesis. PLoS Pathog. 2020;e1008986:16.
Hughes SM, Levy CN, Calienes FL, Stekler JD, Pandey U, Vojtech L, et al. Treatment with commonly used antiretroviral drugs induces a type I/III interferon signature in the gut in the absence of HIV infection. Cell Rep Med. 2020;1:100096.
Yoder AC, Guo K, Dillon SM, Phang T, Lee EJ, Harper MS, et al. The transcriptome of HIV-1 infected intestinal CD4+ T cells exposed to enteric bacteria. PLoS Pathog. 2017;13:e1006226.
Pinacchio C, Scagnolari C, Iebba V, Santinelli L, Innocenti GP, Frasca F, et al. High abundance of genus Prevotella is associated with dysregulation of IFN-I and T cell response in HIV-1-infected patients. AIDS. 2020;34:1467–73.
Pinacchio C, Scheri GC, Statzu M, Santinelli L, Ceccarelli G, Innocenti GP, et al. Type I/II Interferon in HIV-1-infected patients: expression in gut mucosa and in peripheral blood mononuclear cells and its modification upon probiotic supplementation. J Immunol Res. 2018;1738676:2018.
Harper MS, Guo K, Gibbert K, Lee EJ, Dillon SM, Barrett BS, et al. Interferon-alpha subtypes in an ex vivo model of acute HIV-1 infection: expression, potency and effector mechanisms. PLoS Pathog. 2015;e1005254:11.
Chaillon A, Gianella S, Dellicour S, Rawlings SA, Schlub TE, De Oliveira MF, et al. HIV persists throughout deep tissues with repopulation from multiple anatomical sources. J Clin Invest. 2020;130:1699–712.
Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis. 2008;197:714–20.
Ward AR, Mota TM, Jones RB. Immunological approaches to HIV cure. Semin Immunol. 2020. https://doi.org/10.1016/j.smim.2020.101412.
Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202:1553–61.
Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med. 2017;23:1271–6.
Olesen R, Vigano S, Rasmussen TA, Sogaard OS, Ouyang Z, Buzon M, et al. Innate immune activity correlates with CD4 T cell-associated HIV-1 DNA decline during latency-reversing treatment with panobinostat. J Virol. 2015;89:10176–89.
Van der Sluis RM, Zerbato JM, Rhodes JW, Pascoe RD, Solomon A, Kumar NA, et al. Diverse effects of interferon alpha on the establishment and reversal of HIV latency. PLoS Pathog. 2020;e1008151:16.
Wong ME, Jaworowski A, Hearps AC. The HIV reservoir in monocytes and macrophages. Front Immunol. 2019;10:1435.
Zalar A, Figueroa MI, Ruibal-Ares B, Bare P, Cahn P, de Bracco MM, et al. Macrophage HIV-1 infection in duodenal tissue of patients on long term HAART. Antiviral Res. 2010;87:269–71.
Yukl SA, Sinclair E, Somsouk M, Hunt PW, Epling L, Killian M, et al. A comparison of methods for measuring rectal HIV levels suggests that HIV DNA resides in cells other than CD4+ T cells, including myeloid cells. AIDS. 2014;28:439–42.
Cattin A, Wiche Salinas TR, Gosselin A, Planas D, Shacklett B, Cohen EA, et al. HIV-1 is rarely detected in blood and colon myeloid cells during viral-suppressive antiretroviral therapy. AIDS. 2019;33:1293–306.
Calantone N, Wu F, Klase Z, Deleage C, Perkins M, Matsuda K, et al. Tissue myeloid cells in SIV-infected primates acquire viral DNA through phagocytosis of infected T cells. Immunity. 2014;41:493–502.
Acknowledgements
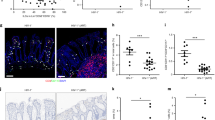
We would like to acknowledge and give our sincere thanks to all the study participants who generously contributed their time and biological samples to the many clinical studies detailed in this review. We would also like to acknowledge and thank Steven Lada for his assistance with the in vitro studies detailed in Fig. 1.
Funding
S.M.D. and C.C.W. are currently supported by funding from NIH (R01AI118983; R01AI134220; R21AG062932). Previously unpublished studies included in this review were supported by R01AI134220. Published studies conducted by Drs. Dillon and Wilson mentioned in this review were supported by R01DK088663, R01AI118983, and R01AI108404.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors that were previously published complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on HIV Pathogenesis and Treatment
Rights and permissions
About this article
Cite this article
Dillon, S.M., Wilson, C.C. Gut Innate Immunity and HIV Pathogenesis. Curr HIV/AIDS Rep 18, 128–138 (2021). https://doi.org/10.1007/s11904-021-00544-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-021-00544-3