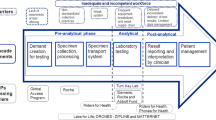
Abstract
Laboratory innovation significantly affects program sustainability of HIV programs in low and middle income countries (LMICs) far beyond its immediate sphere of impact. Innovation in rapid development of diagnostic technologies, improved quality management systems, strengthened laboratory management, affordable external quality assurance and accreditation schemes, and building local capacity have reduced costs, brought quality improvement to point-of-care testing, increased access to testing services, reduced treatment and prevention costs and opened the door to the real possibility of ending the AIDS epidemic. However, for effectively implemented laboratory innovation to contribute to HIV quality program sustainability, it must be implemented within the overall context of the national strategic plan and HIV treatment programs. The high quality of HIV rapid diagnostic test was a breakthrough that made it possible for more persons to learn their HIV status, receive counseling, and if infected to receive treatment. Likewise, the use of dried blood spots made the shipment of samples easier for the assessment of different variables of HIV infection—molecular diagnosis, CD4+ cell counts, HIV antibodies, drug resistance surveillance, and even antiretroviral drug level measurements. Such advancement is critical for to reaching the UNAIDS target of 90-90-90 and for bringing the AIDS epidemic to an end, especially in LMICs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At the World Health Organization (WHO) Regional Office for Africa meeting in Johannesburg, September 2003 [1], there was a roundtable convened to discuss laboratory services in the provision of health care. Forty-eight country representatives participated and at the start of the meeting noted “the primary role of laboratories in health care ……and the disastrous situation in Our Region.” This was neither the first nor the last time such declarations were made regarding laboratory services. For the most part, the chronic weaknesses in laboratory systems in Africa did not change for the better until the mounting crisis of the HIV/AIDS pandemic drew international resources to accompany concern and begin to address the issues of access and quality of laboratory services [2].
In the past three decades, however, measurable progress has been made in quality and capacity of laboratory services and this transformation has been driven to a considerable extent by innovation as well as increased funding [3]. Innovations in diagnostic technology [4], laboratory systems and management and policy have affected diagnostic services, quality assurance, laboratory management, policy development, partnerships, and standards and as a result built infrastructure that underpins sustainability in HIV programs [5••, 6, 7].
Laboratory innovation was championed and influenced HIV care programs through the efforts of the local Ministries of Health and their many players and partners including international agencies , government partners , academically affiliated partners, non-governmental organizations (NGOs), foundations and many other governments, agencies and nations. This has been largely supported by the PEPFAR and Global Fund in low and middle income countries (LMICs) [3].
Innovation in HIV Serology
HIV diagnostics have seen major advances in technology since their introduction as an FDA licensed HIV immunoassay test in 1985 [8]. In particular, numerous technology innovations in rapid diagnostic test (RDT) assays that do not require laboratory equipment to perform reliable tests have been introduced and changed the landscape of diagnostic testing. RDT kits with simple to perform procedures enabled testing outside standard laboratory facilities [9, 10], provided test results while patients were still at the health clinic, and increased access to testing [11]. Increased access with RDTs available in rural settings have made it possible for more persons to learn their HIV status, receive counseling and if infected to receive treatment. It was a breakthrough in the efforts to slow the HIV epidemic and stimulated efforts to develop strategies to identify and provide treatment for HIV-infected persons [12].
Despite the highly accurate test results that can be obtained with RDTs, however, there are challenges to obtaining correct results outside of the traditional laboratory setting and instances of worrisome false positive and false negative test results have been documented. In addition to issues of reliability and accuracy of testing, ethical issues must be addressed [13]. There is expected variation in the performance of testing among non-laboratory test sites just as there are among laboratory sites. However, the challenges of assuring quality testing at non-laboratory settings may be greater as the same good laboratory practices are required in non-laboratory settings to assure quality testing, but training of testers is often insufficient [14, 15•, 16–18].
Use of RDTs requires a national validated testing algorithm to assure accurate test results. In many countries, the recommended WHO testing strategy for HIV testing has not been implemented. A recent survey reported in December 2015 [19••] found that 63 % of national policies worldwide and 72 % in the African Region were not aligned to WHO recommended strategies. Compliance with WHO recommendations has been challenging for LMIC for known reasons, e.g., insufficient funding; lack of trained human resources, and weak laboratory infrastructure. An earlier example of this chronic situation was described in a November 2009 article by Aghokeng et al. [20] who reported on the test results of five rapid HIV tests, three of which were in current use as first-line tests in Cameroon. None of the three assays used in the national algorithm had been evaluated prior to use against local serum specimens as recommended by WHO. One analysis of the study data estimated that up to 6 % of HIV-1 M and up to 80 % of HIV-1 O-infected persons in Cameroon who were tested in this time period would not be detected as infected and therefore not enrolled in treatment due to low test sensitivity. In like manner, low test specificity was estimated to result in up Consolidated Guidelines on HIV Testing Services [21]. This guideline as well as the recently published handbook, Improving the Quality of HIV-Related Point-of-Care Testing [22], are important documents developed at WHO through collaboration with numerous experts with extensive field experience and provide a blueprint for achieving quality testing. Just as important is the leadership and advocacy of national and international professionals in the public and private sectors to energize action and commitment from governments, foundations and the full range of NGOs to support sustainable laboratory capacity.
A recent publication by Bhanu Mehra et al. [23] underscores the continuing challenge and need for standardized training not only for HIV RDT testers but also for staff supervisors in non-laboratory settings, certification of staffs and sites, and regular external quality assurance (EQA). They reported results of comparison of the HIV RDT screening test approved in the India algorithm for diagnosis with the standard HIV ELISA assay. Specimens from 787 consecutive patients at a voluntary counseling and testing (VCT) facility were tested by both RDT and ELISA with Western blot confirmation. There were nine false negative and five false positive test results using the RDT assay compared to ELISA and these discrepancies were confirmed by Western blot. Serial testing using a validated algorithm developed with specimens from the population of interest can minimize false positive and false negative results but the errors reported here and in other studies underscore the need for well established, comprehensive and funded quality assurance programs.
A key and efficient means to establish a sustainable quality assurance program is described in Scaling Up HIV Rapid Testing in Developing Countries: Comprehensive Approach for Implementing Quality Assurance by Parekh et al [10]. This article addresses the full range of key components of a quality assurance program including quality of test kits, selection of test kits, and development of test algorithms, training and certification of testers and sites, and post market evaluation. Two very innovative tools are provided for aiding quality assurance sustainability and effectiveness. First is the detailed description of how to use a standardized log book as a QA tool and the second is the introduction of a dried tube specimen (DTS) for use in proficiency testing programs. The use of the log book is not only an effective means to monitor the quality of testing, but it provides opportunity for meaningful input and review by supervisory staff thereby adding aspects of support through coaching and mentoring to the testers. The development of the DTS for proficiency testing (PT) provides an affordable option for LMIC to implement and have direct control over their HIV PT.
Innovation in HIV CD4 Technologies
Similar to the advances in diagnostics, for HIV immunology was the rapid technology development for measurement of CD4+ T cells using point-of-care testing methods. This technology brought gains in timeliness to identification of HIV-infected persons who were eligible for ART and retention in treatment programs prior to initiation of treatment [24]. Based on scientific data indicating a clinical and public health benefit of earlier treatment the WHO 2013 guidelines raised the threshold for ART initiation to CD4 count of ≤500 cells/μL, with priority given to those with a CD4 count of ≤350 cells/μL. The 2015 early release guideline even goes further to recommend ART initiation to all HIV-infected persons as soon as possible irrespective of the CD4 count. Although more recent evidence indicates CD4 monitoring is no longer required for virologically stable persons on ART and viral load testing has become technically and more affordable for use in LMICs, innovation in CD4 testing diagnostics has remained a key factor in achieving placement of HIV-infected persons on ART thereby improving individual outcomes and preventing HIV spread. As progress was made in treatment and prevention of HIV, clinical monitoring guidelines evolved from use of clinical symptoms and CD4 to now use of viral load. Nevertheless in LMIC where there is still limited ARVs for infected persons, CD4 estimations will continue to play some role not only in monitoring the immunological improvement of patients on treatment but in prioritizing who gets the limited AIDS drugs first. Several studies show comparable performance to the gold standard BD FASCount® of an impressive list of CD4 instruments that flooded the market in the last 5 years including the top technologies: Partec CyFlow®, Alere Pima® analyzer, PointCare Now®, and Guava PCA® [25–28] which has enabled the significant progress in effective treatment and prevention especially in rural areas.
It should be noted that there still remains significant physiological variability in CD4 count that may account for variability of CD4 measurement among the different CD4 platforms. In addition, an excellent review of CD4 enumeration technologies by Peeling et al. [29] clearly articulated the issue of bias that must be considered when these new technologies are validated against reference instruments of BD FASCount® and the FACSCalibur®. At different CD4 count levels, these new technologies could over- or underestimate the CD4 count. This would influence initiation of therapy where some patients would be initiated early and others late. However, trends of CD4 counts over a period of time using the same CD4 platform even with over or under estimation is useful in monitoring immunological recovery during treatment rather than CD4 count at one time point.
Innovation in HIV Viral Detection
Assessment of viral load is one of the best predictors of clinical progression, as well as the main parameter to assess treatment response in HIV-positive patients. It is effective in detecting treatment failure and avoiding the accumulation of mutations that would eventually lead to drug resistance and the need for an early switch to second-line AIDS drugs [30]. Over the last 10 years, the landscape of care and treatment of HIV-infected individuals has been very positive in Sub-Saharan Africa with the increased availability of generic AIDS drugs and rapid scale up of treatment programs. In parallel has been the development of sensitive assays based on real-time PCR technology, reliably quantifying HIV-RNA in circulation using real-time reverse transcriptase–polymerase chain reaction (RT-PCR) [31, 32••]. Although commercial HIV viral load assays differ in terms of principle, they have the following in common: fast, highly sensitive, and reproducible for all the major HIV-1 subtypes and high throughputs with fully automated extraction procedures. However, these are still a challenge to operate in LMIC except in highly developed laboratories with skilled staff.
Recent advances in simplifying viral load estimations have occurred on two fronts: the use of dried blood spots (DBS) to simplify the collection and shipment of blood samples from rural sites to reference laboratories; and second, the development of point-of-care viral load machines as possible alternatives for viral load estimates in LMIC. Several studies have demonstrated that DBS are convenient for viral load measurements using existing viral load platforms from the major companies: COBAS AmpliPrep/COBAS TaqMan® v2.0 (Roche Molecular Systems), Real-Time HIV-1® (Abbott), VERSANT HIV-1 RNA 1.0® assay (kPCR) (Siemens), and Nuclisense real-time NASBA Amplification® (BioMerieux) [33]. The jury is still out in terms of the sensitivity range and correlation with plasma values, and in the detection of the accumulation of resistant forms where the viral load may not be high [34]. The multi-partner WHO Technical and Operational Considerations for Implementing Viral Load Testing report and recommendations [35] should help in standardizing specimen collection, transportation and testing, turnaround time, and quality assurance processes for the use of DBS. This is an important and critical step of its wide use.
Even with the possibility of using DBS, factors limiting access to routine viral load still remains due to the limited number of laboratories with the sophistication to support these automated high throughput machines, the relatively high cost of viral load tests, and reduced sensitivity of using DBS. As a result, there has been increased effort in the development of viral load point-of-care test machines (VL POCT) that have high sensitivity, are simple to operate, are more affordable, not requiring infrastructure investment, reducing, or eliminating the need for sample transportation, and reduced turnaround time with the possibility of same day results for prompt clinical decision-making. Perhaps two of the most advanced technologies that have been launched in recent years are the Alere Q® and SAMBA® but there are very promising VL POCT products in the pipeline. Nevertheless the limitations of VL POCT in terms of reduced throughput and sensitivity may require that each country develops a blueprint of how best to integrate these VL POCT platforms into the national strategic plan and country needs.
Innovation in Accreditation and Quality Assurance
High quality laboratory support is critical for HIV/AIDS treatment, care, and prevention programs as it ensures that clients are accurately diagnosed and treated for HIV and other opportunistic infections such as tuberculosis (TB), and for monitoring the progress of ART, patient adherence, and quality of care. The International Organization for Standards (ISO) is the world’s largest developer of international standards, including the most common standard used by medical laboratories (ISO 15189), as well as the widely used standard for testing and calibration laboratories (ISO 17025), and, the standard for providing general guidelines for proficiency testing (ISO 17043) [36]. Though ISO and other similar forms of accreditation are out of reach for most of the LMICs laboratories due to the complexity of the process and expense, it is important that internationally accepted “best practices” are consistently applied to generate high quality laboratory results to gain the confidence of clinicians and the clients. The more recent WHO-AFRO Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) [37, 38] for laboratories is an attempt to make this process more achievable and affordable to LMIC. SLIPTA has been paired with the CDC developed Strengthening Laboratory Management Toward Accreditation (SLMTA) training tool that uses short courses followed by mentored learning projects to address weaknesses and gaps in laboratory operations. The SLIPTA checklist is used to measure progress in laboratory achievement based on the ISO 15189 requirements for quality and competency. Laboratories are graded in the same 12 quality elements of ISO 15189 of document and records, management review, organization and personnel, client management and customer service, equipment, internal audit, purchasing and inventory, process control and internal and external quality assessment, information management, corrective action, occurrence/incident management and process improvement, and facility and safety. Each section has a total number of points graded on compliance of the laboratory with 275 points total possible. Laboratories receive recognition by award of from one to five stars depending on total points scored [39]. SLMTA and SLPTA are widely accepted by PEPFAR supported countries and implemented by the African Society for Laboratory Medicine (ASLM) [38] to support both public and private laboratories in LMIC. Since the inception of SLIPTA in 2011, it is encouraging that 11 African countries with no accredited laboratory have successfully implemented SLIPTA walking their way to ISO accreditation as shown in Fig. 1. Of the 45 laboratories, 29 (64 %) were at 0–2 stars while 16 (36 %) were already at 3–5 stars following only 1 year of implementing SLIPTA. Tiered laboratory networks with national public health national laboratories must be maintained for the management of quality in Primary Health Centers (PHCs) and by extension POCT test results. A major effort is needed to adopt SLIPTA within national regulatory boards or commissions so that quality improvement efforts are universal and formal accreditation can be achieved by all medical diagnostic laboratories operating within countries. While ISO 15189 accreditation is desirable for national reference laboratories as an independent external validation of laboratory quality, it is neither necessary, affordable or sustainable to attempt to have the majority of laboratories in LMICs seek this form of accreditation.
Progress on SLIPTA by African countries 2011–2013 Legend: In just 2 years, eleven African countries: Cameron, Cote d’Ivoire, Ethiopia, Ghana, Lesotho, Mozambique, Namibia, Nigeria, Rwanda, Tanzania, and Zambia with no ISO-accredited laboratory contributed to 45 laboratories that have progressed from star zero. One laboratory in Nigeria has now attained the highest star score of 5 which is equivalent to ISO 15189. Source of data: ASLM www.aslm.org
Innovation in Policy Development
The Joint United Nations Program on HIV/AIDS (UNAIDS) set ambitious goals to spur action to end the AIDS epidemic (UNAIDS, October 2014), which called for achieving by 2020 that 90 % of all HIV-infected persons would know their HIV status, 90 % of those people who are diagnosed would receive ART and 90 % of all people receiving ART would have viral suppression. Achieving these goals would mean 81 % of people with HIV infection would be receiving ART compared to the 2014 ART coverage of 40 % [40]. Recognizing the critical role that diagnostics serve in effective and sustainable treatment and prevention and the weaknesses in LMICs of diagnostics availability, quality, and affordability, UNAIDS and leading international organizations quickly followed the 90-90-90 target goals with the formation of an active collaboration to develop and drive strategic initiatives to assure that the targets can be met. The Diagnostics Access Initiative [41] partners have agreed to advocate for investing in diagnostic services, facilitate development of accurate forecasting information for the needs and demands for diagnostics, identify new funding options for diagnostics, push for negotiation of optimal pricing for affordability and sustainability in LMICs, and coordinate activities on an ongoing basis with the partners and Ministries of Health.
Under the leadership of the WHO, POCT has been integrated into the Global Health Strategy and attempts are ongoing to standardize their evaluation in the field in a cooperative and transparent way without the inevitable biases associated with evaluations involving manufactures [42••]. Each country is encouraged to conduct their post market evaluations especially of Rapid Test Kits in order to maintain the country’s ownership and oversight. According to the Maputo Declaration on Strengthening of Laboratory Systems, POCT policy needs to be embedded within national strategic laboratory plans [43]. The consolidation and leadership of activities should lie within the Ministries of Health that should form technical task teams to support decision-making as each of these new platforms are validated within the context of the local cultural norms and environs. The composition of such teams should be broad including a cross section of health care providers such as clinicians, laboratory personnel, health economists, procurement, supply and distribution staff, and representative of funders. Strong partnerships with industry would facilitate procurement and maintenance of machines. Ownership of the POCT process, however, needs to extend to users of the assays and the communities that are tested, with creative ways developed for incentivizing healthcare workers conducting the tests to maintain high quality standards; and clear guidelines included in the countries policies.
Conclusion
The remarkable changes brought by innovation in laboratory diagnostics, technology, and policy has changed the landscape of HIV treatment and prevention and made the goal of an end to HIV/AIDS epidemics more than a hope. The availability of laboratory diagnostic testing and point-of-care technologies has brought these essential services to areas which could not be served by standard laboratory networks. The development of high throughput and high complexity instruments coupled with the ease of DBS and electronic information systems have enabled major progress towards eliminating mother to child HIV infections. The design of innovative policy such as the recent UNAIDS 90/90/90 targets has galvanized collaboration as well as innovation to achieve what at first look was an improbable goal but now seems possible. Yet with the substantial resources directed to cutting edge innovation and notable breakthroughs that have fostered progress in HIV treatment and prevention, there remains a lack of appreciation of the need for supporting the core basic elements of laboratory infrastructure that ensure effective implementation and use of these innovations in medical laboratory sciences. These include the following: (1) an adequate laboratory trained workforce, (2) reasonable compensation, (3) facilities that allow for efficient and safe operations, and (4) use of advanced technologies and information systems. Together, these elements enable timely use and analysis of laboratory data and information to inform treatment, prevention, and planning. The sustainability of HIV care and treatment programs depend on this commitment.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Laboratory Services in the Provision of Quality Health Care, Regional Committee for Africa, Fifty-third session, World Health Organization Regional Office for Africa, Johannesburg, South Africa. 2003; 1-5
Global Health Sector Response to HIV, 2000-2015: Focus on Innovations in Africa, 2015
Financing the Response to HIV in Low- and Middle-Income Countries: International Assistance from Donor Governments in 2014, UNAIDS and The Henry J. Kaiser Foundation, July 2015
Biomedical AIDS Research: Recent and Upcoming Advances, Joint United nations Program on HIV/AIDS, 2015
Improving the quality of HIV-related Point-of-care testing: ensuring the reliability and accuracy of test results, WHO, December 2015. This article reviews the context in which the diagnostics must operate, some of the appropriate diagnostic technologies already in distribution, and some emerging technologies that promise to address this challenge. It points to the fact that there needs to be innovation, adaptation, and cost reduction before these technologies can impact health care in the developing world.
WHO Guide for the Stepwise Laboratory Improvement Process Towards Accreditation in the African Region, WHO Regional Office for Africa, 2015
T Spira, ML Lindegren, R Ferris, V Habiyambere and T Ellerbrock, Am J Clin Path 2009:887-894 The WHO/PEPFAR Collaboration to Prepare an Operations Manual for HIV Prevention, Care and Treatment at Primary Health Centers in High-Prevalence, Resource-Constricted Settings: Defining Laboratory Services
FDA HIV Historical Timeline, 1991-1990, http://www.fda.gov/forpatients/illness/hivaids/history/ucm151074.htm
Alemnji G, Nkengasong JN, Parekh BS. HIV testing in developing countries: what is required? Indian J Med Res. 2011;134(6):779–86.
Parekh BS, Kalou MB, Alemnji G, Ou C, Gershy-Damet G, Nkengasong JN. Scaling up rapid HIV testing in developing countries. Am J Clin Pathol. 2010;134(4):573–84.
Consolidated guidelines on HIV testing services, WHO 2015, ISBN 978 92 4 150 892 6
Towards Universal Access, Scaling up priority HIV/AIDS interventions in the health sector, Progress Report 2010, WHO, UNAIDS, UNICEF 2010
CM Obermeyer, S Bott, R Bayer, A Desclaux and R Baggely, HIV testing and care in Burkina Faso, Kenya, Malawi and Uganda, BMC International Health and Human Rights 2013 13(6)
Claassen M, van Zyl GU, Korsman SNJ, Smit L, Cotton MF, Preiser W. Pitfalls with rapid HIV antibody testing in HIV-infected children in the Western Cape, South Africa. J Clin Virol. 2006;37(1):68–71.
Gray RH, Makumbi F, Serwadda D, et al. Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study. Br Med J. 2007;335(188):7612. This article evaluates the limitations of rapid tests for HIV-1. The article recommends ways for limiting false positives; First, weak positive bands on rapid tests for HIV should be confirmed by enzyme immunoassay and western blotting before disclosing the diagnosis. Second, programs using rapid tests routinely should use standard serological assays for quality control.
Wright RJ, Stringer JSA. Rapid testing strategies for HIV-1 serodiagnosis in high-prevalence African settings. Am J Prev Med. 2004;27(1):42–8.
Klarkowski DB, Wazome JM, Lokuge KM, Shanks L, Mills CF, O’Brien DP. The evaluation of a rapid in situ HIV confirmation test in a programme with a high failure rate of the WHO HIV two-test diagnostic algorithm. PLoS ONE. 2009;4(2):e4351.
Klarkowski D, O’Brien DP, Shanks L, Singh KP. Causes of false-positive HIV rapid diagnostic test results. Expert Rev Anti-Infect Ther. 2014;12(1):49–62.
Flynn, David, et al., Uptake of WHO Recommended Testing Strategies, An analysis of national policies in HIV testing strategies, December 2016, USAID, WHO publication. This article points out the difficulty in the assessment of national policies on biomedical interventions with moderate effects such as an integrative package that includes biomedical, behavioural, and structural interventions. Issues to be considered include the nature of control groups and the effect of adherence on the true effectiveness of the intervention.
Aghokeng A et al. Inaccurate diagnosis of HIV-1 group M and O is a key challenge for on-going universal access to antiretroviral treatment and HIV prevention in Cameroon. PLoS ONE. 2009;4(11), e7702.
Consolidated Guidelines on HIV Testing, WHO, July 2015, http://apps.who.int/iris/bitstream/10665/179870/1/9789241508926_eng.pdf?ua=1
Improving the Quality of HIV-Related Point-of-Care Testing: Ensuring the reliability and accuracy of Test Results, December 2015, World Health Organization, http://www.who.int/hiv/pub/toolkits/handbook-point-of-care-testing/en/
B Mehra, S Bhattar, P Bhalla and D Rhawat, Rapid Tests versus ELISA for Screening of HIV Infection: Our Experience from a Voluntary Counselling and Testing Facility of a Tertiary Care Centre in North India, Hindawi Pub. Corp., ISRN AIDS Vol. 2014, Art. ID 296840
Wynberg E, Cooke G, Shroufi A, Reid S, Ford N. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. JIAS. 2014;17:18809.
Zachariah R, Reid SD, Challet P, Massaquoi M, Shouten EJ, Harries AD. Why do we need point-of-care tests for low-income countries? Trop Med Int Health. 2011;16(1):37–41.
Reid SD, Fidler SJ, Cooke GS. Tracking the progress of HIV: the impact of point-of-care tests on HIV antiretroviral therapy. Clin Epidemiol. 2013;5:387–96.
Wu G, Zaman MH. Low-cost tools for diagnosing and monitoring HIV infection in low-resource settings. Bull World Health Organ. 2012;90:914–20.
Wynberg E, Cooke G, Shroufi A, Reid S, Ford N. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc. 2014;17:18809.
Peeling R, Sollis K, Glover S, et al. CD4 enumeration technologies: a systematic review of test performance for determining eligibility for antiretroviral therapy. PLoS ONE. 2015;10(3):e0115019.
Rutherford GW, Anglemyer A, Easterbrook PJ, Horvath T, Vitoria M, Penazzato M, et al. Predicting treatment failure in adults and children on antiretroviral therapy: a systematic review of the performance characteristics of the performance characteristics of the 2010 WHO immunologic and clinical criteria for virologic failure. AIDS. 2014;28 Suppl 2:S161–9.
Wittek M, Sturmer M, Doerr H, Berger A. Molecular assays for monitoring HIV infection and antiretroviral therapy. Expert Rev Mol Diagn. 2007;7:237–46.
Tang N, Huang S, Salituro J, et al. A real time HIV-1 viral load assay for automated quantitation of HIV-1 RNA in genetically diverse group M subtypes A-H, group O and N samples. J Virol Methods. 2007;146:236–45. This article illustrated the power of real time HIV-1 viral load assay in establishing whether raltegravir-containing antiretroviral therapy (ART) intensification reduces HIV levels in the gut for all HIV subtypes.
Scott L, Noble L, Moloi J, et al. An evaluation of the Abbott m2000 Real-Time HIV-1 assay for HIV viral load monitoring in South Africa compared with existing technologies: Roche COBAS Ampliprep/COBAS Amplicor; Roche COBAS Ampliprep/COBAS TaqMan HIV-1, and bioMérieux NucliSENS EasyQ HIV-1. J Clin Microbiol. 2009;47(7):2209–17.
Bertagnolio S, Soto-Ramirez L, Pilon R, et al. HIV-1 drug resistance surveillance using dried whole blood spots. Antivir Ther. 2007;12:107–13.
WHO technical and operational considerations for implementing HIV viral load test: Interim technical update July 2014 www.who.int/hiv/pub/arv/viral-load-testing-technical-update/en/
WHO Guide for the Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA) in the African Region (with checklist) http://www.afro.who.int/en/clusters-a-programmes/hss/blood-safety-laboratories-a-health-technology/blt-highlights/3859-who-guide-for-the-stepwise-laboratory-improvement-process-towards-accreditation-in-the-african-region-with-checklist.html
African Society for Laboratory Medicine (ASLM) www.aslm.org
Katy Yao, Talkmore Maruta, Elizabeth T Luman, John N Nkengasong. The SLMTA programme: Transforming the laboratory landscape in developing countries. www.ajlmonline.org doi:10.4102/ajlm.v312.194
90–90–90 - An ambitious treatment target to help end the AIDS epidemic http://www.unaids.org/en/resources/documents/2014/90-90-90
UNAIDS and partners launch initiative to improve HIV diagnostics. http://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2014/july/20140723dai
WHO Global health strategy on HIV/AIDS: 2011-2015. www.who.int/hiv/pub/hiv_strategy/en. The reference outlines recommended strategy from WHO that promotes a long-term, sustainable HIV response through strengthening health and community systems, tackling the social determinants of health that both drive the epidemic and hinder the response, and protecting and promoting human rights and promoting gender equity as essential elements of the health sector response.
WHO-AFRO The Maputo declaration on strengthening of laboratory systems. http://www.who.int/diagnostics_laboratory/Maputo-Declaration_2008.pdf
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Alash’le Abimiku, Ralph Timperi, and William Blattner declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on The Global Epidemic
Rights and permissions
About this article
Cite this article
Abimiku, A., Timperi, R. & Blattner, W. Laboratory Innovation Towards Quality Program Sustainability. Curr HIV/AIDS Rep 13, 202–208 (2016). https://doi.org/10.1007/s11904-016-0323-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-016-0323-y