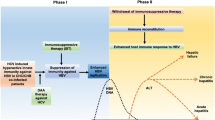
Abstract
Purpose of Review
The hepatitis C virus (HCV) is a major cause of liver-related morbidity and mortality, and a major risk factor of hepatocellular carcinoma (HCC) around the world. Early detection, continued prevention of transmission, and antiviral treatment of chronically infected persons are the pillars to decrease incidence and mortality of HCV. The widespread access to safe and effective direct acting anti-viral agents (DAA) has allowed the elimination of the infection possible in almost all treated patients, thus leading to a significant reduction of liver-related and overall mortality due to HCV in the cured population. Treatment of HCV does not completely eradicate HCC risk in populations with advanced liver disease and those with cofactors known to promote liver carcinogenesis such as diabetes, obesity, and excessive alcohol consumption.
Recent Findings
Molecular-based biomarkers are expected to overcome the limits of liver disease severity, thus improving the identification of screening candidates.
Summary
The implementation of risk-stratified surveillance programs coupled with the identification of biomarkers to predict HCC in HCV cured patents is deemed necessary for implementing the cost-effective management of these patients.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Tan DJH, Setiawan VW, Ng CH, Lim WH, Muthiah MD, Tan EX, et al. Global burden of liver cancer in males and females: changing etiological basis and the growing contribution of NASH. Hepatology. 2023;77:1150–63.
Blach S, Terrault NA, Tacke F, Gamkrelidze I, Craxi A, Tanaka J, et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7:396–415.
Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies 2016–2021: actions for impact. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
Toh MR, Wong EYT, Wong SH, Ng AWT, Loo L-H, Chow PK-H, et al. Global epidemiology and genetics of hepatocellular carcinoma. Gastroenterology. 2023;164:766–82.
Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–606.
Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. Accessed 5 June 2022.
Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL–Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399:61–116.
Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236.
Lockart I, Yeo MGH, Hajarizadeh B, Dore GJ, Danta M. HCC incidence after hepatitis C cure among patients with advanced fibrosis or cirrhosis: a meta-analysis. Hepatology. 2022;76:139–54.
Hsu CC, Gopalakrishna H, Mironova M, Lee M-H, Chen C-J, Yang H-I, et al. Risk of hepatocellular carcinoma after spontaneous clearance of hepatitis C virus and in noncirrhosis chronic hepatitis C patients with sustained virological response: a systematic review. Clin Infect Dis. 2023;77:S245–56.
Hamdane N, Jühling F, Crouchet E, El Saghire H, Thumann C, Oudot MA, et al. HCV-induced epigenetic changes associated with liver cancer risk persist after sustained virologic response. Gastroenterology. 2019;156:2313–2329.e7.
Nahon P, Vo Quang E, Ganne-Carrié N. Stratification of hepatocellular carcinoma risk following HCV eradication or HBV control. J Clin Med. 2021;10:353.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6.
Lemon SM, McGivern DR. Is hepatitis C virus carcinogenic? Gastroenterology. 2012;142:1274–8.
Bandiera S, Billie Bian C, Hoshida Y, Baumert TF, Zeisel MB. Chronic hepatitis C virus infection and pathogenesis of hepatocellular carcinoma. Curr Opin Virol. 2016;20:99–105.
D’souza S, Lau KC, Coffin CS, Patel TR. Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol. 2020;26:5759–83.
Wölfl M, Rutebemberwa A, Mosbruger T, Mao Q, Li H, Netski D, et al. Hepatitis C virus immune escape via exploitation of a hole in the T cell repertoire. J Immunol. 2008;181:6435–46.
Li J-T, Liao Z-X, Ping J, Xu D, Wang H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J Gastroenterol. 2008;43:419–28.
Kanda T, Goto T, Hirotsu Y, Moriyama M, Omata M. Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: a review. Int J Mol Sci. 2019;20:1358.
Quan H, Zhou F, Nie D, Chen Q, Cai X, Shan X, et al. Hepatitis C virus core protein epigenetically silences SFRP1 and enhances HCC aggressiveness by inducing epithelial–mesenchymal transition. Oncogene. 2014;33:2826–35.
Iida N, Mizukoshi E, Yamashita T, Yutani M, Seishima J, Wang Z, et al. Chronic liver disease enables gut Enterococcus faecalis colonization to promote liver carcinogenesis. Nat Can. 2021;2:1039–54.
Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996–1005.e1.
Ioannou GN, Beste LA, Green PK, Singal AG, Tapper EB, Waljee AK, et al. Increased risk for hepatocellular carcinoma persists up to 10 years after HCV eradication in patients with baseline cirrhosis or high FIB-4 scores. Gastroenterology. 2019;157:1264–1278.e4.
Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of hepatocellular carcinoma in patients with HCV-associated cirrhosis treated with direct-acting antiviral agents. Gastroenterology. 2018;155:411–421.e4.
Degasperi E, D’Ambrosio R, Iavarone M, Sangiovanni A, Aghemo A, Soffredini R, et al. Factors associated with increased risk of de novo or recurrent hepatocellular carcinoma in patients with cirrhosis treated with direct-acting antivirals for HCV infection. Clin Gastroenterol Hepatol. 2019;17:1183–1191.e7.
Chen Q, Ayer T, Adee MG, Wang X, Kanwal F, Chhatwal J. Assessment of incidence of and surveillance burden for hepatocellular carcinoma among patients with hepatitis C in the era of direct-acting antiviral agents. JAMA Netw Open. 2020;3:e2021173.
Ciancio A, Ribaldone DG, Spertino M, Risso A, Ferrarotti D, Caviglia GP, et al. Who should not be surveilled for HCC development after successful therapy with DAAS in advanced chronic hepatitis C? Results of a long-term prospective study. Biomedicines. 2023;11:166.
Pons M, Rodríguez-Tajes S, Esteban JI, Mariño Z, Vargas V, Lens S, et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J Hepatol. 2020;72:472–80.
Tahata Y, Sakamori R, Yamada R, Kodama T, Hikita H, Nozaki Y, et al. Risk of hepatocellular carcinoma after sustained virologic response in hepatitis C virus patients without advanced liver fibrosis. Hepatol Res. 2022;52:824–32.
Pawlotsky J-M, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G, et al. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511.
Singal AG, Llovet JM, Yarchoan M, Mehta N, Heimbach JK, Dawson LA, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78:1922–65.
Ioannou GN. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol. 2021;74:458–65.
Kim NJ, Vutien P, Berry K, Ioannou GN. Hepatocellular carcinoma risk declines but remains high enough for screening in the first 7 years after hepatitis C virus cure with direct-acting antivirals in patients with cirrhosis or high fibrosis-4 score. Gastroenterology. 2022;163:1104–1106.e3. Findings from this study suggest that all patients following SVR should undergo HCC screening, except those with low Fib-4 and no pre-treatment cirrhosis.
Nahon P, Bamba-Funck J, Layese R, Trépo E, Zucman-Rossi J, Cagnot C, et al. Integrating genetic variants into clinical models for hepatocellular carcinoma risk stratification in cirrhosis. J Hepatol. 2023;78:584–95.
Huntley C, Torr B, Sud A, Rowlands CF, Way R, Snape K, et al. Utility of polygenic risk scores in UK cancer screening: a modelling analysis. Lancet Oncol. 2023;24:658–68.
Omata M, Cheng A-L, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–70.
Allaire M, Bruix J, Korenjak M, Manes S, Maravic Z, Reeves H, et al. What to do about hepatocellular carcinoma: recommendations for health authorities from the International Liver Cancer Association. JHEP Rep. 2022;4:100578.
Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The Performance of AFP, AFP-3, DCP as Biomarkers For Detection Of Hepatocellular Carcinoma (HCC): A Phase 3 Biomarker Study in the United States. Clin Gastroenterol Hepatol. 2023;21:415–423.e4.
Singal AG, Zhang E, Narasimman M, Rich NE, Waljee AK, Hoshida Y, et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: A meta-analysis. J Hepatol. 2022;77:128–39.
Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance Imaging And Alpha Fetoprotein For Early Detection Of Hepatocellular Carcinoma In Patients With Cirrhosis: A Meta-Analysis. Gastroenterology. 2018;154:1706–1718.e1.
Santi V, Trevisani F, Gramenzi A, Grignaschi A, Mirici-Cappa F, Del PP, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol. 2010;53:291–7.
Trinchet J-C, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: A randomized trial comparing 3- and 6-month periodicities. Hepatology. 2011;54:1987–97.
Omata M, Kanda T, Wei L, Yu M-L, Chuang W-L, Ibrahim A, et al. APASL consensus statements and recommendations for hepatitis C prevention, epidemiology, and laboratory testing. Hepatol Int. 2016;10:681–701.
Mueller PP, Chen Q, Ayer T, Nemutlu GS, Hajjar A, Bethea ED, et al. Duration and cost-effectiveness of hepatocellular carcinoma surveillance in hepatitis C patients after viral eradication. J Hepatol. 2022;77:55–62.
Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71:265–73.
Singal AG, Reddy S, Radadiya aka Patel H, Villarreal D, Khan A, Liu Y, et al. Multicenter Randomized Clinical Trial Of A Mailed Outreach Strategy For Hepatocellular Carcinoma Surveillance. Clin Gastroenterol Hepatol. 2022;20:2818–2825.e1.
Yoon JH, Lee JM, Lee DH, Joo I, Jeon JH, Ahn SJ, et al. A Comparison Of Biannual Two-Phase Low-Dose Liver CT and US for HCC Surveillance In A Group At High Risk of HCC Development. Liver Cancer. 2020;9:503–17.
Kim SY, An J, Lim Y-S, Han S, Lee J-Y, Byun JH, et al. MRI With Liver-Specific Contrast For Surveillance Of Patients With Cirrhosis At High Risk Of Hepatocellular Carcinoma. JAMA Oncol. 2017;3:456.
Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, et al. Role of the GALAD and BALAD-2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol. 2016;14:875–886.e6.
Kim DH, Choi SH, Shim JH, Kim SY, Lee SS, Byun JH, et al. Meta-analysis of the accuracy of abbreviated magnetic resonance imaging for hepatocellular carcinoma surveillance: non-contrast versus hepatobiliary phase-abbreviated magnetic resonance imaging. Cancers (Basel). 2021;13:2975.
Lin N, Lin Y, Xu J, Liu D, Li D, Meng H, et al. A multi-analyte cell-free DNA–based blood test for early detection of hepatocellular carcinoma. Hepatol Commun. 2022;6:1753–63.
Kim AK, Hamilton JP, Lin SY, Chang T-T, Hann H-W, Hu C-T, et al. Urine DNA biomarkers for hepatocellular carcinoma screening. Br J Cancer. 2022;126:1432–8.
Gupta P, Soundararajan R, Patel A, Kumar-M P, Sharma V, Kalra N. Abbreviated MRI for hepatocellular carcinoma screening: A systematic review and meta-analysis. J Hepatol. 2021;75:108–19. Findings from this study suggest that AMRI might be superior than US in certain subgroups of patients, including obese individuals, patients with nonalcoholic steatohepatitis, and those with Child Pugh B or C cirrhosis.
Lee JY, Huo EJ, Weinstein S, Santos C, Monto A, Corvera CU, et al. Evaluation of an abbreviated screening MRI protocol for patients at risk for hepatocellular carcinoma. Abdom Radiol. 2018;43:1627–33.
Yokoo T, Masaki N, Parikh ND, Lane BF, Feng Z, Mendiratta-Lala M, et al. Multicenter validation of abbreviated MRI for detecting early-stage hepatocellular carcinoma. Radiology. 2023;307. https://doi.org/10.1148/radiol.220917.
Nguyen MH, Roberts LR, Engel-Nitz NM, Bancroft T, Ozbay AB, Singal AG. Gaps in hepatocellular carcinoma surveillance in a United States cohort of insured patients with cirrhosis. Curr Med Res Opin. 2022;38:2163–73.
CHAI and The Hepatitis Fund announce pricing breakthrough to reduce cost of viral hepatitis treatment by over 90 percent. The Clinton Health Access Initiative (CHAI). 2023 [Cited 2023 Dec 19]. [Internet]. Available from: https://www.clintonhealthaccess.org/news/chai-and-the-hepatitis-fund-announce-pricing-breakthrough-to-reduce-cost-of-viral-hepatitis-treatment-by-over-90-percent/
Parikh ND, Tayob N, Singal AG. Blood-based biomarkers for hepatocellular carcinoma screening: approaching the end of the ultrasound era? J Hepatol. 2023;78:207–16.
Yeo YH, Samaan JS, Ng WH, Ting P-S, Trivedi H, Vipani A, et al. Assessing the performance of ChatGPT in answering questions regarding cirrhosis and hepatocellular carcinoma. Clin Mol Hepatol. 2023;29:721–32.
Page K, Melia MT, Veenhuis RT, Winter M, Rousseau KE, Massaccesi G, et al. Randomized trial of a vaccine regimen to prevent chronic HCV infection. N Engl J Med. 2021;384:541–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author AA is part of the advisory board of Abbvie, Gilead, MSD, and Intercept. Author AA has received speaker honorarium from Abbvie, Gilead, and Mylan. Authors MC and DP declare that have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aghemo, A., Polverini, D. & Colombo, M. Hepatocellular Carcinoma in the Era of Direct Antiviral Agents Against Hepatitis C Virus. Curr Hepatology Rep (2024). https://doi.org/10.1007/s11901-024-00664-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s11901-024-00664-5