Abstract
Purpose of Review
This review article will examine portal hypertension in alcoholic hepatitis (AH) from both a basic mechanistic and a clinical perspective.
Recent Findings
Alcoholic hepatitis is a major public health problem in the USA, accounting for over 300,000 hospital admissions in a recent year of data (Jinjuvadia et al. J Clin Gastroenterol. 60;49:506–511). Portal hypertension is a key consequence of AH and a driver of liver-related morbidity and mortality. Alcohol may directly mediate portal hypertension via multiple possible mechanisms, including increased portal inflow, increased intrahepatic vasoconstriction, inflammation, and changes in the liver vasculature such as perisinusoidal fibrosis and phlebosclerosis.
Summary
Portal hypertension is a key consequence of AH and a critical area for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol-associated liver disease is a key driver of liver-related mortality in the USA [1]. Alcoholic hepatitis (AH) is the severe, inflammatory manifestation of alcohol-associated liver disease. Recent data collected in a nationwide database from 2000 to 2011 suggest that patients with AH are becoming more severely ill with a higher median Model for End Stage Liver Disease (MELD) score and that these patients have an increasing number of comorbidities as well [2]. More updated epidemiologic data from several states demonstrate an increase in incidence rate of AH among individuals in their 30 s and 40 s, with the highest overall rate in patients aged 40–59 [3]. Additionally, patients with AH are having increasing numbers of complications which can be related to portal hypertension, including hepatic encephalopathy, ascites, hepatorenal syndrome, and gastrointestinal bleeding, presenting critical challenges for clinicians [2].
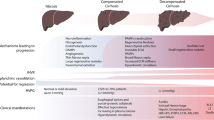
This article aims to review the literature on portal hypertension in AH. Mechanisms of portal hypertension including alterations in portal inflow, changes in nitric oxide and vasoactive mediators, vascular lesions and fibrosis, and inflammation will be examined. The specific role of liver sinusoidal endothelial cells (LSECs) in portal hypertension in this disease will be discussed with respect to these various mechanisms. The article will conclude with an examination of the management of portal hypertension in AH. The diverse potential mechanisms mediating portal hypertension in AH are summarized in Fig. 1.
Possible mechanisms of portal hypertension in alcohol-associated hepatitis: portal hypertension is likely multifactorial, with contributions from increased portal inflow as a direct effect of alcohol, increased intrahepatic vasoconstriction from decreased nitric oxide (NO) production as well as increased endothelin-1, systemic inflammation as well as inflammation within the liver, and increased perisinusoidal fibrosis and phlebosclerosis in the hepatic vasculature
Portal Inflow and Portal Pressure
Because AH occurs in the setting of ongoing alcohol intake, portal hypertension in this context may be due to the direct effects of alcohol on the portal circulation, which has been demonstrated in animal and human studies. Several studies have evaluated the effect of alcohol on portal pressure even in the absence of liver fibrosis or cirrhosis. In one animal study, acute oral ethanol intake was demonstrated to increase portal blood flow by 23–57% with increasing doses of ethanol (3.0 and 4.0 g/kg), which was ameliorated by inhibition of alcohol dehydrogenase, suggesting a role of ethanol metabolism in this effect [4]. Further work demonstrated that in the setting of partial portal vein ligation, ethanol ingestion increased not only portal tributary flow but also hepatic artery blood flow. This study also uncovered a role for adenosine signaling in the increased portal tributary blood flow in the portal vein ligation model following ethanol ingestion, finding that the added portal vein inflow in ethanol-fed, portal vein-ligated animals was inhibited by blocking adenosine signaling [5].
In addition to animal studies, observations have been made in patients regarding the effects of alcohol on portal pressure, and possible mechanisms of alcohol-associated portal hypertension. Luca et. al. performed a study in which patients with alcohol-associated cirrhosis had their hepatic venous pressure gradient (HVPG) measured before and after consumption of identical beverages with and without ethanol. The authors found that ethanol intake increased HVPG and azygos blood flow, confirming that ethanol consumption directly places these patients at risk of portal hypertensive complications [6]. This agrees with an earlier study noting that the histologic lesions characteristic of AH were associated with higher portal pressure than alcohol-associated cirrhosis alone [7]. Another study of patients with chronic alcohol abuse but without cirrhosis or alcohol-associated hepatitis subjected to an intravenous ethanol infusion also demonstrated that ethanol alone can significantly increase portal pressure [8]. In a more recent study in severe AH, HVPG was found to be a key prognostic indicator of survival, with a value less than or equal to 22 mmHg predicting a 13% mortality, while a value greater than 22 mmHg predicted a 66% mortality [9]. In another study, the effect of alcohol consumption on portal pressure was assessed in follow-up after an episode of AH by comparing measurements of HVPG from during the episode of hepatitis with a subsequent measurement a median of 100 days following the baseline measurement. Unsurprisingly, patients who achieved alcohol abstinence or reported only occasional drinking were more likely to have a greater than 20% reduction in HVPG on follow-up measurement than patients who exhibited harmful alcohol use [10].
Nitric Oxide and Other Vasoactive Signals
Specific vasoactive mediators play a mechanistic role in the pathophysiology of portal hypertension in alcoholic hepatitis as well, with a key contribution by LSECs. Liver sinusoidal endothelial cells comprise nearly 90% of the liver endothelial cell (EC) population and play pivotal roles in liver homeostasis [11] by regulating intrahepatic vascular tone and antigen clearance as well as maintaining functions of neighboring immune cells, hepatic stellate cells, and hepatocytes. LSEC dysfunction has been reported in alcohol-associated liver disease [12, 13] and is thought to precede liver injury, and in general LSEC dysfunction is a key factor mediating portal hypertension in liver disease. Chronic alcohol intake decreases nitric oxide production in LSECs [14], a hallmark of EC dysfunction, contributing to an increase in intrahepatic vascular resistance, given that nitric oxide is a key regulator of vascular tone.
In addition to examining the role of ethanol in mediating portal inflow, a study in isolated perfused rat livers showed that ethanol infusion into the portal vein resulted in intrahepatic vasoconstriction and increased portal pressure, which was ameliorated by antagonism of endothelin-1 (a vasoconstrictor peptide synthesized by endothelial cells). When a nitric oxide synthesis inhibitor was infused into the portal vein along with ethanol, vasoconstriction due to ethanol was more sustained, implicating nitric oxide most likely derived from LSECs in this phenomenon as well [15].
Another study of mechanisms of portal hypertension in AH examined patients with decompensated alcohol-associated cirrhosis alone or decompensated alcohol-associated cirrhosis with superimposed AH. The authors noted a significantly higher HVPG in the AH group and assessed whether the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) was increased in the AH group because inhibition of nitric oxide can raise portal pressure. The AH patients did in fact have higher plasma levels of ADMA, and the dimethylarginine score (ADMA plus its stereo-isomer SDMA) was a better predictor of survival than the MELD score and the Maddrey’s Discriminant Function [16].
Inflammation
Translational studies examining the molecular phenomena accompanying portal hypertension in AH have identified several molecules associated with inflammation and vascular dysfunction as predictors and mediators of increased portal pressure. An early inflammatory mediator identified as responsible for portal hypertensive changes in AH was tumor necrosis factor α (TNF-α). In a key study, patients with severe AH were treated with the anti-TNF-α antibody infliximab, and hemodynamic assessment was performed at baseline prior to treatment and repeated 24 h following treatment, as well as prior to discharge in some patients. HVPG was significantly decreased 24 h after treatment, and this decrease was sustained in the patients who agreed to the later hemodynamic assessment. This was accompanied by a decrease in other pro-inflammatory cytokines such as interleukin (IL)-6 and IL-8 as well [17]. Unfortunately, despite this signal that portal pressure may be lowered by this treatment, anti-TNF-α therapy was associated with increased 6-month mortality in AH in a randomized controlled trial and so is not currently recommended for clinical use in this setting [18].
A study examining liver tissue from patients with AH compared with normal control livers demonstrated additional factors correlated with portal hypertension in AH. The median value for HVPG in the AH patients in this study was 20 mmHg, and the authors found that expression of IL-8, C-X-C motif chemokine ligand (CXCL)1, and CXCL5 in the liver were significantly increased in patients with AH and HVPG > 20 mmHg, suggesting a role for these inflammatory factors in the pathogenesis of portal hypertension in AH [19].
An additional factor that seems to play a role in portal hypertension in AH is lipocalin 2 (LCN2). A study of patients with AH with advanced liver fibrosis and portal hypertension revealed that LCN2 expression in the liver was significantly correlated with HVPG as well as the expression of fibrogenic genes COL1A1 and TIMP1, suggesting this molecule plays a key pathogenic role in the progression of alcohol-associated liver disease. Interestingly, the upregulation of hepatic LCN2 was observed in AH but not other chronic liver diseases such as non-alcoholic steatohepatitis (NASH) and hepatitis C(20•).
Turning to the intersection of inflammation and LSECs, endothelial hyperactivation is a form of EC dysfunction, most often referred to as an upregulation of genes involved in inflammation, such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and CD146 (also known as melanoma cell adhesion molecule (MCAM) or cell surface glycoprotein MUC18). These cell adhesion molecules (CAMs) expressed on the surface of LSECs are thought to play a key role in adhesion of neutrophils, macrophages, and lymphocytes [21–23] to LSECs before transmigration from circulation into the liver to induce hepatocyte injury. Inflammatory cytokines and growth factors induce upregulation of CAMs on ECs, thereby promoting inflammatory cell adhesion and trans-endothelial migration [24, 25]. As inflammation progresses, these CAMs on ECs can be cleaved from the cell surface and released into the blood as soluble forms. Studies have suggested that several soluble CAMs could be potential prognostic biomarkers and indicators of disease activity [26–28]. Soluble ICAM-1 and soluble VCAM-1 levels are elevated in patients with different liver diseases with excess alcohol ingestion, including AH and alcohol-associated cirrhosis [29]. Elevated soluble CD146 levels are also observed in patients with cirrhosis due to alcohol, viral infection, or other etiologies, and could serve as a sensitive soluble marker to predict decompensation of cirrhosis [30]. A recent study by Xia et al. [31•] determined plasma levels of soluble CD146, soluble ICAM-1, and soluble VCAM-1 in patients with AH (n = 79), heavy drinkers without clinical evidence of liver disease (HDC, n = 66), then compared to those of healthy control (n = 46). These soluble CAMs were also compared to their clinical parameters and plasma levels of inflammatory cytokines and growth factors that are related to EC activation and function. It was found that these soluble CAMs were highly elevated and related to disease severity of AH. In the HDC group, soluble VCAM-1 was the only CAM that showed a significant elevation compared to a healthy control group. Xia et al. [31•] also determined whether alcohol abstinence could reduce EC hyperactivation in 31 AH patients and 30 HDC at 6-month follow-up as well as 18 AH patients and 25 HDC at 12-month follow-up after alcohol abstinence, and found that alcohol secession did not completely reverse these abnormalities.
While CAMs are important for immune cell adhesion to the EC surface, multiple CXCL chemokines play a key role in the recruitment of inflammatory immune cells such as neutrophils. As discussed above, inflammatory cytokines such as TNF-α are upregulated in AH and induce expression of CXCL1 and CXCL8 chemokines [32], facilitating recruitment of neutrophils to the liver and leading to hepatotoxic injury and inflammation in AH [33–35]. Liu et al. [33] demonstrated the mechanism by which TNF-α signaling leads to downstream transcriptional regulation in AH by characterizing transcriptomic and epigenomic changes in this disease. Several CXCL chemokines including CXCL1, 6, and 8 were found to be upregulated in the liver of AH patients as previously reported [19]. As discussed above, several of these chemokines are also associated with HVPG > 20 mmHg in patients with AH, suggesting a role in mediating portal hypertension. Further, the authors showed that the production of these CXCL chemokines is under the regulation of the TNF-α/NFkB signaling axis via a “super-enhancer” region of the genome comprising multiple enhancers for an array of transcription factors, which regulate multiple target genes [33].
The reticuloendothelial system in the liver refers to the system of phagocytosis and clearance of macromolecules and nanoparticles from the blood circulation, performed by activated Kupffer cells and LSECs. Overactivation of these reticuloendothelial cells in the sinusoids may correlate with disease severity and mortality in patients with severe AH. A prospective study was carried out in 50 severe AH patients (Maddrey’s discriminant function > 32 or MELD > 20) [36]. Of these, 43 patients (82%) who fell into the category of very severe AH (Maddrey’s discriminant function, 76.23 (55.06–104.35)) showed a marked activation of the reticuloendothelial system with elevated levels of von Willebrand factor (vWF), an indicator of EC activation, as well as increased levels of soluble CD163 and ferritin, markers of macrophage activation. In addition, vWF was a predictor of mortality in severe AH. Given that 36 patients (72%) had systemic inflammatory syndrome and that these markers of macrophages and EC activation were measured in plasma, it is still possible that macrophages and ECs in tissues other than the liver may be contributing to these findings.
Interleukin-6 is elevated in alcohol-associated liver disease [17] and known as an indicator of systemic and tissue inflammation. Studies in mice showed that IL-6 is protective against alcohol-induced liver injury [37]. Thus, it was speculated that the elevated IL-6 in alcohol-associated liver disease patients may play a compensatory role in protecting the liver from alcohol-induced liver injury. In general, IL-6 induces signaling through activation of signal transducer and activator of transcription 3 (STAT3) [38]. In ECs, STAT3 has been thought of as anti-inflammatory and shown to protect against lipopolysaccharide (LPS)–induced inflammation [39] and ischemia–reperfusion injury in the heart [40]. Similarly, in LSECs, Gao et al. showed a protective role for STAT3 in ethanol-induced liver injury when endothelial-specific STAT3 knockout (KO) mice were given 4-week Lieber-DeCarli liquid diet containing 5% ethanol or control diet [37]. In contrast to these protective roles of conventional IL-6 signaling, IL-6 trans-signaling in LSECs [41], which is mediated by IL-6 and the soluble IL-6 receptor complex in the blood circulation, has been implicated in endotheliopathy and linked to liver injury in patients with COVID-19 [42, 43]. Thus, activation of STAT3 might not be entirely anti-inflammatory, but may also have an opposing role depending on the activation pathway involved (conventional IL-6 vs. trans-signaling pathway).
The role of infection in exacerbating portal hypertension in AH remains an area in which research is needed. In the landmark STOPAH trial, 12% of patients with severe AH presented with baseline infection [44], and bacterial translocation is an important mechanism of worsening portal hypertension [45]. However, studies on the precise role of infection in portal hypertension in alcohol-associated liver disease are currently lacking.
Vascular Lesions and Fibrosis
The effects of chronic alcohol consumption inducing liver fibrosis on portal hypertension have been evaluated in animal models. In one study in non-human primates, portal pressure in alcohol-fed animals was significantly correlated with perivenular fibrosis in the liver, suggesting a mechanistic role of this vascular lesion in portal hypertension [46]. In a rat model of alcohol-induced liver fibrosis via chronic intragastric feeding, portal pressure was significantly raised and was associated with peri-sinusoidal fibrosis in the alcohol-fed animals, raising the possibility that this sinusoidal lesion is playing a key role in alcohol-induced portal hypertension [47].
Additionally, several studies in patients have examined whether distinct vascular lesions in the liver may play a role in portal hypertension in AH. In one study by Goodman and Ishak, signs of portal hypertension in pre-cirrhotic AH were associated with the degree of veno-occlusive change and phlebosclerosis (perivenular scarring with gradual obliteration of the lumen of terminal hepatic venules) [48]. Another study several years later by Burt and MacSween of patients with AH or alcohol-associated cirrhosis was less clear about the importance of veno-occlusive changes, but did note that phlebosclerosis was a universal finding and increased in severity with progressive degree of liver injury, again suggesting its importance in the progression of liver disease [49].
Management of Portal Hypertension in Alcoholic Hepatitis
The general management of portal hypertensive complications in AH in the acute setting does not differ from other etiologies of portal hypertension and should adhere to best evidence and practice guidelines [50–52]. A specific consideration in AH is the effect of corticosteroid therapy on portal hypertension and its complications. In the foundational study by Maddrey et. al. on corticosteroid therapy in AH, there was no difference in decrease in portal pressure over 4 weeks between the corticosteroid and placebo-treated groups [53]. Clinicians often must consider whether to use corticosteroids in the situation of upper gastrointestinal bleeding in severe AH, and one study provides some guidance. Rudler et. al. retrospectively analyzed survival of severe AH patients treated with corticosteroids with or without upper GI bleeding (> 50% with upper GI bleeding had variceal hemorrhage) and found no difference in survival between the two groups, suggesting that appropriately managed upper GI bleeding from portal hypertension is not an absolute contraindication to corticosteroid use [54].
Therapies to acutely ameliorate portal hypertension specifically in the setting of AH remain experimental. Lowering portal pressure in the setting of variceal hemorrhage should be carried out as per current guidelines with octreotide, non-selective beta blockers, and TIPS as indicated [51]. One single-center, retrospective study has reported an association between non-selective beta blocker use in severe AH and acute kidney injury, but further data are needed before drawing broad conclusions regarding safety in this setting and as with all patients safe use requires attention to patient hemodynamics [55]. While evidence on variceal hemorrhage specifically in AH remains sparse, one study evaluated outcomes of patients with AH undergoing emergency portacaval shunt surgery for bleeding esophageal varices and found no evidence that cirrhosis plus AH had a worse prognosis following surgery than cirrhosis alone [56]. Another study evaluated TIPS placement for hepatorenal syndrome in a small number of patients with severe AH and found that TIPS improved creatinine, blood urea nitrogen, and urine output and may be an area of further study for renal portal hypertensive complications in these patients [57]. An additional highly experimental study carried forward the findings that proinflammatory stimuli such as TNF-α may be causing portal hypertension in AH and sought to ameliorate these changes with albumin dialysis. The authors found that patients receiving albumin dialysis had a significant reduction in HVPG versus those receiving hemofiltration alone, although the mechanism by which this occurred was unclear. This result suggests that albumin dialysis may be worthy of further study for acute lowering of portal hypertension in inflammatory conditions [58].
Conclusion
Alcoholic hepatitis is a pressing clinical concern, and the impact of alcohol-associated liver disease in the USA is predicted to worsen due to the ongoing effects of the COVID-19 pandemic [59]. Portal hypertension in AH is a key therapeutic target for mitigating complications, morbidity, and mortality from the disease. Future research concerning portal hypertension related to AH should address whether anti-inflammatory therapy can be directed to mitigate portal hypertension without adverse outcomes for patients, whether endothelium can be precisely targeted to prevent complications from portal hypertension in AH, and whether non-invasive markers of portal hypertension that can assist with prognosis and identifying patients who will benefit from future therapies in AH can be identified.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362:k2817.
Nguyen TA, DeShazo JP, Thacker LR, Puri P, Sanyal AJ. The worsening profile of alcoholic hepatitis in the United States. Alcohol Clin Exp Res. 2016;40:1295–303.
Doshi SD, Stotts MJ, Hubbard RA, Goldberg DS. The changing burden of alcoholic hepatitis: rising incidence and associations with age, gender, race, and geography. Dig Dis Sci. 2021;66:1707–14.
McKaigney JP, Carmichael FJ, Saldivia V, Israel Y, Orrego H. Role of ethanol metabolism in the ethanol-induced increase in splanchnic circulation. Am J Physiol. 1986;250:G518-523.
Verma-Ansil B, Carmichael FJ, Saldivia V, Varghese G, Orrego H. Effect of ethanol on splanchnic hemodynamics in awake and unrestrained rats with portal hypertension. Hepatology. 1989;10:946–52.
Luca A, Garcia-Pagan JC, Bosch J, Feu F, Caballeria J, Groszmann RJ, Rodes J. Effects of ethanol consumption on hepatic hemodynamics in patients with alcoholic cirrhosis. Gastroenterology. 1997;112:1284–9.
Poynard T, Degott C, Munoz C, Lebrec D. Relationship between degree of portal hypertension and liver histologic lesions in patients with alcoholic cirrhosis Effect of acute alcoholic hepatitis on portal hypertension. Dig Dis Sci. 1987;32:337–43.
Silva G, Fluxa F, Bresky G, Backhouse C, Palma M, Ruiz M, Hirsch S, et al. Splanchnic and systemic hemodynamics in early abstinence and after ethanol administration in non-cirrhotic alcoholic patients. J Hepatol. 1994;20:494–9.
Rincon D, Lo Iacono O, Ripoll C, Gomez-Camarero J, Salcedo M, Catalina MV, Hernando A, et al. Prognostic value of hepatic venous pressure gradient for in-hospital mortality of patients with severe acute alcoholic hepatitis. Aliment Pharmacol Ther. 2007;25:841–8.
Spahr L, Goossens N, Furrer F, Dupuis M, Vijgen S, Elkrief L, Giostra E, et al. A return to harmful alcohol consumption impacts on portal hemodynamic changes following alcoholic hepatitis. Eur J Gastroenterol Hepatol 2018;30:967-974. This reference demonstrates the key role of treating alcohol use disorder and reducing harmful alcohol use in managing portal hypertension in alcohol-associated hepatitis.
Su T, Yang Y, Lai S, Jeong J, Jung Y, McConnell M, Utsumi T, et al. Single-cell transcriptomics reveals zone-specific alterations of liver sinusoidal endothelial cells in cirrhosis. Cell Mol Gastroenterol Hepatol. 2021;11:1139–61.
McCuskey RS, Bethea NW, Wong J, McCuskey MK, Abril ER, Wang X, Ito Y, et al. Ethanol binging exacerbates sinusoidal endothelial and parenchymal injury elicited by acetaminophen. J Hepatol. 2005;42:371–7.
Nanji AA, Tahan SR, Khwaja S, Yacoub LK, Sadrzadeh SM. Elevated plasma levels of hyaluronic acid indicate endothelial cell dysfunction in the initial stages of alcoholic liver disease in the rat. J Hepatol. 1996;24:368–74.
Yang Y, Sangwung P, Kondo R, Jung Y, McConnell MJ, Jeong J, Utsumi T, et al. Alcohol-induced Hsp90 acetylation is a novel driver of liver sinusoidal endothelial dysfunction and alcohol-related liver disease. J Hepatol. 2021;75:377–86.
Oshita M, Takei Y, Kawano S, Yoshihara H, Hijioka T, Fukui H, Goto M, et al. Roles of endothelin-1 and nitric oxide in the mechanism for ethanol-induced vasoconstriction in rat liver. J Clin Invest. 1993;91:1337–42.
Mookerjee RP, Malaki M, Davies NA, Hodges SJ, Dalton RN, Turner C, Sen S, et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology. 2007;45:62–71.
Mookerjee RP, Sen S, Davies NA, Hodges SJ, Williams R, Jalan R. Tumour necrosis factor alpha is an important mediator of portal and systemic haemodynamic derangements in alcoholic hepatitis. Gut. 2003;52:1182–7.
Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–60.
Dominguez M, Miquel R, Colmenero J, Moreno M, Garcia-Pagan JC, Bosch J, Arroyo V, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–50.
• Chen J, Argemi J, Odena G, Xu MJ, Cai Y, Massey V, Parrish A, et al. Hepatic lipocalin 2 promotes liver fibrosis and portal hypertension. Sci Rep. 2020;10:15558. This reference defines an important mechanism of liver fibrosis and portal hypertension in alcohol-associated hepatitis.
Bautista AP. Neutrophilic infiltration in alcoholic hepatitis. Alcohol. 2002;27:17–21.
Haydon G, Lalor PF, Hubscher SG, Adams DH. Lymphocyte recruitment to the liver in alcoholic liver disease. Alcohol. 2002;27:29–36.
Sandahl TD, Gronbaek H, Moller HJ, Stoy S, Thomsen KL, Dige AK, Agnholt J, et al. Hepatic macrophage activation and the LPS pathway in patients with alcoholic hepatitis: a prospective cohort study. Am J Gastroenterol. 2014;109:1749–56.
Langer HF, Chavakis T. Leukocyte-endothelial interactions in inflammation. J Cell Mol Med. 2009;13:1211–20.
Shaik-Dasthagirisaheb YB, Varvara G, Murmura G, Saggini A, Potalivo G, Caraffa A, Antinolfi P, et al. Vascular endothelial growth factor (VEGF), mast cells and inflammation. Int J Immunopathol Pharmacol. 2013;26:327–35.
Rothlein R, Mainolfi EA, Czajkowski M, Marlin SD. A form of circulating ICAM-1 in human serum. J Immunol. 1991;147:3788–93.
Sasseville VG, Newman WA, Lackner AA, Smith MO, Lausen NC, Beall D, Ringler DJ. Elevated vascular cell adhesion molecule-1 in AIDS encephalitis induced by simian immunodeficiency virus. Am J Pathol. 1992;141:1021–30.
Yuan J, Guo Q, Qureshi AR, Anderstam B, Eriksson M, Heimburger O, Barany P, et al. Circulating vascular endothelial growth factor (VEGF) and its soluble receptor 1 (sVEGFR-1) are associated with inflammation and mortality in incident dialysis patients. Nephrol Dial Transplant. 2013;28:2356–63.
Adams DH, Burra P, Hubscher SG, Elias E, Newman W. Endothelial activation and circulating vascular adhesion molecules in alcoholic liver disease. Hepatology. 1994;19:588–94.
Nomikou E, Alexopoulou A, Vasilieva L, Agiasotelli D, Pavlou E, Theodossiades G, Dourakis SP. Soluble CD146, a novel endothelial marker, is related to the severity of liver disease. Scand J Gastroenterol. 2015;50:577–83.
• Xia Y, Yang J, Sanyal AJ, Shah VH, Chalasani NP, Yu Q, Zheng X, et al. Persistent hyperactivation of endothelial cells in patients with alcoholic hepatitis. Alcohol Clin Exp Res. 2020;44:1075–87. This reference defines a role for endothelial hyperactivation in alcohol-associated hepatitis.
Gao B, Ahmad MF, Nagy LE, Tsukamoto H. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol. 2019;70:249–59.
Liu M, Cao S, He L, Gao J, Arab JP, Cui H, Xuan W, et al. Super enhancer regulation of cytokine-induced chemokine production in alcoholic hepatitis. Nat Commun. 2021;12:4560.
Mathurin P, Mendenhall CL, Carithers RL Jr, Ramond MJ, Maddrey WC, Garstide P, Rueff B, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomized placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. 2002;36:480–7.
Sehrawat TS, Liu M, Shah VH. The knowns and unknowns of treatment for alcoholic hepatitis. Lancet Gastroenterol Hepatol. 2020;5:494–506.
Vijayalekshmi B, Sharma A, Prabhu SB, Nair SC, Mammen J, Goel A, Zachariah UG, et al. Reticuloendothelial activation correlates with disease severity and predicts mortality in severe alcoholic hepatitis. Eur J Gastroenterol Hepatol. 2021;33:e329–34.
Miller AM, Wang H, Park O, Horiguchi N, Lafdil F, Mukhopadhyay P, Moh A, et al. Anti-inflammatory and anti-apoptotic roles of endothelial cell STAT3 in alcoholic liver injury. Alcohol Clin Exp Res. 2010;34:719–25.
Wegenka UM, Buschmann J, Lutticken C, Heinrich PC, Horn F. Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol. 1993;13:276–88.
Kano A, Wolfgang MJ, Gao Q, Jacoby J, Chai GX, Hansen W, Iwamoto Y, et al. Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. J Exp Med. 2003;198:1517–25.
Wang M, Zhang W, Crisostomo P, Markel T, Meldrum KK, Fu XY, Meldrum DR. Endothelial STAT3 plays a critical role in generalized myocardial proinflammatory and proapoptotic signaling. Am J Physiol Heart Circ Physiol. 2007;293:H2101-2108.
Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J. 1994;300(Pt 2):281–90.
McConnell MJ, Kondo R, Kawaguchi N, Iwakiri Y. Covid-19 and liver injury: role of inflammatory endotheliopathy, platelet dysfunction, and thrombosis. Hepatol Commun. 2022;6:255–69.
McConnell MJ, Kawaguchi N, Kondo R, Sonzogni A, Licini L, Valle C, Bonaffini PA, et al. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. J Hepatol. 2021;75:647–58.
Thursz MR, Forrest EH, Ryder S, investigators S. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;373:282–283.
Arab JP, Martin-Mateos RM, Shah VH. Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int. 2018;12:24–33.
Miyakawa H, Iida S, Leo MA, Greenstein RJ, Zimmon DS, Lieber CS. Pathogenesis of precirrhotic portal hypertension in alcohol-fed baboons. Gastroenterology. 1985;88:143–50.
Li J, Niu JZ, Wang JF, Li Y, Tao XH. Pathological mechanisms of alcohol-induced hepatic portal hypertension in early stage fibrosis rat model. World J Gastroenterol. 2005;11:6483–8.
Goodman ZD, Ishak KG. Occlusive venous lesions in alcoholic liver disease A study of 200 cases. Gastroenterology. 1982;83:786–96.
Burt AD, MacSween RN. Hepatic vein lesions in alcoholic liver disease: retrospective biopsy and necropsy study. J Clin Pathol. 1986;39:63–7.
Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71:306–33.
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–35.
Biggins SW, Angeli P, Garcia-Tsao G, Gines P, Ling SC, Nadim MK, Wong F, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1014–48.
Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–9.
Rudler M, Mouri S, Charlotte F, Lebray P, Capocci R, Benosman H, Poynard T, et al. Prognosis of treated severe alcoholic hepatitis in patients with gastrointestinal bleeding. J Hepatol. 2015;62:816–21.
Serste T, Njimi H, Degre D, Deltenre P, Schreiber J, Lepida A, Trepo E, et al. The use of beta-blockers is associated with the occurrence of acute kidney injury in severe alcoholic hepatitis. Liver Int. 2015;35:1974–82.
Bell RH Jr, Miyai K, Orloff MJ. Outcome in cirrhotic patients with acute alcoholic hepatitis after emergency portacaval shunt for bleeding esophageal varices. Am J Surg. 1984;147:78–84.
Testino G, Leone S, Ferro C, Borro P. Severe acute alcoholic hepatitis and hepatorenal syndrome: role of transjugular intrahepatic portosystemic stent shunt. J Med Life. 2012;5:203–5.
Sen S, Mookerjee RP, Cheshire LM, Davies NA, Williams R, Jalan R. Albumin dialysis reduces portal pressure acutely in patients with severe alcoholic hepatitis. J Hepatol. 2005;43:142–8.
Julien J, Ayer T, Tapper EB, Barbosa C, Dowd WN, Chhatwal J. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: a modeling study. Hepatology. 2022;75:1480–90.
Jinjuvadia R, Liangpunsakul S, Translational R. Evolving alcoholic hepatitis treatment C. Trends in alcoholic hepatitis-related hospitalizations, financial burden, and mortality in the United States. J Clin Gastroenterol. 2015;49:506–11.
Funding
NIH grants (R01DK117597, R01AA025342) to YI; NIH grants (K08AA029182) to MM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McConnell, M.J., Iwakiri, Y. Portal Hypertension in Alcohol-Associated Hepatitis. Curr Hepatology Rep 22, 67–73 (2023). https://doi.org/10.1007/s11901-023-00601-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-023-00601-y