Abstract
Purpose of Review
Portal hypertension is responsible of the main complications of cirrhosis, which carries a high mortality. Recent treatments have improved prognosis, but this is still far from ideal. This paper reviews new potential therapeutic targets unveiled by advances of key pathophysiologic processes.
Recent Findings
Recent research highlighted the importance of suppressing etiologic factors and a safe lifestyle and outlined new mechanisms modulating portal pressure. These include intrahepatic abnormalities linked to inflammation, fibrogenesis, vascular occlusion, parenchymal extinction, and angiogenesis; impaired regeneration; increased hepatic vascular tone due to sinusoidal endothelial dysfunction with insufficient NO availability; and paracrine liver cell crosstalk. Moreover, pathways such as the gut-liver axis modulate splanchnic vasodilatation and systemic inflammation, exacerbate liver fibrosis, and are being targeted by therapy. We have summarized studies of new agents addressing these targets.
Summary
New agents, alone or in combination, allow acting in complementary mechanisms offering a more profound effect on portal hypertension while simultaneously limiting disease progression and favoring regression of fibrosis and of cirrhosis. Major changes in treatment paradigms are anticipated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Portal hypertension (PH) is a major consequence of liver cirrhosis (LC) where its complications are key determining prognosis [1]. These complications include the development of esophageal varices (that can rupture causing massive gastrointestinal bleeding), formation of ascites and its complications (spontaneous bacterial peritonitis, hepatorenal syndrome), and hepatic encephalopathy. The prognosis of cirrhosis is good before the development of complications related to PH. In this stage of compensated cirrhosis, the median survival is about 12 years. However, cirrhosis becomes a highly lethal disease, with a median survival of about 2 years once the patients have developed ascites, bleeding, or encephalopathy, which is known as the decompensated stage of the disease [2]. The two main mechanisms of PH are increased intrahepatic vascular resistance (IHVR) and splanchnic vasodilatation with increased inflow of blood in the portal system [3]. IHVR (produced by structural remodeling of the liver architecture, increasing hepatic vascular tone due to hepatic endothelial dysfunction) is the initiating and more important factor determining PH, while splanchnic hyperemia develops later, as an adaptive response to maintain liver perfusion challenged by the development of portal-systemic collaterals and by the impaired metabolic exchange at the disturbed hepatic microcirculation. In advanced stages, the splanchnic vasodilation represents an important mechanism maintaining and aggravating PH. Moreover, as splanchnic vasodilation progresses, it determines a state of “effective hypovolemia” that leads to systemic hypotension, expansion of the plasma volume and increased cardiac output, the so-called hyperdynamic syndrome of cirrhosis, which plays an important role in the pathophysiology of ascites and associated renal abnormalities (hyponatremia, hepatorenal syndrome, acute kidney injury). Of note, in decompensated cirrhosis, systemic inflammation becomes an important factor driving progression of cirrhosis to further decompensation, acute-on-chronic liver failure (ACLF) and death or liver transplantation.
PH becomes clinically significant (CSPH) when the porto-caval pressure gradient, measured clinically by the hepatic vein pressure gradient (HVPG) is equal or above 10 mmHg [4]. Patients with CSPH are at increased risk of developing decompensation [5] and hepatocellular carcinoma [6, 7]. The importance of subclinical PH (HVPG from 5.5 to 9.5 mmHg) is still a matter of debate [8] but nonetheless indicates underlying severe liver disease.
Cirrhosis of the liver is responsible of about 90% of cases of PH in western countries, so we will focus on this disease. Cirrhosis is the result of a prolonged and progressive scarring process (liver fibrosis), caused by chronic injury and inflammation. Fibrosis leads to marked architectural liver disturbances, which are aggravated by loss of parenchymal cells due to vascular occlusion and ensuing tissue collapse, which makes that attempts of regeneration lead to the formation of regenerating nodules separated by fibrous tracts, which represents the hallmark of cirrhosis. Impaired liver regeneration and continued fibrogenesis further aggravate the process. Recent evidence suggests that cirrhosis is potentially reversible, but is not yet defined if, why, and when cirrhosis reaches a point of no-return [9, 10]. Reversibility is possible before CSPH, but unlikely after decompensation or in the presence of co-morbidities.
Treatment of PH at early stages is based primarily on controlling the etiological cause of the liver disease, to enhance the chance of reversing the architectural disturbances leading to PH. In addition, several studies have highlighted the role of relatively simple measures such as a safe lifestyle [11] (including maintaining a body mass index between 18 and 28, abstaining from alcohol, and moderate aerobic exercise), which favorably influence liver disease and PH. In addition, agents with the potential to enhance fibrosis regression may be paramount to achieve clinical improvement.
Current pharmacological treatment for PH is based on the use of drugs that act mainly on the increased portal vein inflow, which for short treatments include the intravenous drugs terlipressin [12] (a long-acting vasopressin analog), somatostatin [13], and analogs (octreotide) [14], while for long-term administration, the key agents are still the non-selective beta-blockers propranolol [15], nadolol [16], and, more recently, carvedilol [17, 18]. Despite the marked impact of these drugs ameliorating the prognosis of PH, still a substantial fraction of patients are insufficiently covered by them and require the association of other drugs or of endoscopic, interventional radiology, or surgical procedures.
It is remarkable that to date, not a single agent acting on IHVR has been approved for PH. Novel therapeutic approaches cover a wide range of targets in rapid development. This review focuses on the new agents under development or being studied for repurposing for PH. Notably, this is specially challenging in the aging population since aging favors disease progression and hampers response to many drugs [19].
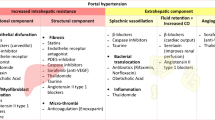
Pathophysiological Basis of Therapy: Intra and Extra-hepatic Targets (Fig. 1)
Progression of PH. Liver fibrosis leads progression first to a low-risk, long-lasting stage known as compensated cirrhosis and thereafter to the high-risk and short duration decompensated stage. A series of factors contribute to disease progression and to increased portal pressure. Initially this is mostly linked to fibrogenesis, structural liver changes and increased hepatic vascular tone, while after development of clinically significant portal hypertension (CSPH, defined by a HVPG ≥ 10 mmHg), portal-systemic collaterals, and hyperkinetic circulation, systemic inflammation (prompted among others by abnormalities of the gut-liver axis and translocation of bacterial products, DAMPs and PAMPs) becomes the main factor determining clinical complications and prognosis. Clinical stages also determine the aim of therapy, the choice of therapeutic agents, and the likelihood of regression upon removal or effective treatment of etiological factors. Abbreviations: intrahepatic vascular resistance (IHVR), liver transplantation (LT), acute on chronic liver failure (ACLF), damage associated molecular patterns (DAMPs), pattern associated molecular patterns (PAMPs), extra cellular matrix (ECM), spontaneous bacterial peritonitis (SBP), hepatopulmonary syndrome (HPS), portopulmonary hypertension (POPH), cirrhotic cardiomyopathy (CCM), acute kidney injury (AKI), hepatorenal syndrome (HRS)
As commented, the initial mechanism leading to PH is the IHVR to portal blood flow. This is due to a combination of structural changes and increased vascular tone. The structural component is determined by progressive fibrosis, vascular occlusion with ensuing parenchymal extinction and tissue collapse, which are mediated by chronic liver injury/repair, oxidative stress, release of damage associated molecular patterns (DAMPs) and pro-inflammatory cytokines, activation of hepatic stellate cells (HSCs), and macrophages. These processes can be targeted with specific drugs, which is a relevant area of research.
Splanchnic vasodilation and increased portal inflow represent the main extra-hepatic factor aggravating PH. This is mediated by increased NO availability [20], decreased response to vasoconstrictors [21], and the vasculogenesis in splanchnic organs via VEGF-VEGF-R2 signaling [22]. Splanchnic vasodilation is minimal or absent until development of CSPH. Then, portal-systemic collaterals begin to form, driven by increased portal pressure and VEGF-dependent angiogenesis in areas of anatomical connections between the portal and systemic circulations. Functional and anatomic intrahepatic shunts promote secretion of VEGF, which contributes to the formation of collaterals and to splanchnic vasodilation by stimulating the release of NO at the splanchnic arterioles and promoting splanchnic vasculogenesis [23]. Additionally, translocation of intestinal bacterial products and bacteria to the portal circulation may further contribute to upregulate eNOS and worsen splanchnic vasodilation [24] while enhancing the hepatic inflammation through pathogen-associated molecular patterns (PAMPs) [25]. Other vasodilators, including carbon monoxide [26], glucagon [27], and endocannabinoids [28], may aggravate splanchnic arterial vasodilation [29]. Importantly, the splanchnic vasodilation is not a primary factor in PH, but an adaptive response. Curing liver disease or transplantation results in deactivation of the splanchnic vasodilation.
In addition to structural abnormalities (fibrosis and disruption of liver vascular architecture), an increased liver vascular tone further increases IHVR and is thought to represent about 30–35% of the total IHVR. So, interfering with this component has the potential to decrease portal pressure by over 30%, which should be enough for preventing most complications from PH [3]. The hepatic sinusoid is a very complex network which is highly orchestrated by the liver sinusoidal endothelial cells (LSECs) due to their role as a vasoactive and permeabilized plate between the sinusoidal lumen and the parenchyma [30]. Increased liver vascular tone is mainly caused by LSECs dysfunction [31], which is characterized by decreased release of vasodilatory mediators such as nitric oxide (NO) [32], enhanced release, and response to endogenous vasoconstrictors (including the adrenergic system, renin-angiotensin system, prostanoids, cysteinyl-leukotrienes, thromboxane, and endothelins). Loss of LSECs fenestrations (a process known as pseudo-capillarization) marks an important step in the disease as it is responsible for the impaired oxygenation of hepatocytes, favored by accumulation of ECM components such as fibronectins, laminin, forming a basal membrane (absent in the normal liver sinusoids) that further impair the metabolic exchange between circulating blood and hepatocytes [33]. Oxidative stress, increased levels of asymmetrical dimethylarginine (ADMA) and decreased synthesis of tetrahydrobiopterin further decrease NO availability by uncoupling or inactivating endothelial NO-synthase (eNOS) and NO scavenging. Interfering with the chain of events leading to impair NO availability is therefore a relevant therapeutic target. Besides, dysfunctional LSECs become proliferative, pro-inflammatory, and pro-thrombotic and may contribute to vascular occlusion and parenchymal extinction, which has led to introducing anti-coagulants as potential treatments for PH.
The mechanisms leading to endothelial dysfunction are interconnected with those causing HSCs activation and fibrogenesis due to crosstalk between LSECs and HSCs, thus explaining how drugs improving endothelial dysfunction may also improve fibrogenesis (as it happens with statins and antioxidants). Moreover, some extrahepatic stimuli, specifically from the gut-liver axis, are also involved in activation of HSCs and explain the beneficial effects of Farnesoid X Receptor agonists, such as obeticholic acid. Hepatocytes finally undergo necroptosis; cell death stimulates the release of pro-inflammatory elements and contributes to HSC activation [10]. The vascular architecture is remodeled under mechanical and biochemical stimuli that increase liver stiffness, which may further enhance and perpetuate liver fibrosis in a winch-like process.
Under these driving forces, the liver undergoes dramatic changes, the percentage of fibrotic tissue increasing at the expense of the functional parenchymal mass. Disrupted microcirculation generates areas of the parenchyma that become less perfused and together with parenchymal extinction progressively worsens IHVR. As stated before, initial progression of PH is dominated by structural elements, while thereafter extrahepatic factors increasingly contribute to aggravate PH by increasing both the portal blood inflow and the hepatic vascular tone [34].
Therapeutic Agents for Liver Cirrhosis and Portal Hypertension
The complications of PH do not appear until the HVPG reaches values ≥ 10 mmHg [35]. During the compensated stage, the aim of the treatment of PH is to prevent decompensation. Protection is maximal when the HVPG is decreased below 10 mmHg, but significant protection is already obtained when HVPG decreases by over 10% of baseline [17]. After decompensation, a greater portal pressure reduction is required to protect from repeat or new complications from PH; protection is significant when achieving an HVPG decrease over 20% of baseline and almost optimal when HVPG decreases ≤ 12 mmHg. Baveno VII recommends a different approach for each sub-stage, starting in compensated cirrhosis without CSPH, followed by compensated patients with CSPH, decompensated patients without ascites, and decompensated patients with ascites.
Current drugs used for clinical management of LC patients are based on general therapy (lifestyle intervention), control of etiological factors, and use of drugs that counteract the splanchnic and systemic vasodilation. Up to now, this is achieved mainly by the oral administration of non-selective beta-blockers (NSBB) (like propranolol, and nadolol) for long-term therapy or somatostatin and analogs (mainly octreotide), and vasopressin derivatives such as terlipressin for short-term administration (for instance, for acute variceal bleeding and hepatorenal syndrome). The most recent therapeutic innovation is carvedilol, a NSBB that has intrinsic anti-adrenergic activity, this makes that it decreases portal venous inflow, but also the intrahepatic vascular tone, resulting in an enhanced reduction in HVPG even at low doses. This makes carvedilol the NSBB of choice [17, 18, 36]. Carvedilol can prevent ascites and clinical decompensation in patients with cirrhosis and PH and also markedly improves survival in meta-analysis. This has represented a change of paradigm in the treatment of PH, which is no longer limited to correct or prevent variceal hemorrhage, but to prevent all the complications of PH. The best use of these agents and current therapeutic strategies for PH management are established by the International Baveno VII Consensus conference [37], the European Association for the Study of the Liver (EASL) guideline for decompensated cirrhosis [38], and the American Association for the Study of Liver Disease (AASLD) [4, 39].
Intrahepatic Targets
In this section, we discuss promising strategies to reduce structural and functional alterations leading to IHVR; a detailed list of drugs that act on these processes is shown in Table 1.
Antifibrotics
Structural changes and fibrogenesis contribute to progression of LC and PH. Although there is still not any approved antifibrotic drug, numerous efforts have been spent in developing candidate agents over the last decade. These efforts made it possible to clarify targets and pathways involved in liver fibrogenesis. Transforming growth factor β1 (TGF1) is a major contributor to HSCs activation, for which many different strategies have been proposed in the past. Monoclonal antibodies such as fresolimumab, currently in phase II clinical trial, can neutralize all isoforms of TFGβ [106]. Lysil-oxidase-like 2 (LOXL2) [107] plays a critical role in collagen cross-linking, rendering it more difficult to reabsorb. Simtuzumab is a monoclonal antibody against LOXL2 that, despite the initial preclinical results, showed no effect in phase II RCTs for primary sclerosing cholangitis, NASH fibrosis or cirrhosis, and HCV/HIV [50, 107, 108]. However, targeting LOX family members, still appears a promising strategy [109]. Galectin-3, from the lectin family, is a regulator of mRNA splicing and is associated with HSCs activation and hepatocellular carcinoma [110]. Finally, belapectin, an inhibitor of galectin-3, showed encouraging preclinical but controversial results in clinical trials [111, 112].
Indirect strategies for HSCs deactivation have raised considerable attention; antidiabetic drugs showed antifibrotic activity by reducing oxidative stress, inflammation, and consequently HSCs activation, such as antibodies against leptin receptor [91], metformin [113] and glucagon-like peptide-1 (GLP-1) receptor agonists (liraglutide, semaglutide) [48, 90, 114]. GLP-1a is used to treat type II diabetes, a co-morbidity frequently present in patients with NASH and that has been shown to be a suitable therapy for NASH resolution [115]. Additionally, the GLP-2 receptor, such as teduglutide, showed to be involved in the control of HSCs activation in a preclinical study based on mice treated with a high fat diet [105]. Therefore, GLP-1 and GLP-2 could be good candidate targets for therapy. Taurine is an essential amino acid that showed a reduction in HSCs contraction and activation [116]. A small randomized clinical study suggested intrahepatic activity with a reduction of HPGV by ~ 10% in 58% of CSPH patients [117].
Other drugs also have pleiotropic effects, including antifibrotic activity. Peroxisome proliferation-activated receptors (PPAR-α, β, γ/δ) are nuclear hormone receptors which regulates cholesterol synthesis, whose deregulation is involved in inflammation, insulin resistance, and fibrogenesis. Re-activation of these receptors showed encouraging results in reversing liver fibrosis, inflammation, steatosis, and extrahepatic complications of chronic liver disease [93, 118,119,120,121,122,123]. Among the many drugs targeting some of the PPAR, the pan-PPAR agonist lanifibranor, an indole sulfonamide derivative, has attracted much attention, since on top of our studies demonstrating marked effects in experimental cirrhosis [94]. Moreover, clinical studies suggest that lanifibranor may promote fibrosis regression in patients with NASH, although further studies are needed to confirm a therapeutic benefit in patients with advanced fibrosis [124]. PPARγ may reduce splanchnic angiogenesis and formation of portosystemic collaterals, as suggested by preclinical studies using aleglitazar, a dual PPAR α/γ agonist [93]. Telmisartan, a PPARγ agonist with antagonist properties on angiotensisn II type 1 receptor (AT1R), ameliorated PH by reducing inflammation, fibrosis, and vascular remodeling in bile-duct ligation and TAA cirrhotic rat models [96].
Recently, the cannabinoid receptors CB1 and CB2, which mediate glucose and lipid metabolism and insulin signaling, and are overexpressed in hepatic fibrosis [125], have been proposed as potential targets for the treatment of PH and NASH [126].
Structural and functional changes are intimately linked. Strategies against functional alterations such as reversing the changes in LSECs phenotype could contribute to deactivation of HSCs via crosstalk. For instance, soluble guanylyl cyclase (sGC) which is highly expressed in most liver cells, causes vasodilation via cyclic guanosine monophosphate (cGMP) production [127, 128]. sGC is physiologically activated by NO to cause vasodilation. Moreover, sGC can be targeted directly by drugs that act as stimulators or activators of sGC. Preclinical studies showed encouraging results preventing LSECs capillarization and reducing HSCs activation [129,130,131]. Riociguat, an sGMP stimulator that appears safe in advanced cirrhosis [132], has shown significant reductions in portal pressure, fibrosis, necroinflammation, and sinusoidal remodeling in bile duct-ligated rats [128], and it is close to enter phase II clinical trials. An alternative to Riociguat, is IW-1973, which showed similar properties together with reduced hepatic steatosis in a NASH model [133, 134]. Additionally, the sGC activator BAY 60–2770 showed promising results in a toxic-cirrhotic rat model [135]. Recently, it has been proposed that the protein ProAgio, by inducing the integrinαvβ3-mediated HSCs apoptosis, may decrease liver fibrosis and reduce IHVR [101]. This type of approach acts on mechanosensing-dependent mechanisms that among others drive nuclear stretching and contribute to cell dysfunction as ECM stiffness increases with progressive liver fibrosis [102].
Thromboxane is a vasoconstrictor molecule which contributes to the increased vascular tone in advanced chronic liver disease (ACLD). Terutroban [78], an eicosanoid inhibitor that blocks thromboxane prostanoid receptor, reduced portal pressure by decreasing intrahepatic vascular resistance in experimental cirrhosis. However, ifetroban (a molecule with similar effects) was not found to modify these parameters in patients with cirrhosis treated for 3 months in a recent small RCT [136]. Finally, the dual cyclooxygenase-2 (COX2) soluble epoxide hydrolase inhibitor PTUPB was recently found to reduce liver fibrosis and PH in cirrhotic rats [104]. Previous experimental studies already shown that COX derived prostanoids modulate hepatic vascular tone in cirrhotic rats [137].
Anticoagulants
Occlusion of small hepatic veins as a result of endothelial injury is thought to cause parenchymal extinction contributing to tissue collapse and architectural distortion during the progression of cirrhosis [138]. This is in part the basis for proposing anticoagulants in patients with cirrhosis. Enoxaparin, a low molecular weight heparin like and rivaroxaban (a direct oral acting anticoagulant), decreases IHVR in preclinical studies in cirrhotic rat models with rats [95, 139]. In patients with cirrhosis on a waiting list for liver transplantation, 1-year treatment with enoxaparin prevented hepatic decompensation, decreased the episodes of portal vein thrombosis, decreased systemic inflammation and markers of bacterial translocation, and improved survival [140]. Finally, aspirin and other anti-platelet agents use was found to be associated with a decreased odds of hepatic fibrosis in patients at risk in a recent meta-analysis [141, 142].
Agents Increasing the Bioavailability of NO in the Hepatic Circulation
Statins
Statins are drugs with pleiotropic effects on vascular biology that go beyond their lipid lowering capacity. Statins have been shown to act on several pathways, including Rho/ROCK and KLF2 [143], causing antifibrotic, anti-inflammatory, antioxidant, anti-proliferative, anti-coagulant, and vasculoprotective effects in experimental animals with several forms of LC and markedly increasing NO availability [144]. In clinical trials, simvastatin has been shown to decrease HVPG, improve liver function, and improve survival after variceal hemorrhage [145,146,147]. Moreover, the addition of statins (simvastatin/atorvastatin) to standard NSBB (propranolol) treatment improves the HVPG response [148,149,150]. Ongoing trials are currently assessing if some potential effects of statin delaying disease progression, decompensation, and death noticed in epidemiological studies [147] can be confirmed in randomized controlled studies [151, 152]. Despite concerns regarding risk of hepatic or muscular toxicity in ACLD patients, their safety profile has been shown to be acceptable even in decompensated Child–Pugh patients when administered at low doses (10–20 mg/day of simvastatin) [153]. Targeted delivery to LSEC, tested in preclinical models, may further enhance efficacy while decreasing the risk of adverse events [154].
Phosphodiesterase-5 (PDE5) Inhibitors
PDE5 is an enzyme which converts vasodilating cGMP into its inactive form and is increased in the cirrhotic liver. Its modulation using PDE5-inhibitors may have potential in the treatment of ACLD, as highlighted in a recent review [155]. In the clinical setting, results have been mixed, with some studies showing no change in HVPG [59, 156], while others observed a decrease [41, 60]. This class of drugs might be studied in early stage cirrhosis, but not in more advanced patients due to its effects decreasing systemic arterial pressure, which may be deleterious [59, 157].
Tetrahydrobiopterin (BH4) is a cofactor for NO synthesis which is downregulated in cirrhotic livers. However, a trial of a synthetic analog of BH4, sapropterin, did not demonstrate any beneficial hemodynamic or functional effect over 2 weeks of treatment [40].
Antioxidants
LSECs inflammation and injury are accompanied by increased oxidative stress which contributes to LSECs capillarization. Oxidative stress via ROS production in all liver cells decreases the bioavailability of NO [158] by scavenging it to form peroxynitrate, contributing to increase the hepatic vascular tone. Strategies based on antioxidants have tested intravenous ascorbic acid, recombinant human manganese superoxide dismutase (rMnSOD), and dark chocolate. SOD activity is reduced in cirrhosis [159], and a preclinical study showed that rMnSOD administration to cirrhotic rats decreased IHVR, portal pressure, and liver fibrosis. It further abolished endothelial dysfunction without affecting the systemic circulation [83]. Flavonoids are polyphenolic secondary metabolites of plants and are commonly assumed with the regular diet. Dark chocolate is reach in flavonoids, has a powerful antioxidant activity and increases NO bioavailability [160], and attenuates the increase in portal pressure associated with post-prandial hyperemia in patients with cirrhosis [46]. Other strategies targeting oxidative stress were based on the inhibition of NADPH, such as GKT137831 [161], angiotensin blocking agents (discussed in functional targets below). Resveratrol, a natural anti-oxidant with multiple effects on vascular biology, has also been found to decrease portal pressure, decrease fibrosis, and improve NO availability in rats with experimental cirrhosis [47, 162].
Renin–Angiotensin–Aldosterone System (RAAS)
Inhibitors include angiotensin-converting-enzyme inhibitors (ACEIs), angiotensin-receptor blockers (ARBs), and aldosterone antagonists (AAs). In advanced cirrhosis, after developing a hyperkinetic circulation, RAAS is activated in response to splanchnic and systemic vasodilation and contributes to sodium and water retention. Moreover, angiotensin II is a powerful vasoconstrictor that in HSCs has profibrotic effects [163]. Spironolactone is key in the management of ascites [38] and contributes to decrease the HVPG [164]. Trials of ACEIs or ARBs are heterogeneous regarding population, study drug, dosage, and treatment time and include small cohorts. A meta-analysis of RAAS modulators suggests a benefit of ARBs/ACEIs in early disease (Child–Pugh A) but raises concerns in more advanced patients due to hypotension-related complications [163]. Additional approaches for acting on RAAS include the MasR agonist and Janus-kinase-2 (JAK2) inhibitor. MasR agonist is a non-peptide agonist of angiotensin [1,2,3,4,5,6,7] able to reduce portal pressure by upregulation of NO and regulation of Rho-kinase [87]. JAK2 regulates RHOA/Roh-kinase activation from angiotensin II extrahepatically. Initial studies suggest that ruxolitinib, an inhibitor of JAK1 and JAK2, may cause a reduction of HVPG in cirrhosis [165].
Farnesoid X Receptors (FXR) Agonists
The FXR is a nuclear transcription factor responsive to bile acids. It is normally expressed in the liver but also in the intestine and kidney [166]. In preclinical models, steroidal (obeticholic acid (OCA)) or non-steroidal (cilofexor) FXR agonists decreased portal pressure, fibrosis, bacterial translocation and improved endothelial dysfunction via increased NO release [62, 63, 167,168,169]. In the clinical setting, obeticholic acid administered for 7 days to alcohol-induced chronic liver disease patients caused an HVPG reduction in some patients [170]; however, this agent may precipitate decompensation and death in advanced cirrhotic patients [171]. Recently, PX20606 reduced PH and showed anti-angiogenic effect [63].
Anti-angiogenics
In PH progression, angiogenesis is stimulated by several stimuli. Impaired microcirculation due to distorted intrahepatic circulation and sinusoidal pseudo-capillarization, and fibrosis stimulates expression of hypoxia-inducible factors (HIFs) and inflammation, with ensuing release of vascular endothelial grow factor (VEGF) and other angiogenic factors (placental growth factor (P1GF) and platelet-derived growth factor (PDGF)). The elevated pressure causes LSECs stretching and phenotypic dysregulation and activation of the Raf/MEK/ERK pathway [22, 172]. Among the anti-angiogenic compounds, sorafenib [173], sunitinib [174], regorafenib [175], and bivanib [176] reduced portal pressure, splanchnic neovascularization, and portosystemic shunting. Sorafenib, first multikinase inhibitor demonstrated to improve overall survival of HCC patients [177], has an anti-angiogenic activity by blocking the autophosphorylation of several receptors’ tyrosine kinases such as VEGFR1, 2, and 3; PDGFRβ; c-Kit; and RET and by inhibiting Raf kinase isoforms [178]. Despite being very effective in experimental cirrhosis, when used clinically, it has shown controversial results [179].
Rho-Kinase Inhibitors
Rho-kinase (ROCK) activity is correlated with activation of HSCs and endothelial dysfunction [31]. A clinical trial of fasudil, a ROCK inhibitor, has demonstrated an acute decrease in HVPG. However, it also caused systemic side-effects by significantly decreasing arterial pressure and systemic vascular resistance [180]. Future strategies may employ special delivery methods directly targeting HSCs [99].
Endothelin Antagonists
Endothelin is a potent vasoconstrictor molecule produced by the endothelium, which is increased in patients with cirrhosis. Moreover, in cirrhosis, there may be a change in the distribution and responsiveness of the different endothelin receptors [181]. This might explain the mixed results obtained so far with non-selective antagonists in clinical trials. While dual endothelin antagonists had beneficial effects on HRS and POPH [56], they did not significantly reduce HVPG [74, 182]. Specifically, blocking the ETA receptor might be a more promising strategy [43], and currently there is an ongoing open label trial of ambrisentan [42].
Novel Extrahepatic Targets
There are promising strategies to modulate extrahepatic targets that may influence PH, either by means of decreasing the portal blood flow or by modulating extrahepatic mechanisms that finally influence liver disease (Table 2).
Vasodilatatory and Antifibrotic
Relaxin is a vasodilatory and antifibrotic protein that is produced mainly by the ovary and breast in females and in the prostate in males. Important vasodilatory and antifibrotic properties have already been shown in pre-clinical studies [192,193,194], including a decrease in portal pressure. However, a small clinical trial with IV serelaxin, a human relaxin-2 analog, failed to show any decrease in HVPG after a 2-h infusion [183]. Innovative delivery methods may perhaps hold promise for serelaxin as a therapeutic agent [195].
Human Albumin
Serum albumin is produced by the liver and is markedly decreased in decompensated LC. Moreover, it is structurally and functionally abnormal, jeopardizing its antioxidant, scavenging, and immunomodulatory non-oncotic properties [196]. Consequently, albumin is considered a potential agent for the treatment of decompensated cirrhosis. A clinical trial of long-term treatment with weekly albumin infusion showed efficacy and improved survival [197]. However, there are concerns with the possibility of causing volume overload, especially in patients with NASH that frequently have associated cardiovascular disease. Such adverse effects were documented in a recent trial aimed at increasing albumin levels above 30 g/L in patients with decompensated cirrhosis [198]. A recent preclinical study in cirrhotic rats exploring the potential effect of the ROCK inhibitor coupled to a peptide-modified albumin carrier showed a reduced portal pressure and enhanced renal perfusion [99].
Targeting Gut-Liver Axis
Bacterial translocation is a frequent event in decompensated cirrhosis with ascites. Translocation may cause endotoxemia and may be preceded by translocation of bacterial products, DAMPs and PAMPs, with associated pro-inflammatory responses that may enhance progression of the liver disease, cause systemic inflammation, and in the case of bacterial translocation result in sepsis or severe bacterial infections, such as spontaneous bacterial peritonitis.
The increased levels of endotoxin and pro-inflammatory cytokines contribute to endothelial dysfunction and vasoconstriction via ET-1 and vasoconstrictory prostanoids [199]. Limiting bacterial overgrowth and translocation, by means of non-absorbable antibiotics, NSBBs (that accelerate intestinal transit time and decrease intestinal permeability) and fecal transplantation may contribute to reduce liver inflammation and injury in LC [200]. Rifaximin [201] did not significantly decreased HVPG in patients with LC but decreased complications from PH [202]. Although rifaximin efficacy remains controversial [203], more studies are ongoing to clarify its application in LC [204]. Another approach to reduce bacterial overgrowth and translocation is administering probiotics. However, there is no evidence for now that probiotics have an impact on PH [205].
Cell Therapy: Parenchymal Extinction
Liver regeneration and fibrogenesis occur in parallel in chronic liver disease, although if liver injury continues, the process leads to the formation of cirrhosis, with regenerating liver nodules surrounded by fibrotic tracts, with a profoundly disturbed liver vascular architecture and IHVR. LSEC as well as quiescent HSCs and their crosstalk are important elements in support of the regenerative process. Cell therapy based on bone marrow transplantation was a promising approach to stimulate regeneration [206]. Bone marrow stem cells are mononucleated cells that include many different types of stem cells such as hematopoietic stem cells and mesenchymal stem cells. Hematopoietic cells can differentiate into hepatocytes, and a recent study showed improvements in liver function in patients with LC [207, 208]. Mesenchymal stem cells are easier to obtain and can exert different functions and present low levels of immunogenicity. A recent clinical trial in phase II showed that administration of mesenchymal stem cells improved liver fibrosis and Child–Pugh score in patients with alcoholic cirrhosis [209]. A novel approach used placental stem cells, with promising results in CCl4-cirrhotic rats [210].
A recent clinical trial studied the infusion of mature autologous monocyte-derived macrophages in patients with compensated cirrhosis with a change in MELD score at 90-day as primary outcome [98]. Cell therapy has also been shown to regulate microbial growth and function in the context of fibrosis [97]. This may be a promising opportunity for novel antifibrotic therapies.
Conclusions
Knowledge of the PH mechanism underwent continuous development since the concept of splanchnic vasodilation contributing to increase portal pressure was introduced in the 1980s, providing for the first time a rationale for using splanchnic vasoconstrictors for the complications of PH. Since then, understanding the molecular mechanisms of disease progression and careful clinical observations have allowed identifying multiple targets to improve treatment and patients’ management. This has been paramount to ameliorate short and long-term prognosis of patients with cirrhosis, for which we now have disease-modifying therapies. While the current therapy and management are mainly focused on the prevention of the complications of PH and the decompensation of cirrhosis, the novel strategies are centered in reducing the IHVR and in preventing/reversing fibrosis.
Future therapy for PH is likely to involve multiple strategies aimed at acting on different mechanisms that act together and frequently synergistically promoting cirrhosis progression. New therapies that have shown promising results include the following: (i) statins, PPAR agonists, GLP-1 agonists, sGC activators and stimulators, enoxaparin, ribaroxaban, and dual or pan-FXR receptor agonists. Multiple RCTs are in progress, which makes the field of PH and cirrhosis a fascinating one, where rapid changes in management and prognosis are expected soon.
Data Availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol. 2013;7(2):141–55.
Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71.
Bosch J, Groszmann RJ, Shah VH. Evolution in the understanding of the pathophysiological basis of portal hypertension: how changes in paradigm are leading to successful new treatments. J Hepatol. 2015;62(1 Suppl):S121–30.
Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–35.
Garcia-Tsao G, Groszmann RJ, Fisher RL, Conn HO, Atterbury CE, Glickman M. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology. 1985;5(3):419–24.
Baiges A, Hernandez-Gea V, Bosch J. Pharmacologic prevention of variceal bleeding and rebleeding. Hepatol Int. 2018;12(Suppl 1):68–80.
Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Dig Dis. 2016;34(4):382–6.
Baffy G, Bosch J. Overlooked subclinical portal hypertension in non-cirrhotic NAFLD: is it real and how to measure it? J Hepatol. 2022;76(2):458–63.
Bonis PA, Friedman SL, Kaplan MM. Is liver fibrosis reversible? N Engl J Med. 2001;344(6):452–4.
Kisseleva T, Brenner D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. 2021;18(3):151–66.
Berzigotti A, Albillos A, Villanueva C, Genesca J, Ardevol A, Augustin S, et al. Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: the SportDiet study. Hepatology. 2017;65(4):1293–305.
Papaluca T, Gow P. Terlipressin: Current and emerging indications in chronic liver disease. J Gastroenterol Hepatol. 2018;33(3):591–8.
Abraldes JG, Bosch J. Somatostatin and analogues in portal hypertension. Hepatology. 2002;35(6):1305–12.
Seo YS, Park SY, Kim MY, Kim JH, Park JY, Yim HJ, et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology. 2014;60(3):954–63.
Villanueva C, Albillos A, Genesca J, Abraldes JG, Calleja JL, Aracil C, et al. Development of hyperdynamic circulation and response to beta-blockers in compensated cirrhosis with portal hypertension. Hepatology. 2016;63(1):197–206.
Lo GH, Chen WC, Wang HM, Lee CC. Controlled trial of ligation plus nadolol versus nadolol alone for the prevention of first variceal bleeding. Hepatology. 2010;52(1):230–7.
Villanueva C, Albillos A, Genesca J, Garcia-Pagan JC, Calleja JL, Aracil C, et al. beta blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393(10181):1597–608.
Villanueva C, Torres F, Sarin SK, Shah HA, Tripathi D, Brujats A, et al. Carvedilol reduces the risk of decompensation and mortality in patients with compensated cirrhosis in a competing-risk meta-analysis. J Hepatol. 2022;77(4):1014–25. https://doi.org/10.1016/j.jhep.2022.05.021.
Maeso-Diaz R, Ortega-Ribera M, Fernandez-Iglesias A, Hide D, Munoz L, Hessheimer AJ, et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell. 2018;17(6):e12829.
Angeli P, Fernandez-Varo G, Dalla Libera V, Fasolato S, Galioto A, Arroyo V, et al. The role of nitric oxide in the pathogenesis of systemic and splanchnic vasodilation in cirrhotic rats before and after the onset of ascites. Liver Int. 2005;25(2):429–37.
Colle IO, De Vriese AS, Van Vlierberghe HR, Lameire NH, De Vos MM. Vascular hyporesponsiveness in the mesenteric artery of anaesthetized rats with cirrhosis and portal hypertension: an in-vivo study. Eur J Gastroenterol Hepatol. 2004;16(2):139–45.
Fernandez M, Semela D, Bruix J, Colle I, Pinzani M, Bosch J. Angiogenesis in liver disease. J Hepatol. 2009;50(3):604–20.
Gana JC, Serrano CA, Ling SC. Angiogenesis and portal-systemic collaterals in portal hypertension. Ann Hepatol. 2016;15(3):303–13.
Gracia-Sancho J, Caparros E, Fernandez-Iglesias A, Frances R. Role of liver sinusoidal endothelial cells in liver diseases. Nat Rev Gastroenterol Hepatol. 2021;18(6):411–31.
Casulleras M, Zhang IW, Lopez-Vicario C, Claria J. Leukocytes, systemic inflammation and immunopathology in acute-on-chronic liver failure. Cells. 2020;9(12):2632. https://doi.org/10.3390/cells9122632.
Fernandez M, Bonkovsky HL. Increased heme oxygenase-1 gene expression in liver cells and splanchnic organs from portal hypertensive rats. Hepatology. 1999;29(6):1672–9.
Kravetz D, Bosch J, Arderiu MT, Pizcueta MP, Casamitjana R, Rivera F, et al. Effects of somatostatin on splanchnic hemodynamics and plasma glucagon in portal hypertensive rats. Am J Physiol. 1988;254(3 Pt 1):G322–8.
Batkai S, Jarai Z, Wagner JA, Goparaju SK, Varga K, Liu J, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7(7):827–32.
Gatta A, Bolognesi M, Merkel C. Vasoactive factors and hemodynamic mechanisms in the pathophysiology of portal hypertension in cirrhosis. Mol Aspects Med. 2008;29(1–2):119–29.
Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol. 2016;65(3):608–17.
Gracia-Sancho J, Marrone G, Fernandez-Iglesias A. Hepatic microcirculation and mechanisms of portal hypertension. Nat Rev Gastroenterol Hepatol. 2019;16(4):221–34.
Zafra C, Abraldes JG, Turnes J, Berzigotti A, Fernandez M, Garca-Pagan JC, et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology. 2004;126(3):749–55.
Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127(6 Pt 2):2037–48.
Engelmann C, Claria J, Szabo G, Bosch J, Bernardi M. Pathophysiology of decompensated cirrhosis: portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol. 2021;75(Suppl 1):S49–66.
Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6(10):573–82.
Bosch J. Carvedilol for portal hypertension in patients with cirrhosis. Hepatology. 2010;51(6):2214–8.
de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VIIF. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–74.
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–60.
Bunchorntavakul C, Reddy KR. Pharmacologic management of portal hypertension. Clin Liver Dis. 2019;23(4):713–36.
Reverter E, Mesonero F, Seijo S, Martinez J, Abraldes JG, Penas B, et al. Effects of sapropterin on portal and systemic hemodynamics in patients with cirrhosis and portal hypertension: a bicentric double-blind placebo-controlled study. Am J Gastroenterol. 2015;110(7):985–92.
Kreisel W, Deibert P, Kupcinskas L, Sumskiene J, Appenrodt B, Roth S, et al. The phosphodiesterase-5-inhibitor udenafil lowers portal pressure in compensated preascitic liver cirrhosis. A dose-finding phase-II-study. Dig Liver Dis. 2015;47(2):144–50.
Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74.
Zipprich A, Gittinger F, Winkler M, Dollinger MM, Ripoll C. Effect of ET-A blockade on portal pressure and hepatic arterial perfusion in patients with cirrhosis: a proof of concept study. Liver Int. 2021;41(3):554–61.
Tofteng F, Larsen FS. The effect of indomethacin on intracranial pressure, cerebral perfusion and extracellular lactate and glutamate concentrations in patients with fulminant hepatic failure. J Cereb Blood Flow Metab. 2004;24(7):798–804.
Ansley JD, Isaacs JW, Rikkers LF, Kutner MH, Nordlinger BM, Rudman D. Quantitative tests of nitrogen metabolism in cirrhosis: relation to other manifestations of liver disease. Gastroenterology. 1978;75(4):570–9.
De Gottardi A, Berzigotti A, Seijo S, D’Amico M, Thormann W, Abraldes JG, et al. Postprandial effects of dark chocolate on portal hypertension in patients with cirrhosis: results of a phase 2, double-blind, randomized controlled trial. Am J Clin Nutr. 2012;96(3):584–90.
Ma Z, Sheng L, Li J, Qian J, Wu G, Wang Z, et al. Resveratrol alleviates hepatic fibrosis in associated with decreased endoplasmic reticulum stress-mediated apoptosis and inflammation. Inflammation. 2022;45(2):812–23.
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–24.
Berzigotti A, Bellot P, De Gottardi A, Garcia-Pagan JC, Gagnon C, Spenard J, et al. NCX-1000, a nitric oxide-releasing derivative of UDCA, does not decrease portal pressure in patients with cirrhosis: results of a randomized, double-blind, dose-escalating study. Am J Gastroenterol. 2010;105(5):1094–101.
Harrison SA, Abdelmalek MF, Caldwell S, Shiffman ML, Diehl AM, Ghalib R, et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology. 2018;155(4):1140–53.
Artru F, Louvet A, Ruiz I, Levesque E, Labreuche J, Ursic-Bedoya J, et al. Liver transplantation in the most severely ill cirrhotic patients: a multicenter study in acute-on-chronic liver failure grade 3. J Hepatol. 2017;67(4):708–15.
Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, et al. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374(9699):1423–31.
Harrison SA, Wong VW, Okanoue T, Bzowej N, Vuppalanchi R, Younes Z, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: results from randomized phase III STELLAR trials. J Hepatol. 2020;73(1):26–39.
Paton A, Saunders JB. ABC of alcohol. Asking the right questions. Br Med J (Clin Res Ed). 1981;283(6304):1458–9.
Anstee QM, Neuschwander-Tetri BA, Wong VW, Abdelmalek MF, Younossi ZM, Yuan J, et al. Cenicriviroc for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis: AURORA Phase 3 study design. Contemp Clin Trials. 2020;89:105922.
Sitbon O, Bosch J, Cottreel E, Csonka D, de Groote P, Hoeper MM, et al. Macitentan for the treatment of portopulmonary hypertension (PORTICO): a multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet Respir Med. 2019;7(7):594–604.
Agasti AK, Mahajan AU, Phadke AY, Nathani PJ, Sawant P. Comparative randomized study on efficacy of losartan versus propranolol in lowering portal pressure in decompensated chronic liver disease. J Dig Dis. 2013;14(5):266–71.
Hernandez-Guerra M, Garcia-Pagan JC, Turnes J, Bellot P, Deulofeu R, Abraldes JG, et al. Ascorbic acid improves the intrahepatic endothelial dysfunction of patients with cirrhosis and portal hypertension. Hepatology. 2006;43(3):485–91.
Tandon P, Inayat I, Tal M, Spector M, Shea M, Groszmann RJ, et al. Sildenafil has no effect on portal pressure but lowers arterial pressure in patients with compensated cirrhosis. Clin Gastroenterol Hepatol. 2010;8(6):546–9.
Deibert P, Schumacher YO, Ruecker G, Opitz OG, Blum HE, Rossle M, et al. Effect of vardenafil, an inhibitor of phosphodiesterase-5, on portal haemodynamics in normal and cirrhotic liver – results of a pilot study. Aliment Pharmacol Ther. 2006;23(1):121–8.
Mookerjee R, Rosselli M, Pieri G, Beecher-Jones T, Hooshmand-Rad R, Chouhan M, et al. O15 Effects of the FXR agonist obeticholic acid on hepatic venous pressure gradient (HVPG) in alcoholic cirrhosis: a proof of concept phase 2a study. J Hepatol. 2014;60(1):S7–8.
Schwabl P, Hambruch E, Budas GR, Supper P, Burnet M, Liles JT, et al. The non-steroidal FXR agonist cilofexor improves portal hypertension and reduces hepatic fibrosis in a rat NASH model. Biomedicines. 2021;9(1):60. https://doi.org/10.3390/biomedicines9010060.
Schwabl P, Hambruch E, Seeland BA, Hayden H, Wagner M, Garnys L, et al. The FXR agonist PX20606 ameliorates portal hypertension by targeting vascular remodelling and sinusoidal dysfunction. J Hepatol. 2017;66(4):724–33.
Chong LW, Hsu YC, Lee TF, Lin Y, Chiu YT, Yang KC, et al. Fluvastatin attenuates hepatic steatosis-induced fibrogenesis in rats through inhibiting paracrine effect of hepatocyte on hepatic stellate cells. BMC Gastroenterol. 2015;15:22.
Biecker E, Trebicka J, Kang A, Hennenberg M, Sauerbruch T, Heller J. Treatment of bile duct-ligated rats with the nitric oxide synthase transcription enhancer AVE 9488 ameliorates portal hypertension. Liver Int. 2008;28(3):331–8.
Duong HT, Dong Z, Su L, Boyer C, George J, Davis TP, et al. The use of nanoparticles to deliver nitric oxide to hepatic stellate cells for treating liver fibrosis and portal hypertension. Small. 2015;11(19):2291–304.
Brusilovskaya K, Konigshofer P, Lampach D, Szodl A, Supper P, Bauer D, et al. Soluble guanylyl cyclase stimulation and phosphodiesterase-5 inhibition improve portal hypertension and reduce liver fibrosis in bile duct-ligated rats. United Eur Gastroenterol J. 2020;8(10):1174–85.
Zhang R, Chen J, Liu D, Wang Y. Urotensin II receptor antagonist reduces hepatic resistance and portal pressure through enhanced eNOS-dependent HSC vasodilatation in CCl4-induced cirrhotic rats. Front Med. 2019;13(3):398–408.
Feng HQ, Weymouth ND, Rockey DC. Endothelin antagonism in portal hypertensive mice: implications for endothelin receptor-specific signaling in liver disease. Am J Physiol Gastrointest Liver Physiol. 2009;297(1):G27-33.
van der Graaff D, Chotkoe S, De Winter B, De Man J, Casteleyn C, Timmermans JP, et al. Vasoconstrictor antagonism improves functional and structural vascular alterations and liver damage in rats with early NAFLD. JHEP Rep. 2022;4(2):100412.
Kojima H, Yamao J, Tsujimoto T, Uemura M, Takaya A, Fukui H. Mixed endothelin receptor antagonist, SB209670, decreases portal pressure in biliary cirrhotic rats in vivo by reducing portal venous system resistance. J Hepatol. 2000;32(1):43–50.
Culshaw G, Binnie D, Dhaun N, Hadoke P, Bailey M, Webb DJ. The acute pressure natriuresis response is suppressed by selective ETA receptor blockade. Clin Sci (Lond). 2021;136(1):15–28. https://doi.org/10.1042/CS20210937.
Fox BM, Becker BK, Loria AS, Hyndman KA, Jin C, Clark H, et al. Acute pressor response to psychosocial stress is dependent on endothelium-derived endothelin-1. J Am Heart Assoc. 2018;7(4):e007863. https://doi.org/10.1161/JAHA.117.007863.
Tripathi D, Therapondos G, Ferguson JW, Newby DE, Webb DJ, Hayes PC. Endothelin-1 contributes to maintenance of systemic but not portal haemodynamics in patients with early cirrhosis: a randomised controlled trial. Gut. 2006;55(9):1290–5.
Eizayaga FX, Aguejouf O, Belon P, Doutremepuich C. Platelet aggregation in portal hypertension and its modification by ultra-low doses of aspirin. Pathophysiol Haemost Thromb. 2005;34(1):29–34.
Tai Y, Zhao C, Zhang L, Tang S, Jia X, Tong H, et al. Celecoxib reduces hepatic vascular resistance in portal hypertension by amelioration of endothelial oxidative stress. J Cell Mol Med. 2021;25(22):10389–402.
Laleman W, Van Landeghem L, Van der Elst I, Zeegers M, Fevery J, Nevens F. Nitroflurbiprofen, a nitric oxide-releasing cyclooxygenase inhibitor, improves cirrhotic portal hypertension in rats. Gastroenterology. 2007;132(2):709–19.
Rosado E, Rodriguez-Vilarrupla A, Gracia-Sancho J, Tripathi D, Garcia-Caldero H, Bosch J, et al. Terutroban, a TP-receptor antagonist, reduces portal pressure in cirrhotic rats. Hepatology. 2013;58(4):1424–35.
Steib CJ, Bilzer M, op den Winkel M, Pfeiler S, Hartmann AC, Hennenberg M, et al. Treatment with the leukotriene inhibitor montelukast for 10 days attenuates portal hypertension in rat liver cirrhosis. Hepatology. 2010;51(6):2086–96.
Murad HA, Gazzaz ZJ, Ali SS, Ibraheem MS. Candesartan, rather than losartan, improves motor dysfunction in thioacetamide-induced chronic liver failure in rats. Braz J Med Biol Res. 2017;50(11):e6665.
Wei L, Yang J, Wang M, Xu SN, Liang HM, Zhou Q. Sodium ferulate lowers portal pressure in rats with secondary biliary cirrhosis through the RhoA/Rho-kinase signaling pathway: a preliminary study. Int J Mol Med. 2014;34(5):1257–67.
Garcia-Caldero H, Rodriguez-Vilarrupla A, Gracia-Sancho J, Divi M, Lavina B, Bosch J, et al. Tempol administration, a superoxide dismutase mimetic, reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2011;54(4):660–5.
Guillaume M, Rodriguez-Vilarrupla A, Gracia-Sancho J, Rosado E, Mancini A, Bosch J, et al. Recombinant human manganese superoxide dismutase reduces liver fibrosis and portal pressure in CCl4-cirrhotic rats. J Hepatol. 2013;58(2):240–6. https://doi.org/10.1016/j.jhep.2012.09.010.
Rahman MM, Muse AY, Khan D, Ahmed IH, Subhan N, Reza HM, et al. Apocynin prevented inflammation and oxidative stress in carbon tetra chloride induced hepatic dysfunction in rats. Biomed Pharmacother. 2017;92:421–8.
Vilaseca M, Garcia-Caldero H, Lafoz E, Ruart M, Lopez-Sanjurjo CI, Murphy MP, et al. Mitochondria-targeted antioxidant mitoquinone deactivates human and rat hepatic stellate cells and reduces portal hypertension in cirrhotic rats. Liver Int. 2017;37(7):1002–12.
Gunarathne LS, Angus PW, Herath CB. Blockade of Mas receptor or Mas-related G-protein coupled receptor type D reduces portal pressure in cirrhotic but not in non-cirrhotic portal hypertensive rats. Front Physiol. 2019;10:1169.
Klein S, Herath CB, Schierwagen R, Grace J, Haltenhof T, Uschner FE, et al. Hemodynamic Effects of the Non-Peptidic Angiotensin-(1–7) Agonist AVE0991 in Liver Cirrhosis. PLoS ONE. 2015;10(9):e0138732.
Klein S, Kleine CE, Pieper A, Granzow M, Gautsch S, Himmit M, et al. TGR(mREN2)27 rats develop non-alcoholic fatty liver disease-associated portal hypertension responsive to modulations of Janus-kinase 2 and Mas receptor. Sci Rep. 2019;9(1):11598.
Hsu SJ, Wang SS, Huo TI, Lee FY, Huang HC, Chang CC, et al. The impact of spironolactone on the severity of portal-systemic collaterals and hepatic encephalopathy in cirrhotic rats. J Pharmacol Exp Ther. 2015;355(1):117–24.
de Mesquita FC, Guixe-Muntet S, Fernandez-Iglesias A, Maeso-Diaz R, Vila S, Hide D, et al. Liraglutide improves liver microvascular dysfunction in cirrhosis: evidence from translational studies. Sci Rep. 2017;7(1):3255.
Delgado MG, Gracia-Sancho J, Marrone G, Rodriguez-Vilarrupla A, Deulofeu R, Abraldes JG, et al. Leptin receptor blockade reduces intrahepatic vascular resistance and portal pressure in an experimental model of rat liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2013;305(7):G496-502.
Mandala A, Chen WJ, Armstrong A, Malhotra MR, Chavalmane S, McCommis KS, et al. PPARalpha agonist fenofibrate attenuates iron-induced liver injury in mice by modulating the Sirt3 and beta-catenin signaling. Am J Physiol Gastrointest Liver Physiol. 2021;321(4):G262–9.
Tsai HC, Li TH, Huang CC, Huang SF, Liu RS, Yang YY, et al. Beneficial effects of the peroxisome proliferator-activated receptor alpha/gamma agonist aleglitazar on progressive hepatic and splanchnic abnormalities in cirrhotic rats with portal hypertension. Am J Pathol. 2018;188(7):1608–24.
Boyer-Diaz Z, Aristu-Zabalza P, Andres-Rozas M, Robert C, Ortega-Ribera M, Fernandez-Iglesias A, et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J Hepatol. 2021;74(5):1188–99.
Cerini F, Vilaseca M, Lafoz E, Garcia-Irigoyen O, Garcia-Caldero H, Tripathi DM, et al. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J Hepatol. 2016;64(4):834–42.
Zheng L, Zhao Z, Lin J, Li H, Wu G, Qi X, et al. Telmisartan relieves liver fibrosis and portal hypertension by improving vascular remodeling and sinusoidal dysfunction. Eur J Pharmacol. 2022;915:174713.
Lett MJ, Mehta H, Keogh A, Jaeger T, Jacquet M, Powell K, et al. Stimulatory MAIT cell antigens reach the circulation and are efficiently metabolised and presented by human liver cells. Gut. 2022;71(12):2526–38. https://doi.org/10.1136/gutjnl-2021-324478.
Brennan PN, MacMillan M, Manship T, Moroni F, Glover A, Graham C, et al. Study protocol: a multicentre, open-label, parallel-group, phase 2, randomised controlled trial of autologous macrophage therapy for liver cirrhosis (MATCH). BMJ Open. 2021;11(11):e053190.
Klein S, Frohn F, Magdaleno F, Reker-Smit C, Schierwagen R, Schierwagen I, et al. Rho-kinase inhibitor coupled to peptide-modified albumin carrier reduces portal pressure and increases renal perfusion in cirrhotic rats. Sci Rep. 2019;9(1):2256.
Lee KC, Hsu WF, Hsieh YC, Chan CC, Yang YY, Huang YH, et al. Dabigatran reduces liver fibrosis in thioacetamide-injured rats. Dig Dis Sci. 2019;64(1):102–12.
Turaga RC, Satyanarayana G, Sharma M, Yang JJ, Wang S, Liu C, et al. Targeting integrin alphavbeta3 by a rationally designed protein for chronic liver disease treatment. Commun Biol. 2021;4(1):1087.
Guixe-Muntet S, Ortega-Ribera M, Wang C, Selicean S, Andreu I, Kechagia JZ, et al. Nuclear deformation mediates liver cell mechanosensing in cirrhosis. JHEP Rep. 2020;2(5):100145.
Gentilini P, Laffi G, La Villa G, Romanelli RG, Buzzelli G, Casini-Raggi V, et al. Long course and prognostic factors of virus-induced cirrhosis of the liver. Am J Gastroenterol. 1997;92(1):66–72.
Zhao Z, Zhang C, Lin J, Zheng L, Li H, Qi X, et al. COX-2/sEH dual inhibitor PTUPB alleviates CCl 4 -induced liver fibrosis and portal hypertension. Front Med (Lausanne). 2021;8:761517.
Fuchs S, Yusta B, Baggio LL, Varin EM, Matthews D, Drucker DJ. Loss of Glp2r signaling activates hepatic stellate cells and exacerbates diet-induced steatohepatitis in mice. JCI Insight. 2020;5(8).
Formenti SC, Hawtin RE, Dixit N, Evensen E, Lee P, Goldberg JD, et al. Baseline T cell dysfunction by single cell network profiling in metastatic breast cancer patients. J Immunother Cancer. 2019;7(1):177.
Muir AJ, Levy C, Janssen HLA, Montano-Loza AJ, Shiffman ML, Caldwell S, et al. Simtuzumab for primary sclerosing cholangitis: phase 2 study results with insights on the natural history of the disease. Hepatology. 2019;69(2):684–98.
Meissner EG, McLaughlin M, Matthews L, Gharib AM, Wood BJ, Levy E, et al. Simtuzumab treatment of advanced liver fibrosis in HIV and HCV-infected adults: results of a 6-month open-label safety trial. Liver Int. 2016;36(12):1783–92.
Chen W, Yang A, Jia J, Popov YV, Schuppan D, You H. Lysyl Oxidase (LOX) Family members: rationale and their potential as therapeutic targets for liver fibrosis. Hepatology. 2020;72(2):729–41.
Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol. 2013;19(47):8831–49.
Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS ONE. 2013;8(10):e75361.
Chalasani N, Abdelmalek MF, Garcia-Tsao G, Vuppalanchi R, Alkhouri N, Rinella M, et al. Effects of belapectin, an inhibitor of galectin-3, in patients with nonalcoholic steatohepatitis with cirrhosis and portal hypertension. Gastroenterology. 2020;158(5):1334-45.e5.
Tripathi DM, Erice E, Lafoz E, Garcia-Caldero H, Sarin SK, Bosch J, et al. Metformin reduces hepatic resistance and portal pressure in cirrhotic rats. Am J Physiol Gastrointest Liver Physiol. 2015;309(5):G301–9.
Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne). 2019;10:155.
Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–90.
Liang J, Deng X, Lin ZX, Zhao LC, Zhang XL. Attenuation of portal hypertension by natural taurine in rats with liver cirrhosis. World J Gastroenterol. 2009;15(36):4529–37.
Schwarzer R, Kivaranovic D, Mandorfer M, Paternostro R, Wolrab D, Heinisch B, et al. Randomised clinical study: the effects of oral taurine 6 g/day vs placebo on portal hypertension. Aliment Pharmacol Ther. 2018;47(1):86–94.
Iwaisako K, Haimerl M, Paik YH, Taura K, Kodama Y, Sirlin C, et al. Protection from liver fibrosis by a peroxisome proliferator-activated receptor delta agonist. Proc Natl Acad Sci U S A. 2012;109(21):E1369–76.
Rodriguez-Vilarrupla A, Lavina B, Garcia-Caldero H, Russo L, Rosado E, Roglans N, et al. PPARalpha activation improves endothelial dysfunction and reduces fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2012;56(5):1033–9.
Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58(6):1941–52.
Haczeyni F, Wang H, Barn V, Mridha AR, Yeh MM, Haigh WG, et al. The selective peroxisome proliferator-activated receptor-delta agonist seladelpar reverses nonalcoholic steatohepatitis pathology by abrogating lipotoxicity in diabetic obese mice. Hepatol Commun. 2017;1(7):663–74.
Jain MR, Giri SR, Bhoi B, Trivedi C, Rath A, Rathod R, et al. Dual PPARalpha/gamma agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int. 2018;38(6):1084–94.
Daniels SJ, Leeming DJ, Detlefsen S, Bruun MF, Hjuler ST, Henriksen K, et al. Biochemical and histological characterisation of an experimental rodent model of non-alcoholic steatohepatitis - effects of a peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist and a glucagon-like peptide-1 analogue. Biomed Pharmacother. 2019;111:926–33.
Francque SM, Bedossa P, Ratziu V, Anstee QM, Bugianesi E, Sanyal AJ, et al. A randomized, controlled trial of the Pan-PPAR agonist lanifibranor in NASH. N Engl J Med. 2021;385(17):1547–58.
Coch L, Mejias M, Berzigotti A, Garcia-Pras E, Gallego J, Bosch J, et al. Disruption of negative feedback loop between vasohibin-1 and vascular endothelial growth factor decreases portal pressure, angiogenesis, and fibrosis in cirrhotic rats. Hepatology. 2014;60(2):633–47.
Bazwinsky-Wutschke I, Zipprich A, Dehghani F. Endocannabinoid system in hepatic glucose metabolism, fatty liver disease, and cirrhosis. Int J Mol Sci. 2019;20(10):2516. https://doi.org/10.3390/ijms20102516.
Theilig F, Bostanjoglo M, Pavenstadt H, Grupp C, Holland G, Slosarek I, et al. Cellular distribution and function of soluble guanylyl cyclase in rat kidney and liver. J Am Soc Nephrol. 2001;12(11):2209–20.
Schwabl P, Brusilovskaya K, Supper P, Bauer D, Konigshofer P, Riedl F, et al. The soluble guanylate cyclase stimulator riociguat reduces fibrogenesis and portal pressure in cirrhotic rats. Sci Rep. 2018;8(1):9372.
Xie G, Wang X, Wang L, Wang L, Atkinson RD, Kanel GC, et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology. 2012;142(4):918-27.e6.
DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015;61(5):1740–6.
Deleve LD, Wang X, Guo Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology. 2008;48(3):920–30.
Saleh S, Becker C, Frey R, Muck W. Population pharmacokinetics of single-dose riociguat in patients with renal or hepatic impairment. Pulm Circ. 2016;6(Suppl 1):S75-85.
Flores-Costa R, Alcaraz-Quiles J, Titos E, Lopez-Vicario C, Casulleras M, Duran-Guell M, et al. The soluble guanylate cyclase stimulator IW-1973 prevents inflammation and fibrosis in experimental non-alcoholic steatohepatitis. Br J Pharmacol. 2018;175(6):953–67.
Flores-Costa R, Duran-Guell M, Casulleras M, Lopez-Vicario C, Alcaraz-Quiles J, Diaz A, et al. Stimulation of soluble guanylate cyclase exerts antiinflammatory actions in the liver through a VASP/NF-kappaB/NLRP3 inflammasome circuit. Proc Natl Acad Sci U S A. 2020;117(45):28263–74.
Knorr A, Hirth-Dietrich C, Alonso-Alija C, Harter M, Hahn M, Keim Y, et al. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60–2770 in experimental liver fibrosis. Arzneimittelforschung. 2008;58(2):71–80.
ClinicalTrials.gov [Internet]. Study to assess safety and efficacy of ifetroban for treatment of portal hypertension in cirrhotic patients. Bethesda (MD): National Library of Medicine (US). 2016. https://ClinicalTrials.gov/show/NCT02802228.
Graupera M, Garcia-Pagan JC, Abraldes JG, Peralta C, Bragulat M, Corominola H, et al. Cyclooxygenase-derived products modulate the increased intrahepatic resistance of cirrhotic rat livers. Hepatology. 2003;37(1):172–81.
Wanless IR. The role of vascular injury and congestion in the pathogenesis of cirrhosis: the congestive escalator and the parenchymal extinction sequence. Curr Hepatol Rep. 2020;19(1):40–53.
Vilaseca M, Garcia-Caldero H, Lafoz E, Garcia-Irigoyen O, Avila MA, Reverter JC, et al. The anticoagulant rivaroxaban lowers portal hypertension in cirrhotic rats mainly by deactivating hepatic stellate cells. Hepatology. 2017;65(6):2031–44.
Villa E, Camma C, Marietta M, Luongo M, Critelli R, Colopi S, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology. 2012;143(5):1253-60.e4.
Leonardi F, Maria N, Villa E. Anticoagulation in cirrhosis: a new paradigm? Clin Mol Hepatol. 2017;23(1):13–21.
Iqbal U, Dennis BB, Li AA, Cholankeril G, Kim D, Khan MA, et al. Use of anti-platelet agents in the prevention of hepatic fibrosis in patients at risk for chronic liver disease: a systematic review and meta-analysis. Hepatol Int. 2019;13(1):84–90.
Marrone G, Maeso-Diaz R, Garcia-Cardena G, Abraldes JG, Garcia-Pagan JC, Bosch J, et al. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut. 2015;64(9):1434–43.
Bosch J, Gracia-Sancho J, Abraldes JG. Cirrhosis as new indication for statins. Gut. 2020;69(5):953–62.
Abraldes JG, Albillos A, Banares R, Turnes J, Gonzalez R, Garcia-Pagan JC, et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136(5):1651–8.
Abraldes JG, Villanueva C, Aracil C, Turnes J, Hernandez-Guerra M, Genesca J, et al. Addition of simvastatin to standard therapy for the prevention of variceal rebleeding does not reduce rebleeding but increases survival in patients with cirrhosis. Gastroenterology. 2016;150(5):1160-70.e3.
Pollo-Flores P, Soldan M, Santos UC, Kunz DG, Mattos DE, da Silva AC, et al. Three months of simvastatin therapy vs. placebo for severe portal hypertension in cirrhosis: a randomized controlled trial. Dig Liver Dis. 2015;47(11):957–63.
Wani ZA, Mohapatra S, Khan AA, Mohapatra A, Yatoo GN. Addition of simvastatin to carvedilol non responders: a new pharmacological therapy for treatment of portal hypertension. World J Hepatol. 2017;9(5):270–7.
Bishnu S, Ahammed SM, Sarkar A, Hembram J, Chatterjee S, Das K, et al. Effects of atorvastatin on portal hemodynamics and clinical outcomes in patients with cirrhosis with portal hypertension: a proof-of-concept study. Eur J Gastroenterol Hepatol. 2018;30(1):54–9.
Trebicka J, Hennenberg M, Odenthal M, Shir K, Klein S, Granzow M, et al. Atorvastatin attenuates hepatic fibrosis in rats after bile duct ligation via decreased turnover of hepatic stellate cells. J Hepatol. 2010;53(4):702–12.
Kimer N, Gronbaek H, Fred RG, Hansen T, Deshmukh AS, Mann M, et al. Atorvastatin for prevention of disease progression and hospitalisation in liver cirrhosis: protocol for a randomised, double-blind, placebo-controlled trial. BMJ Open. 2020;10(1):e035284.
Ventura-Cots M, Arranz JA, Simon-Talero M, Torrens M, Blanco A, Riudor E, et al. Safety of ornithine phenylacetate in cirrhotic decompensated patients: an open-label, dose-escalating, single-cohort study. J Clin Gastroenterol. 2013;47(10):881–7.
Pose E, Napoleone L, Amin A, Campion D, Jimenez C, Piano S, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5(1):31–41.
Hide D, Gil M, Andrade F, Rafael D, Raurell I, Bravo M, et al. Simvastatin-loaded polymeric micelles are more effective and less toxic than conventional statins in a pre-clinical model of advanced chronic liver disease. Nanomedicine. 2020;29:102267.
Kreisel W, Lazaro A, Trebicka J, Grosse Perdekamp M, Schmitt-Graeff A, Deibert P. Cyclic GMP in liver cirrhosis-role in pathophysiology of portal hypertension and therapeutic implications. Int J Mol Sci. 2021;22(19):10372. https://doi.org/10.3390/ijms221910372.
Lee KC, Yang YY, Wang YW, Hou MC, Lee FY, Lin HC, et al. Acute administration of sildenafil enhances hepatic cyclic guanosine monophosphate production and reduces hepatic sinusoid resistance in cirrhotic patients. Hepatol Res. 2008;38(12):1186–93.
Schaffner D, Lazaro A, Deibert P, Hasselblatt P, Stoll P, Fauth L, et al. Analysis of the nitric oxide-cyclic guanosine monophosphate pathway in experimental liver cirrhosis suggests phosphodiesterase-5 as potential target to treat portal hypertension. World J Gastroenterol. 2018;24(38):4356–68.
Matei V, Rodriguez-Vilarrupla A, Deulofeu R, Colomer D, Fernandez M, Bosch J, et al. The eNOS cofactor tetrahydrobiopterin improves endothelial dysfunction in livers of rats with CCl4 cirrhosis. Hepatology. 2006;44(1):44–52.
Gracia-Sancho J, Lavina B, Rodriguez-Vilarrupla A, Garcia-Caldero H, Fernandez M, Bosch J, et al. Increased oxidative stress in cirrhotic rat livers: a potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology. 2008;47(4):1248–56.
Taubert D, Roesen R, Lehmann C, Jung N, Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298(1):49–60.
Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56(6):2316–27.
Di Pascoli M, Divi M, Rodriguez-Vilarrupla A, Rosado E, Gracia-Sancho J, Vilaseca M, et al. Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2013;58(5):904–10.
Tandon P, Abraldes JG, Berzigotti A, Garcia-Pagan JC, Bosch J. Renin-angiotensin-aldosterone inhibitors in the reduction of portal pressure: a systematic review and meta-analysis. J Hepatol. 2010;53(2):273–82.
Garcia-Pagan JC, Salmeron JM, Feu F, Luca A, Gines P, Pizcueta P, et al. Effects of low-sodium diet and spironolactone on portal pressure in patients with compensated cirrhosis. Hepatology. 1994;19(5):1095–9.
Klein S, Rick J, Lehmann J, Schierwagen R, Schierwagen IG, Verbeke L, et al. Janus-kinase-2 relates directly to portal hypertension and to complications in rodent and human cirrhosis. Gut. 2017;66(1):145–55.
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89(1):147–91.
Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol. 2019;71(6):1126–40.
Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59(6):2286–98.
Mookerjee RP, Mehta G, Balasubramaniyan V, Mohamed Fel Z, Davies N, Sharma V, et al. Hepatic dimethylarginine-dimethylaminohydrolase1 is reduced in cirrhosis and is a target for therapy in portal hypertension. J Hepatol. 2015;62(2):325–31.
Mookerjee R, Rosselli M, Pieri G, Beecher-Jones T, Hooshmand-Rad R, Chouhan M, et al. O15 Effects of the FXR agonist obeticholic acid on hepatic venous pressure gradient (HVPG) in alcoholic cirrhosis: a proof of concept phase 2a study. J Hepatol. 2014;60(1, Supplement):S7–8.
John BV, Schwartz K, Levy C, Dahman B, Deng Y, Martin P, et al. Impact of obeticholic acid exposure on decompensation and mortality in primary biliary cholangitis and cirrhosis. Hepatol Commun. 2021;5(8):1426–36.
Turco L, Garcia-Tsao G. Portal hypertension: pathogenesis and diagnosis. Clin Liver Dis. 2019;23(4):573–87.
D’Amico M, Mejias M, Garcia-Pras E, Abraldes JG, Garcia-Pagan JC, Fernandez M, et al. Effects of the combined administration of propranolol plus sorafenib on portal hypertension in cirrhotic rats. Am J Physiol Gastrointest Liver Physiol. 2012;302(10):G1191–8.
Majumder S, Piguet AC, Dufour JF, Chatterjee S. Study of the cellular mechanism of sunitinib mediated inactivation of activated hepatic stellate cells and its implications in angiogenesis. Eur J Pharmacol. 2013;705(1–3):86–95.
Uschner FE, Schueller F, Nikolova I, Klein S, Schierwagen R, Magdaleno F, et al. The multikinase inhibitor regorafenib decreases angiogenesis and improves portal hypertension. Oncotarget. 2018;9(90):36220–37.
Lin HC, Huang YT, Yang YY, Lee PC, Hwang LH, Lee WP, et al. Beneficial effects of dual vascular endothelial growth factor receptor/fibroblast growth factor receptor inhibitor brivanib alaninate in cirrhotic portal hypertensive rats. J Gastroenterol Hepatol. 2014;29(5):1073–82.
Worns MA, Galle PR. HCC therapies–lessons learned. Nat Rev Gastroenterol Hepatol. 2014;11(7):447–52.
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5(10):835–44.
Guadalupe Gracia-Tsao MBF, Rajender Reddy K, Loo N, Bari K, Augustin S, Ciarleglio M, et al. Placebo-controlled, randomized, pilot study of the effect of sorafenib on portal pressure in patients with cirrhosis, portal hypertension and ablated hepatocellular carcinoma (HCC). Hepatology. 2015;62(S1):574A-94A.
Fukuda T, Narahara Y, Kanazawa H, Matsushita Y, Kidokoro H, Itokawa N, et al. Effects of fasudil on the portal and systemic hemodynamics of patients with cirrhosis. J Gastroenterol Hepatol. 2014;29(2):325–9.
Ling L, Kuc RE, Maguire JJ, Davie NJ, Webb DJ, Gibbs P, et al. Comparison of endothelin receptors in normal versus cirrhotic human liver and in the liver from endothelial cell-specific ETB knockout mice. Life Sci. 2012;91(13–14):716–22.
Lebrec D, Bosch J, Jalan R, Dudley FJ, Jessic R, Moreau R, et al. Hemodynamics and pharmacokinetics of tezosentan, a dual endothelin receptor antagonist, in patients with cirrhosis. Eur J Clin Pharmacol. 2012;68(5):533–41.
Gifford FJ, Dunne PDJ, Weir G, Ireland H, Graham C, Tuck S, et al. A phase 2 randomised controlled trial of serelaxin to lower portal pressure in cirrhosis (STOPP). Trials. 2020;21(1):260.
Fernandez J, Claria J, Amoros A, Aguilar F, Castro M, Casulleras M, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149–62.
Rittig N, Aagaard NK, Villadsen GE, Sandahl TD, Jessen N, Gronbaek H, et al. Randomised clinical study: acute effects of metformin versus placebo on portal pressure in patients with cirrhosis and portal hypertension. Aliment Pharmacol Ther. 2021;54(3):320–8.
Zhou W, Yang Y, Mei C, Dong P, Mu S, Wu H, et al. Inhibition of Rho-kinase downregulates Th17 cells and ameliorates hepatic fibrosis by Schistosoma japonicum infection. Cells. 2019;8(10):1262. https://doi.org/10.3390/cells8101262.
Wang W, Yan J, Wang H, Shi M, Zhang M, Yang W, et al. Rapamycin ameliorates inflammation and fibrosis in the early phase of cirrhotic portal hypertension in rats through inhibition of mTORC1 but not mTORC2. PLoS ONE. 2014;9(1):e83908.
Zhang CH, Zheng L, Gui L, Lin JY, Zhu YM, Deng WS, et al. Soluble epoxide hydrolase inhibition with t-TUCB alleviates liver fibrosis and portal pressure in carbon tetrachloride-induced cirrhosis in rats. Clin Res Hepatol Gastroenterol. 2018;42(2):118–25.
Boey A, Leong SQ, Bhave S, Ho HK. Cerium oxide nanoparticles alleviate hepatic fibrosis phenotypes in vitro. Int J Mol Sci. 2021;22(21):11777. https://doi.org/10.3390/ijms222111777.
Gomez-Hurtado I, Zapater P, Portune K, Juanola O, Fernandez-Iglesias A, Gonzalez-Navajas JM, et al. Improved hemodynamic and liver function in portal hypertensive cirrhotic rats after administration of B. pseudocatenulatum CECT 7765. Eur J Nutr. 2019;58(4):1647–58.
Rashid SK, Idris-Khodja N, Auger C, Alhosin M, Boehm N, Oswald-Mammosser M, et al. Probiotics (VSL#3) prevent endothelial dysfunction in rats with portal hypertension: role of the angiotensin system. PLoS ONE. 2014;9(5):e97458.
Fallowfield JA, Hayden AL, Snowdon VK, Aucott RL, Stutchfield BM, Mole DJ, et al. Relaxin modulates human and rat hepatic myofibroblast function and ameliorates portal hypertension in vivo. Hepatology. 2014;59(4):1492–504.
Lee KC, Hsieh YC, Chan CC, Sun HJ, Huang YH, Hou MC, et al. Human relaxin-2 attenuates hepatic steatosis and fibrosis in mice with non-alcoholic fatty liver disease. Lab Invest. 2019;99(8):1203–16.
Bennett RG, Heimann DG, Singh S, Simpson RL, Tuma DJ. Relaxin decreases the severity of established hepatic fibrosis in mice. Liver Int. 2014;34(3):416–26.
Hu M, Wang Y, Liu Z, Yu Z, Guan K, Liu M, et al. Hepatic macrophages act as a central hub for relaxin-mediated alleviation of liver fibrosis. Nat Nanotechnol. 2021;16(4):466–77.
Bernardi M, Angeli P, Claria J, Moreau R, Gines P, Jalan R, et al. Albumin in decompensated cirrhosis: new concepts and perspectives. Gut. 2020;69(6):1127–38.
Caraceni P, Riggio O, Angeli P, Alessandria C, Neri S, Foschi FG, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417–29.
China L, Freemantle N, Forrest E, Kallis Y, Ryder SD, Wright G, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808–17.
Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41(3):422–33.
Lim YL, Kim MY, Jang YO, Baik SK, Kwon SO. Rifaximin and propranolol combination therapy is more effective than propranolol monotherapy for the reduction of portal pressure: an open randomized controlled pilot study. Gut Liver. 2017;11(5):702–10.
Mendoza YP, Rodrigues SG, Bosch J, Berzigotti A. Effect of poorly absorbable antibiotics on hepatic venous pressure gradient in cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(9):958–65.
Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28(3):450–5.
Kimer N, Pedersen JS, Busk TM, Gluud LL, Hobolth L, Krag A, et al. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: a randomized, double-blind, placebo-controlled trial. Hepatology. 2017;65(2):592–603.
Kang SH, Kim MY, Baik SK. Novelties in the pathophysiology and management of portal hypertension: new treatments on the horizon. Hepatol Int. 2018;12(Suppl 1):112–21.
Jayakumar S, Carbonneau M, Hotte N, Befus AD, St Laurent C, Owen R, et al. VSL#3 (R) probiotic therapy does not reduce portal pressures in patients with decompensated cirrhosis. Liver Int. 2013;33(10):1470–7.
Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126(4):955–63.
King A, Barton D, Beard HA, Than N, Moore J, Corbett C, et al. REpeated AutoLogous Infusions of STem cells In Cirrhosis (REALISTIC): a multicentre, phase II, open-label, randomised controlled trial of repeated autologous infusions of granulocyte colony-stimulating factor (GCSF) mobilised CD133+ bone marrow stem cells in patients with cirrhosis. A study protocol for a randomised controlled trial. BMJ Open. 2015;5(3):e007700.
Newsome PN, Fox R, King AL, Barton D, Than NN, Moore J, et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3(1):25–36.
Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, et al. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64(6):2185–97.
Pietrosi G, Fernandez-Iglesias A, Pampalone M, Ortega-Ribera M, Lozano JJ, Garcia-Caldero H, et al. Human amniotic stem cells improve hepatic microvascular dysfunction and portal hypertension in cirrhotic rats. Liver Int. 2020;40(10):2500–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. JB has been a consultant for Gilead, Actelion, Ambys, BMB, Surrozen, BioVie, and Boehringer Ingelheim and received speaker fees from Gore. He is an unpaid member of the Board of BLB. The remaining authors have no financial disclosures.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Portal Hypertension and Liver Transplantation
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Felli, E., Nulan, Y., Selicean, S. et al. Emerging Therapeutic Targets for Portal Hypertension. Curr Hepatology Rep 22, 51–66 (2023). https://doi.org/10.1007/s11901-023-00598-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-023-00598-4