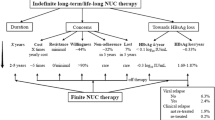
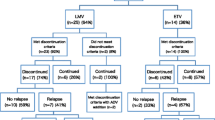
Abstract
Therapy with currently available oral nucleos(t)ide analogs (NA) in patients with chronic hepatitis B (CHB, HBeAg-positive or -negative) should ultimately aim at preventing progression to cirrhosis and hepatocellular carcinoma. Since these hard to achieve clinical outcomes evolve over several decades, a number of intermediate therapy end-points i.e. virological, biochemical, serological and histological have been proposed. For HBeAg-positive CHB, durable HBeAg seroconversion remains the gold standard clinical end-point while for HBeAg-negative CHB sustained HBV DNA suppression and HBsAg loss represent the desirable goals to be reached. In this review, the distinguishing features and therapeutic end-points of HBeAg-positive vs—negative CHB are critically presented.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Chotiyaputta W, Lok AS. Endpoints of hepatitis B treatment. J Viral Hepat. 2010;17:675–84. A recent comprehensive review on the different endpoints of therapy in chronic hepatitis B.
Lok ASF, McMahon BJ. AASLD Practice Guidelines. Chronic hepatitis B: Update 2009. Accessed at www.aasld.org on September 12, 2011
European Association For The Study Of The Liver. EASL clinical practice guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–42.
Feld JJ, Wong DK, Heathcote EJ. Endpoints of therapy in chronic hepatitis B. Hepatology. 2009;49:S96–S102.
Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001;34:617–24.
Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–51.
Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N Engl J Med. 2005;352:2673–81.
Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e antigen-negative chronic hepatitis B: natural history and treatment. Semin Liver Dis. 2006;26:130–41.
Ayoub WS, Keeffe EB. Review article: current antiviral therapy of chronic hepatitis B. Aliment Pharmacol Ther. 2011;34:1145–58.
Fung J, Lai CL, Seto WK, et al. Nucleoside/nucleotide analogues in the treatment of chronic hepatitis B. J Antimicrob Chemother. 2011;66:2715–25.
• Hadziyannis SJ. Natural history of chronic hepatitis B in euro-mediterranean and African countries. J Hepatol. 2011;55:183–91. A recent review on the natural history of predominant HBeAg-negative chronic hepatitis B.
Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:S13–21.
Carman WF, Jacyna MR, Hadziyannis S, et al. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989;2:588–91.
Hadziyannis SJ. Unrevealing the natural course of the so-called “inactive HBsAg or HBV carrier state”. Hepatol Int. 2007;1:281–4.
Hadziyannis SJ. Hepatitis B e antigen negative chronic hepatitis B: from clinical recognition to pathogenesis and treatment. Viral Hepat Rev. 1995;1:7–36.
Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–52.
Liaw YF, Lau GK, Kao JH, et al. Hepatitis B e antigen seroconversion: a critical event in chronic hepatitis B virus infection. Dig Dis Sci. 2010;55:2727–34.
Lau GK. Current treatments for patients with HBeAg-positive chronic hepatitis B virus infection: a comparison focusing on HBeAg seroconversion. Liver Int. 2010;30:512–20.
Heathcote EJ, Marcellin P, Buti M, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140:132–43.
Chang TT, Lai CL, Kew YS, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422–30.
Chang TT, Liaw YF, Wu SS, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–93.
Kim HC, Nam CM, Jee SH, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983.
Rapti I, Dimou E, Mitsoula P, et al. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45:307–13.
Hadziyannis SJ, Sevastianos V, Rapti I. Outcome of HBeAg-negative chronic hepatitis B (CHB) 5 years after discontinuation of long term adefovir dipivoxil (ADV) treatment. J Hepatol. 2009;50 Suppl 1:S9–S10.
Disclosure
Dr. S. Hadziyannis has served on the Global Advisory Board of Gilead and has been a consultant and grant recipient for Roche and Gilead. He has received honoraria from Novartis, Pfizer, BMS, Roche, Gilead and Merck, and travel and accommodation expenses from Roche, Gilead, BMS, and Novartis; Dr. D. Vassilopoulos has received honoraria and travel and accommodation expenses from Roche, Abbott, Merck/Schering-Plough, Wyeth/Pfizer, UCB, and Novartis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hadziyannis, S.J., Vassilopoulos, D. What Should Be the Endpoints of Oral Therapy in HBeAg-positive Versus HBeAg-negative Patients with Chronic Hepatitis B. Curr Hepatitis Rep 11, 65–69 (2012). https://doi.org/10.1007/s11901-012-0124-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-012-0124-8