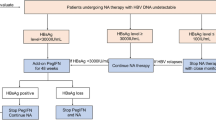
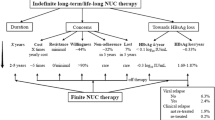
Abstract
Purpose of Review
The strategy of finite nucleos(t)ide analogue (Nuc) therapy in HBeAg-negative patients has been increasingly accepted globally, and when to stop therapy has become an important issue. This review aimed to address this issue.
Recent Findings
The incidence of off-therapy relapse is lower with lower end-of-therapy (EOT) HBsAg level. Pretherapy HBV DNA < 20,000 IU/mL, on-treatment HBV DNA undetectable by week 12, treatment duration > 3 years, on-treatment HBsAg decline > 1 log10 IU/mL, and EOT HBsAg < 100 IU/mL are also factors for increasing off-therapy HBsAg loss. Off-therapy hepatitis flare may occasionally deteriorate to hepatic decompensation, which can be prevented/rescued by timely retreatment whereas “no-retreatment” is an important factor for HBsAg loss.
Summary
All patients meeting recommended stopping criteria are eligible for Nuc withdrawal. The favorable factors for low relapse rate and high HBsAg loss rate can be used to encourage patients to stop Nuc therapy and adhere to off-Nuc monitoring plan.
Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- AASLD:
-
American Association for the Study of Liver Diseases
- aHR:
-
Adjusted hazard ratio
- ALT:
-
Alanine aminotransferase
- APASL:
-
Asian-Pacific Association for the Study of the Liver
- cccDNA:
-
Covalently closed circular DNA
- CHB:
-
Chronic hepatitis B
- CR:
-
Clinical relapse
- EASL:
-
European Association for the Study of the Liver
- EOT:
-
End of therapy
- ETV:
-
Entecavir
- HBeAg:
-
Hepatitis B e antigen
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HBV-LC:
-
HBV-related cirrhosis
- HCC:
-
Hepatocellular carcinoma
- IC:
-
Inactive carrier
- iDNA:
-
Integrated HBV DNA
- Nuc:
-
Nucleos(t)ide analogue
- qHBsAg:
-
Quantitative HBsAg
- SR:
-
Sustained response
- TAF:
-
Tenofovir alafenamide
- TDF:
-
Tenofovir disoproxil fumarate
- ULN:
-
Upper limit of normal
- VR:
-
Virologic relapse
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Liaw YF. Clinical utility of HBV surface antigen quantitation in HBV e antigen-negative chronic HBV infection. Nat Rev Gastroenterol Hepatol. 2019;16:631–41. A comprehensive review on the natural history, pathogenesis, and the clinical utility of qHBsAg in chronic hepatitis B.
Liaw YF. Impact of therapy on the long term outcome of chronic hepatitis B. Clin Liver Dis. 2013;17:413–23.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98.
European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–98.
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–99.
Liaw YF. Finite nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B: an emerging paradigm shift. Hepatol Int. 2019;13:665–73. A review on the rationales and paradigm shift from indefinite to finite Nuc therapy.
Berg T, Lampertico P. The times they are a-changing - a refined proposal for finite HBV nucleos(t)ide analogue therapy. J Hepatol. 2021;75:474–80. A review with a conclusion that increasing HBsAg loss is the main justification for finite Nuc therapy (stop-to-cure).
Liaw YF, Chien RN. Finite Nuc therapy in HBeAg-negative chronic hepatitis B: from an “option” to an “active recommendation.” Kaohsiung J Med Sci. 2022;38:295–301. A review on the paradigm shift from “option” to “proactive recommendation” for finite Nuc therapy.
Levrero M, Pollicino T, Petersen J, Belloni L, Raimondo G, Dandri M. Control of cccDNA function in hepatitis B virus infection. J Hepatol. 2009;51:581–92.
Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, Liu CJ, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531–61. The first clear Nuc stopping rule in HBeAg-negative patients with chronic hepatitis B.
Liaw YF. Hepatitis B flare: the good, the bad and the ugly. Expert Rev Gastroenterol Hepatol. 2022;16:1043–51.
Liu YC, Jeng WJ, Peng CW, Chien RN, Liaw YF. Off-tenofovir hepatitis flares in HBeAg-negative patients occur earlier, more frequent and severe than those off-entecavir therapy. Liver Int. 2022;42:551–60.
Sonneveld MJ, Chiu SM, Park JY, Brakenhoff SM, Kaewdech A, Seto WK, et al. Lower pretreatment HBV DNA levels are associated with better off-treatment outcomes after nucleo(s)tide analogue withdrawal in patients with HBeAg-negative chronic hepatitis B: a multicentre cohort study. JHEP Rep. 2023;5:100790.
Jeng WJ, Chien RN, Chen YC, Lin CL, Wu CY, Liu YC, et al. Hepatocellular carcinoma reduced, HBsAg loss increased and survival improved after finite therapy in hepatitis B patients with cirrhosis. Hepatology. 2023. https://doi.org/10.1097/HEP.0000000000000575. A large study in HBV/LC showing > 50% reduction in HCC and mortality as well as a > 10-fold increase in HBsAg loss.
Jeng WJ, Chen YC, Sheen IS, Lin CL, Hu TH, Chien RN, et al. Clinical relapse after cessation of tenofovir therapy in HBeAg-negative patients. Clin Gastroenterol Hepatol. 2016;14:1813–20.
Su TH, Yang HC, Tseng TC, Liou JM, Liu CH, Chen CL, et al. Distinct relapse rates and risk predictors after discontinuing tenofovir and entecavir therapy. J Infect Dis. 2018;217:1193–201.
Chen CH, Hsu YC, Lu SN, Hung CH, Wang JH, Lee CM, et al. The incidence and predictors of HBV relapse after cessation of tenofovir therapy in chronic hepatitis B patients. J Viral Hepat. 2018;25:590–7.
HönerZuSiederdissen C, Hui AJ, Sukeepaisarnjaroen W, Tangkijvanich P, Su WW, Nieto GEG, et al. Contrasting timing of virological relapse after discontinuation of tenofovir or entecavir in hepatitis B e antigen-negative patients. J Infect Dis. 2018;218:1480–4.
Liu YC, Jeng WJ, Peng CW, Chien RN, Liaw YF. The role of off-therapy viral kinetics in the timing and severity of flares in hepatitis B e antigen-negative patients. Clin Gastroenterol Hepatol. 2023;21:1533–41.
Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2018;68:425–34. A large study on the incidence and factors of off-Nuc HBsAg loss.
van Bömmel F, Stein K, Heyne R, Petersen J, Buggisch P, Berg C, et al. A multicenter randomized-controlled trial of nucleos(t)ide analogue cessation in HBeAg-negative chronic hepatitis B. J Hepatol. 2023;78:926–36.
Broquetas T, Hernandez JJ, Garcia-Retortillo M, Canillas L, Puigvehí M, Cañete N, et al. On-therapy HBsAg kinetics can predict HBsAg loss after nucleos(t)ide analogues interruption in HBeAg-negative patients. The cup is half full and half empty. Dig Liver Dis. 2022;54:1044–51.
Chang ML, Jeng WJ, Liaw YF. Clinical events after cessation of lamivudine therapy in patients recovered from hepatitis B flare with hepatic decompensation. Clin Gastroenterol Hepatol. 2015;13:979–86. A large study in Nuc cessation in patients with pretherapy hepatic decompensation.
Jeng WJ, Liaw YF. Finite antiviral therapy in chronic hepatitis B patients with cirrhosis. Semin Liver Dis. 2021;41:349–57.
Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, et al. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol. 2017;67:918–24.
Chang ML, Liaw YF, Hadziyannis SJ. Systematic review: cessation of long-term nucleos(t)ide analogue therapy in patients with hepatitis B e antigen-negative chronic hepatitis B. Aliment Pharmacol Ther. 2015;42:243–57.
Papatheodoridis G, Vlachogiannakos I, Cholongitas E, Wursthorn K, Thomadakis C, Touloumi G, et al. Discontinuation of oral antivirals in chronic hepatitis B: a systematic review. Hepatology. 2016;63:1481–92.
Song DS, Jang JW, Yoo SH, Kwon JH, Nam SW, Bae SH, et al. Improving the prediction of relapse after nucleos(t)ide analogue discontinuation in patients with chronic hepatitis B. Clin Infect Dis. 2021;73:e892–903.
Jeng WJ, Chen CH, Chen YC, Sheen IS, Chien RN, Liaw YF. Off therapy durability of response to entecavir therapy in hepatitis B e antigen negative chronic hepatitis B patients. J Hepatol. 2020;73:S881.
Peng CW, Jeng WJ, Yang HI, Liu YC, Chien RN, Liaw YF. A switch from tenofovir to entecavir prior to hepatitis B treatment cessation is associated with a reduced risk of off-therapy relapse: an observational study. J Gastroenterol Hepatol. 2022;37:2164–72.
Chang ML, Chien RN, Liaw YF. Evidence-based management of oral nucleos(t)ide analogue withdrawal in virally suppressed patients with chronic HBV infection. Curr Hepaology Rep. 2022;21:52–8. A comprehensive review on the management of finite Nuc therapy.
Liaw YF. Hepatitis B flare after cessation of nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B: to retreat or not to retreat. Hepatology. 2021;73:843–52. A review on off-Nuc hepatitis flares and the role of combined HBsAg/ALT kinetics for retreatment decision.
Jeng WJ, Liu YC, Peng CW, Chien RN, Liaw YF. Highly significant differences in HBsAg kinetics among patients with two types of hepatitis B flare with and without retreatment. J Antimicrob Chemother. 2022;77:205–12.
Grudda T, Hwang HS, Taddese M, Quinn J, Sulkowski MS, Sterling RK, et al. Integrated hepatitis B virus DNA maintains surface antigen production during antiviral treatment. J Clin Invest. 2022;132:e161818.
Luo M, Zhou B, Hou J, Jiang D. Biomarkers for predicting nucleos(t)ide analogs discontinuation and hepatitis B virus recurrence after drug withdrawal in chronic hepatitis B patients. Hepatol Res. 2022;52:337–51.
Adraneda C, Tan YC, Yeo EJ, Kew GS, Khakpoor A, Lim SG. A critique and systematic review of the clinical utility of hepatitis B core-related antigen. J Hepatol. 2023;78:731–41.
Acknowledgements
The author thanks Ms. Su-Chiung Chu for her secretary assistance and preparation of the manuscript.
Funding
YFL has been supported by grants from Chang Gung Medical Research Fund (CMRPG1K0101-3, CMRPG1K0111-3, CMRPG1N0111) and the Prosperous Foundation, Taipei, Taiwan.
Author information
Authors and Affiliations
Contributions
Yun-Fan Liaw wrote the whole manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
Not applicable (a review article).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liaw, YF. When to Stop Antiviral Therapy in HBeAg-Negative Patients with Chronic Hepatitis B?. Curr Hepatology Rep 23, 221–226 (2024). https://doi.org/10.1007/s11901-024-00663-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11901-024-00663-6