Abstract
Purpose of Review
Due to lack of pediatric-specific data, the management of chronic myeloid leukemia (CML) in pediatric, adolescents, and young adults is guided by adult CML evidence-based recommendations. Pediatric CML presents differently than adult CML and is often a more aggressive disease with different biological and host factors, yet there is sparse literature on how to address those differences.
Recent Findings
Over the past two decades, tyrosine kinase inhibitors (TKIs) have changed the way CML is treated. There are currently three FDA-approved TKIs (imatinib, dasatinib, and nilotinib) for pediatric patients. When choosing which TKI to begin treatment with, there are many factors that should be considered on a case-to-case basis to obtain optimal outcomes. The safety profiles for long-term TKI use in pediatrics require further study. Unlike adults, children are still actively growing during TKI use, and the effect on development can be detrimental. TKI therapy is not recommended during pregnancy with variable but significant risk of fetal abnormalities and miscarriage, warranting counseling for young female patients prior to beginning TKIs. Attempts for treatment-free remission (TFR) by planned TKI cessation in eligible adult patients in deep and sustained molecular remission are now done as a standard of practice. However, data is sparse in the pediatric population. There is currently an ongoing Children’s Oncology Group (COG) study to determine the feasibility of TFR as a treatment goal.
Summary
Further research and additional pediatric trials are needed to characterize the unique aspects of CML in children and adolescents and optimize outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm that results from translocation t(9:22) and the resultant BCR-ABL1 fusion. CML is rare in pediatrics, adolescents, and young adults. It comprises approximately 2–3% of all pediatric leukemias in those under 15 years old and approximately 9% of all leukemias in those between 15 and 19 years old [1, 2]. Given its lower incidence, most of the data used to guide management is derived from the adult population. However, there is no clear evidence that risk assessment tools and adult treatment guidelines can be applied to pediatric patients. There are data suggesting that CML is often more aggressive in pediatric patients for many different reasons, such as underlying biology and host factors, necessitating integration into optimal treatment of CML in younger individuals [3, 4].
Over the last two decades, tyrosine kinase inhibitors (TKIs) have revolutionized the way CML is treated, including the role of hematopoietic stem cell transplant (HSCT). HSCT is now typically reserved for those patients who cannot tolerate or are resistant to TKIs, or those who present or progress to blast crisis [2]. CML is now beyond simply a chronic disease, given the novel paradigm of long-term treatment with TKIs, often for decades to keep disease under control. Such a paradigm is of even greater importance to younger individuals.
Unlike adults, children are actively developing and growing while undergoing TKI therapy. Long-term side effects of treatment of chronic CML with TKI over decades in children and young adults are still unknown [5]. In adult patients, recommendations to consider when potentially discontinuing TKIs are included in The National Comprehensive Cancer Network (NCCN) Guidelines [6] and European Leukemia Net (ELN) guidelines [7]. Pediatric patients would benefit, potentially even more so, from discontinuing TKI therapy at the earliest point possible to limit the potential developmental side effects. However, there are sparse data supporting or guiding the timing of such an endeavor in younger patients [2]. This review will encompass the specific treatment and management considerations for children and young adults with CML, and how to best transition them to adult care.
Consideration of Differences in Children and Young Adults
Pediatric patients with CML present differently than adults, usually manifesting with more aggressive disease features (Table 1); the biology of CML in children and adolescents thus should not be assumed to be the same as in adults [3]. Children and adolescents typically present with a higher white blood cell (WBC) count and more circulating blasts at diagnosis. Median WBC counts in adults at diagnosis range from 50 to 70 × 109/L [8], but in children and adolescents, the initial WBC count is often as high as 300 × 109/L [9]. Children and adolescents more often have significant splenomegaly and profound anemia, and unfortunately present in an advanced stage of CML — either in the accelerated or blast phase [4].
Approximately 5–15% of both children and adults harbor additional cytogenetic abnormalities (ACAs) including variant translocations, additional chromosomal abnormalities, and complex karyotypes [10, 11]. There is data suggesting ACAs in children may not be associated with inferior outcomes unlike adults. From a genomic standpoint, pediatric patients also appear to have a more complex profile and may harbor a higher proportion of altered oncogenes. CML in children has different breakpoint patterns in the BCR gene, specifically a higher proportion within the Alu repeat regions. They show a distribution of BCR-ABL1 breakpoints that resembles Philadelphia chromosome positive acute lymphoblastic leukemia, which all may correlate with more aggressive disease [2, 3]. Several mutations have been implicated in CML across all ages, including ASXL1, in addition to deregulated genes in the PI3K/AKT/WNT/beta-catenin, Sonic Hedgehog, and MAPK pathways [12]. ASXL1 mutations may be more frequent in pediatric patients with CML compared with adults. One report found that 29% of pediatric and young adult patients with chronic phase CML had an ASXL1 mutation compared with 7–13% of adults [13]. Recent data demonstrated that CD34 + cells from children with chronic CML have different transcriptomes compared to adults. RNA-sequencing results showed that genes in the Rho-GTPase pathway were significantly downregulated in pediatric CML, but not adult CML patient CD34 + cells [14].
There are no standard pediatric specific guidelines in the care of pediatric and adolescent CML patients; thus, many pediatric oncologists are forced to rely heavily on recommendations for adult CML. Treatment guidelines (like NCCN and ELN) which guide therapy changes and define failure, based on cytogenetic and molecular responses to TKI therapy, have never been validated in children [4, 5]. The majority of prognostic scoring systems, like Sokal, EURO, EUTOS, and Hasford, which are used in adults, have not been validated in pediatric populations and thus may be unreliable in general. Interestingly, a study from the International Registry for Childhood Chronic Myeloid Leukemia recently evaluated 4 prognostic scoring systems (Sokal, Euro, EUTOS, and EUTOS Long-Term Survival) in 350 pediatric patients with newly diagnosed chronic phase CML treated with imatinib. The EUTOS Long-Term survival score showed better differentiation of progression-free survival than the other scoring systems. Future studies should examine whether children in the high-risk group might benefit from the use of second-generation TKIs as initial therapy [15].
It is important to note that children may require a lengthier course of TKI therapy, simply given a dramatically longer life expectancy, creating many obstacles in pediatric and adolescent patients. Adolescents and young adults are known to be less compliant with routine medication administration, which is not ideal when a medication may be used for decades. Longer use of TKIs, especially in cases of suboptimal response, may foster greater risk of resistance permitting disease progression to advanced stages of CML. Despite the advent of TFR, across all ages, the safety (and efficacy) of very long term TKI use has not been studied and is unknown. Immune dysfunction, thyroid, cardiac, liver, and fertility issues have all been reported in adults with CML, and particular focus is being paid to cardiovascular and vascular risk over time; there are no long-term data for adverse effects of TKI therapy in children [3, 4].
Choosing Initial Treatment
TKIs have become the standard of care in CML patients in the chronic phase (CP); therefore, HSCT is no longer the definitive therapy for cure. There are three FDA-approved TKIs for first-line CML therapy use in pediatrics — imatinib (approved in 2003), dasatinib (approved in 2017), and nilotinib (approved in 2018). Bosutinib and ponatinib are both currently approved for adults, and the novel “STAMP” (allosteric inhibitor targeting the myristoyl pocket of ABL1) asciminib was approved for adults by the FDA in 2021 [16, 17]. There are open clinical trials for use in children [5, 18] for these three drugs (NCT04258943, NCT03934372, NCT04925479, respectively).
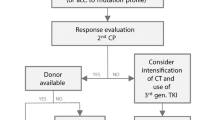
The TKI chosen for treatment should be based upon the aggregate of multiple factors leading to the best compliance and outcome (Table 2). Imatinib and dasatinib are given once a day with or without food, while nilotinib is given twice a day without food 2 h prior and 1 h after administration, potentially difficult for a young child or adolescent. Imatinib has generic formulations available, lending financial desirability to the health care system and theoretically for patients, given the potential of multiple decades of treatment for young patients. The second-generation TKIs (dasatinib and nilotinib) have been shown to induce a faster and deeper molecular response in adult patients in randomized trials [19, 20]. Although there are no randomized trials in pediatrics due to the small number of patients, some published studies show similar trends [2, 5, 21,22,23]. Although a faster and deeper molecular response has not been shown to have a survival benefit, it may be beneficial by accelerating the timeframe and number of eligible patients for attempt at discontinuation of TKI therapy to achieve TFR [2]. There is not currently enough data to use ACAs or prognostic scoring systems to guide initial treatment in pediatric patients. Given the faster and deeper response with second generation TKIs in adults, some providers may elect to start with a second generation TKI because there are few serious side effects such as cardiovascular events in pediatric patients, if they anticipate it will be affordable.
Although TKIs are well tolerated in general, severe side effects associated with second- and third-generation TKIs have been reported in adult patients (Table 2). Cardiovascular side effects, specifically vaso-occlusive events, are of particular concern with the use of second- and third-generation TKIs in adults [24]. While this can cause provider reluctance to use second- and third-generation TKIs especially in older adults with preexisting morbidities [25], there are no serious side effects published within the pediatric literature to date [21, 22]. In addition, comorbidities in children are less common; therefore, there may be less concern for these side effects with later generation TKIs. In conclusion, many factors, including efficacy, cost, availability, toxicity profiles, and comorbidities, must be taken into account when choosing the best TKI for a specific pediatric patient with newly diagnosed CML in CP [2, 5].
There are no current TKI response criteria available to guide the specific management of pediatric patients. Pediatric providers thus resort to using the adult recommendations, such as the NCCN guidelines or ELN criteria [2]. These response criteria recommendations are based on adult data but likely correlate equally to disease modification and risk reduction and may reasonably be used for pediatric patients [1].
Management of Refractory and Intolerant Disease
The most common cause of refractory disease in pediatric CML patients is noncompliance, especially in the adolescent and young adult age groups; the same is often cited in adults. Noncompliance/nonadherence often occurs early, often after 1 year of treatment given good response and patients feeling less burdened by their disease [1]. It is important to emphasize compliance at every visit with not only the patient, but also the caregivers, parents, or others. If a patient is not responding well to TKI therapy, noncompliance should be suspected and queried before investigating for resistance and switching TKIs [2]. TKI plasma trough levels are not routinely measured for compliance. If noncompliance is suspected but not confirmed by the patient, it may be possible to send a TKI plasma trough level for some TKIs to ensure an adequate drug level [1]. Lastly, review of concomitant medications, in addition to supplements, nontraditional medications, over-the-counter products, and dietary habits, is important to rule out drug-drug interactions, many of which may reduce patient exposure to their CML medication.
It is also important to review the potential and actual barriers for compliance with each patient to facilitate solutions. It is imperative to provide and review adherence tools, such as using a pillbox, setting a phone alarm, or marking administration on a calendar; having a routine schedule will lead to better compliance. Potential side effects of TKIs should be reviewed at each visit. As an example, GI toxicity with imatinib may manifest as diarrhea; having this adverse event chronically, even if not higher grade or constant, may not seem “dire,” but may specifically impact a patient’s extracurricular activities, a strong impetus to stop/reduce medication. Financial burden of the medication should also be reviewed; in younger patients, this is often the responsibility of others, and all parties need to understand the importance of consistent treatment. Addressing all the potential obstacles in a transparent and facilitating manner will only provide for better compliance and outcome in the long-term [1, 2].
If noncompliance has been ruled out, and the patient is still not responding to TKI therapy, resistance should be a concern. BCR-ABL1 mutation analysis is indicated for resistance; NCCN guidelines recommend that mutational analysis be sent after initiating TKI therapy if there is a suboptimal response to therapy, failure of prior response, or escalation into advance stage CML [1]. Based on specific types of resistance, TKI choice may be tailored to optimize disease sensitivity (Table 2).
Issues with Side Effects in Children and Young Adults
Two decades after the approval of the first generation TKI imatinib for children, the long-term side effects in children and young adults remain less well understood. Pediatric patients often begin TKIs before or during puberty, which can cause detrimental effects on growth and development, but the exact mechanism is unclear [21, 26,27,28]. TKIs not only inhibit BCR-ABL1, but other targets as well, including PDGFR-beta signaling, resulting in reduced osteoclast activity and recruitment of chondrocytes in the growth plate, which is one of the proposed mechanisms for growth delay. This adversely affects overall bone metabolism and linear growth [1]. Thyroid and gonadal dysfunction are also seen and should be routinely monitored [2]. Metabolic derangements, particularly diabetes mellitus, have also been documented with nilotinib use, so routine blood glucose levels should be checked [1].
All patients being treated with TKI therapy should be educated on reproductive health and safe sexual practices. TKIs are teratogenic and have been shown to cause fetal abnormalities or spontaneous abortions, particularly in the first trimester [6]. Females should be counseled on safe sexual practices to avoid pregnancy entirely while on TKI therapy [4]. There should be a prolonged washout period after TKI discontinuation before attempting to conceive and therapy should be held throughout the pregnancy. If treatment is needed during pregnancy, interferon therapy (interferon alfa a2 or peginterferon alfa a2) can be considered, especially in the second trimester of pregnancy and beyond [6, 29]. TKI therapy can be resumed immediately after pregnancy, but mothers are advised not to breastfeed as it has been shown that imatinib and likely other TKIs can be transmitted through breast milk [1]. There have been no reported adverse effects or congenital abnormalities in the offspring of males who were undergoing TKI therapy at the time of conception [30]. There is sparse literature on fertility and the ability to reproduce after a prolonged TKI treatment course [4].
Treatment-Free Remission in Pediatric Patients
There are very limited data regarding the feasibility of discontinuing TKIs in the pediatric and adolescent population once deep and sustained molecular remission is achieved. There are strict NCCN guidelines for discontinuing TKIs in adults, which include: age ≥ 18 years old, in chronic phase CML with no history of advance stage CML, on TKI therapy for at least 3 years, stable molecular response (MR4 or better) for ≥ 2 years, access to reliable qPCR testing, and frequent molecular monitoring [6]. It is important to note that within 6 months of stopping TKI therapy, 50–60% of patients do not stay in treatment-free remission (TFR) and have disease recurrence. This can be reversed by reinitiating TKI therapy, with remarkably high rates of return to remission/deep remission; patients with unsuccessful TFR may then require longer/indefinite duration of therapy [5]. There also have been reported clinical side effects consistent with “withdrawal syndrome” upon stopping TKIs, such as musculoskeletal pain and neurocognitive deficits. Withdrawal side effects could be more severe in pediatric patients and require prospective trials for further investigation [2]. In pediatrics, there is an ongoing Children’s Oncology Group study to assess feasibility of TFR (NCT03817398). Given that data remains limited in pediatrics, we currently do not recommend routine discontinuation of TKIs outside of a clinical trial.
Transitioning from Pediatric to Adult Care
Transitioning pediatric patients to adult CML care should be fluid and tailored as it may vary on a case-by-case basis depending on the needs of that individual at the time. Transition to an adult clinic may be considered when patient is 18 years of age but may take place by the time a patient is 21 years of age. This transition is unique given the long-term relationship between patient and provider teams and chronicity of active treatment. Like any medical transition, clear communication between the teams to ensure seamless care is paramount [1].
Conclusions
Pediatric CML care is ultimately based upon adult guidelines. There are documented host and molecular differences in CML between pediatric and adult patients, but how those differences affect treatment and prognosis are poorly understood. While TKIs have completely changed the way CML is treated and improved overall survival, there is little known about the side effects of decadelong TKI use. Additional study and pediatric trials are needed to characterize the unique aspects of CML in children, adolescents, and young adults.
References
Athale U, Hijiya N, Patterson BC, Bergsagel J, Andolina JR, Bittencourt H, Schultz KR, Burke MJ, Redell MS, Kolb EA, Johnston DL. Management of chronic myeloid leukemia in children and adolescents: recommendations from the Children’s Oncology Group CML Working Group. Pediatr Blood Cancer. 2019;66(9): e27827.
Hijiya N, Suttorp M. How I treat chronic myeloid leukemia in children and adolescents. Blood. 2019;133(22):2374–84.
Hijiya N, Schultz KR, Metzler M, Millot F, Suttorp M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood. 2016;127(4):392–9.
Hijiya N, Millot F, Suttorp M. Chronic myeloid leukemia in children: clinical findings, management, and unanswered questions. Pediatr Clin North Am. 2015;62(1):107–19.
Phillips LN, Hijiya N. Tyrosine kinase inhibitors and beyond for chronic myeloid leukemia in children. Paediatr Drugs. 2021;23(3):241–51.
National Comprehensive Cancer Network (NCCN) Clinical practice guidelines in oncology-chronic myeloid leukemia (ver 2, 2022); 2022.
Hochhaus A, Baccarani M, Silver RT, Schiffer C, Apperley JF, Cervantes F, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–84.
Castagnetti F, Gugliotta G, Baccarani M, et al. Differences among young adults, adults and elderly chronic myeloid leukemia patients. Ann Oncol. 2015;26(1):185–92.
Millot F, Guilhot J, Baruchel A, et al. Impact of early molecular response in children with chronic myeloid leukemia treated in the French Glivec phase 4 study. Blood. 2014;124(15):2408–10.
Millot F, Dupraz C, Guilhot J, et al. Additional cytogenetic abnormalities and variant t(9;22) at the diagnosis of childhood chronic myeloid leukemia: The experience of the International Registry for Chronic Myeloid Leukemia in Children and Adolescents. Cancer. 2017;123(18):3609–16.
Karow A, Göhring G, Sembill S, et al. The cytogenetic landscape of pediatric chronic myeloid leukemia diagnosed in chronic phase. Cancers (Basel). 2022;14(7):1712.
de CássiaViu Carrara R, Fontes AM, Abraham KJ, et al. Expression differences of genes in the PI3K/AKT, WNT/b-catenin, SHH, NOTCH and MAPK signaling pathways in CD34+ hematopoietic cells obtained from chronic phase patients with chronic myeloid leukemia and from healthy controls. Clin Transl Oncol. 2018;20(4):542–9.
Ernst T, Busch M, Rinke J, et al. Frequent ASXL1 mutations in children and young adults with chronic myeloid leukemia. Leukemia. 2018;32(9):2046–9.
Youn M, Smith SM, Lee AG, et al. Comparison of the transcriptomic signatures in pediatric and adult CML. Cancers (Basel). 2021;13(24):6263.
Millot F, Guilhot J, Suttorp M, et al. Prognostic discrimination based on the EUTOS long-term survival score within the International Registry for Chronic Myeloid Leukemia in children and adolescents. Haematologica. 2017;102(10):1704–8.
Hughes TP, Mauro MJ, Cortes JE, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381(24):2315–26.
Réa D, Mauro MJ, Boquimpani C, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138(21):2031–41.
Pennesi E, Brivio E, Willemse ME, et al. A phase I/II study of bosutinib in pediatric patients with resistant/intolerant or newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia, Study ITCC (Innovative Therapies for Children with Cancer European Consortium) 054 and COG (Children’s Oncology Group Consortium) AAML1921: Results from the Phase I Trial in Resistant/Intolerant Patients. Blood. 2021;138(Supplement 1):2558–2558.
Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044–54.
Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: The dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients Trial. J Clin Oncol. 2016;34(20):2333–40.
Hijiya N, Maschan A, Rizzari C, et al. Phase 2 study of nilotinib in pediatric patients with Philadelphia chromosome-positive chronic myeloid leukemia. Blood. 2019;134(23):2036–45.
Gore L, Kearns PR, de Martino ML, et al. Dasatinib in pediatric patients with chronic myeloid leukemia in chronic phase: results from a phase II trial. J Clin Oncol. 2018;36(13):1330–8.
Millot F, Baruchel A, Guilhot J, et al. Imatinib is effective in children with previously untreated chronic myelogenous leukemia in early chronic phase: results of the French national phase IV trial. J Clin Oncol. 2011;29(20):2827–32.
Barber MC, Mauro MJ, Moslehi J. Cardiovascular care of patients with chronic myeloid leukemia (CML) on tyrosine kinase inhibitor (TKI) therapy. Hematology Am Soc Hematol Educ Program. 2017;2017(1):110–4.
Cortes J. How to manage CML patients with comorbidities. Blood. 2020;136(22):2507–12.
Patterson BC, Samis J, Gore L, et al. Growth rate and endocrine effects of dasatinib therapy observed in a retrospective analysis if a phase 3 clinic trial for pediatric patients with chronic myeloid leukemia in the chronic phase (CML-CP) European Hematology Association Congress. Amsterdam, Netherlands; 2019.
Samis J, Lee P, Zimmerman D, Arceci RJ, Suttorp M, Hijiya N. Recognizing endocrinopathies associated with tyrosine kinase inhibitor therapy in children with chronic myelogenous leukemia. Pediatr Blood Cancer. 2016;63(8):1332–8.
Tauer JT, Nowasz C, Sedlacek P, de Bont ESJM, Aleinikova OV, Suttorp M. Impairment of longitudinal growth by tyrosine kinase inhibitor (TKI) treatment — data from a large pediatric cohort with chronic myeloid leukemia (CML). Blood. 2014;124(21):522–522.
Berman E. Pregnancy in patients with chronic myeloid leukemia. J Natl Compr Canc Netw. 2018;16(5S):660–2.
Palani R, Milojkovic D, Apperley JF. Managing pregnancy in chronic myeloid leukaemia. Ann Hematol. 2015;94(Suppl 2):S167–76.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
N/A
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal studies performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Kathleen M. Sakamoto and Nobuko Hijiya are co-senior authors.
This article is part of the Topical Collection on Chronic Myeloid Leukemias
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ford, M., Mauro, M., Aftandilian, C. et al. Management of Chronic Myeloid Leukemia in Children and Young Adults. Curr Hematol Malig Rep 17, 121–126 (2022). https://doi.org/10.1007/s11899-022-00673-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-022-00673-5