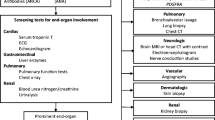
Abstract
Primary eosinophilic disorders include hypereosinophilic syndrome (HES); chronic eosinophilic leukemia, not otherwise categorized (CEL-NOC); platelet-derived growth factor receptor (PDGFR)-rearranged myeloid neoplasms; and other myeloid malignancies associated with prominent blood eosinophilia. According to the World Health Organization consensus criteria, the diagnosis of HES requires the absence of clonal cytogenetic or molecular markers of an underlying myeloid or lymphoid neoplasm. CEL-NOC constitutes an HES-like phenotype associated with an abnormal karyotype or excess blasts in blood (> 2%) or bone marrow (> 5%). HES and CEL-NOC are considered distinct from molecularly defined eosinophilic disorders, such as those associated with activating mutations of PDGFR (PDGFRA and PDGFRB) and fibroblast growth factor receptor-1. This is an important distinction because PDGFR-mutated but not other eosinophilic neoplasms are effectively treated with imatinib. Current management in HES includes observation only for asymptomatic patients with no evidence of organ damage, systemic corticosteroid therapy for acute control of symptoms, and interferon-alfa-2a or hydroxyurea as steroid-sparing agents. In patients with HES who are refractory to usual therapy and have life-threatening disease complications, the use of investigational drugs such as alemtuzumab or mepolizumab might be considered, but data on long-term efficacy and safety are limited.
Similar content being viewed by others
References and Recommended Reading
Denburg JA, Telizyn S, Messner H, et al.: Heterogeneity of human peripheral blood eosinophil-type colonies: evidence for a common basophil-eosinophil progenitor. Blood 1985, 66:312–318.
Boyce JA, Friend D, Matsumoto R, et al.: Differentiation in vitro of hybrid eosinophil/basophil granulocytes: autocrine function of an eosinophil developmental intermediate. J Exp Med 1995, 182:49–57.
Iwasaki H, Mizuno S, Mayfield R, et al.: Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J Exp Med 2005, 201:1891–1897.
McNagny K, Graf T: Making eosinophils through subtle shifts in transcription factor expression. J Exp Med 2002, 195:F43–F47.
Hirasawa R, Shimizu R, Takahashi S, et al.: Essential and instructive roles of GATA factors in eosinophil development. J Exp Med 2002, 195:1379–1386.
Yu C, Cantor AB, Yang H, et al.: Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med 2002, 195:1387–1395.
Sanderson CJ: Interleukin-5, eosinophils, and disease. Blood 1992, 79:3101–3109.
Clutterbuck EJ, Hirst EM, Sanderson CJ: Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF. Blood 1989, 73:1504–1512.
Collins PD, Marleau S, Griffiths-Johnson DA, et al.: Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J Exp Med 1995, 182:1169–1174.
Dent LA, Strath M, Mellor AL, Sanderson CJ: Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med 1990, 172:1425–1431.
Basten A, Beeson PB: Mechanism of eosinophilia. II. Role of the lymphocyte. J Exp Med 1970, 131:1288–1305.
Nielsen K, Fogh L, Andersen S: Eosinophil response to migrating Ascaris suum larvae in normal and congenitally thymus-less mice. Acta Pathol Microbiol Scand [B] Microbiol Immunol 1974, 82:919–920.
Hsu CK, Hsu SH, Whitney RA Jr, Hansen CT: Immunopathology of schistosomiasis in athymic mice. Nature 1976, 262:397–399.
Romagnani S: Th1 and Th2 in human diseases. Clin Immunol Immunopathol 1996, 80:225–235.
Brigden M, Graydon C: Eosinophilia detected by automated blood cell counting in ambulatory North American outpatients. Incidence and clinical significance. Arch Pathol Lab Med 1997, 121:963–967.
Brito-Babapulle F: The eosinophilias, including the idiopathic hypereosinophilic syndrome. Br J Haematol 2003, 121:203–223.
Tefferi A, Patnaik MM, Pardanani A: Eosinophilia: secondary, clonal and idiopathic. Br J Haematol 2006, 133:468–492.
Bain B, Pierre R, Imbert M, et al.: Chronic eosinophilic leukemia and the hypereosinophilic syndrome. In World Health Organization Classification Of Tumors: Tumors of the Hematopoietic and Lymphoid Tissues. Edited by Jaffe ES, Harris NL, Stein H, Vardiman JW. Lyon: International Agency for Research on Cancer Press; 2001:29–31.
Simon HU, Plotz SG, Dummer R, Blaser K: Abnormal clones of T cells producing interleukin-5 in idiopathic eosinophilia. N Engl J Med 1999, 341:1112–1120.
Butterfield JH: Diverse clinical outcomes of eosinophilic patients with T-cell receptor gene rearrangements: the emerging diagnostic importance of molecular genetics testing. Am J Hematol 2001, 68:81–86.
Chang HW, Leong KH, Koh DR, Lee SH: Clonality of isolated eosinophils in the hypereosinophilic syndrome. Blood 1999, 93:1651–1657.
Bain BJ: Relationship between idiopathic hypereosinophilic syndrome, eosinophilic leukemia, and systemic mastocytosis. Am J Hematol 2004, 77:82–85.
Tefferi A, Vardiman JW: Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 2007, In press.
Malcovati L, La Starza R, Merante S, et al.: Hypereosinophilic syndrome and cyclic oscillations in blood cell counts. A clonal disorder of hematopoiesis originating in a pluripotent stem cell. Haematologica 2004, 89:497–499.
Brown NJ, Stein RS: Idiopathic hypereosinophilic syndrome progressing to acute myelomonocytic leukemia with chloromas. South Med J 1989, 82:1303–1305.
Owen J, Scott JG: Transition of the hypereosinophilic syndrome to myelomonocytic leukemia. Can Med Assoc J 1979, 121:1489–1491.
Yoo TJ, Orman SV, Patil SR, et al.: Evolution to eosinophilic leukemia with a t(5:11) translocation in a patient with idiopathic hypereosinophilic syndrome. Cancer Genet Cytogenet 1984, 11:389–384.
Needleman SW, Mane SM, Gutheil JC, et al.: Hypereosinophilic syndrome with evolution to myeloproliferative disorder: temporal relationship to loss of Y chromosome and c-N-ras activation. Hematol Pathol 1990, 4:149–155.
Klion AD, Bochner BS, Gleich GJ, et al.: Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report. J Allergy Clin Immunol 2006, 117:1292–1302.
Sato Y, Taniguchi R, Yamada T, et al.: Measurement of serum concentrations of cardiac troponin T in patients with hypereosinophilic syndrome: a sensitive non-invasive marker of cardiac disorder. Intern Med 2000, 39:350.
Pitini V, Arrigo C, Azzarello D, et al.: Serum concentration of cardiac troponin T in patients with hypereosinophilic syndrome treated with imatinib is predictive of adverse outcomes. Blood 2003, 102:3456–3457; author reply 3457.
Butterfield JH, Gleich GJ: Interferon-alpha treatment of six patients with the idiopathic hypereosinophilic syndrome. Ann Intern Med 1994, 121:648–653.
Baratta L, Afeltra A, Delfino M, et al.: Favorable response to high-dose interferon-alpha in idiopathic hypereosinophilic syndrome with restrictive cardiomyopathy—case report and literature review. Angiology 2002, 53:465–470.
Yoon TY, Ahn GB, Chang SH: Complete remission of hypereosinophilic syndrome after interferon-alpha therapy: report of a case and literature review. J Dermatol 2000, 27:110–115.
Ceretelli S, Capochiani E, Petrini M: Interferon-alpha in the idiopathic hypereosinophilic syndrome: consideration of five cases. Ann Hematol 1998, 77:161–164.
Parrillo JE, Fauci AS, Wolff SM: Therapy of the hypereosinophilic syndrome. Ann Intern Med 1978, 89:167–172.
Pardanani A, Brockman SR, Paternoster SF, et al.: FIP1L1-PDGFRA fusion: prevalence and clinicopathologic correlates in 89 consecutive patients with moderate to severe eosinophilia. Blood 2004, 104:3038–3045.
Sefcick A, Sowter D, DasGupta E, et al.: Alemtuzumab therapy for refractory idiopathic hypereosinophilic syndrome. Br J Haematol 2004, 124:558–559.
Pitini V, Teti D, Arrigo C, Righi M: Alemtuzumab therapy for refractory idiopathic hypereosinophilic syndrome with abnormal T cells: a case report. Br J Haematol 2004, 127:477.
Alinari L, Lapalombella R, Andritsos L, et al.: Alemtuzumab (Campath-1H) in the treatment of chronic lymphocytic leukemia. Oncogene 2007, 26:3644–3653.
Elsner J, Hochstetter R, Spiekermann K, Kapp A: Surface and mRNA expression of the CD52 antigen by human eosinophils but not by neutrophils. Blood 1996, 88:4684–4693.
Au WY, Leung AY, Tse EW, et al.: High incidence of tuberculosis after alemtuzumab treatment in Hong Kong Chinese patients. Leuk Res 2007, In press.
Peleg AY, Husain S, Kwak EJ, et al.: Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis 2007, 44:204–212.
Koury MJ, Newman JH, Murray JJ: Reversal of hypereosinophilic syndrome and lymphomatoid papulosis with mepolizumab and imatinib. Am J Med 2003, 115:587–589.
Plotz SG, Simon HU, Darsow U, et al.: Use of an antiinterleukin-5 antibody in the hypereosinophilic syndrome with eosinophilic dermatitis. N Engl J Med 2003, 349:2334–2339.
Klion AD, Law MA, Noel P, et al.: Safety and efficacy of the monoclonal anti-interleukin-5 antibody SCH55700 in the treatment of patients with hypereosinophilic syndrome. Blood 2004, 103:2939–2941.
Kim YJ, Prussin C, Martin B, et al.: Rebound eosinophilia after treatment of hypereosinophilic syndrome and eosinophilic gastroenteritis with monoclonal anti-IL-5 antibody SCH55700. J Allergy Clin Immunol 2004, 114:1449–1455.
Jabbour E, Verstovsek S, Giles F, et al.: 2-Chlorodeoxyadenosine and cytarabine combination therapy for idiopathic hypereosinophilic syndrome. Cancer 2005, 104:541–546.
Cools J, DeAngelo DJ, Gotlib J, et al.: A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med 2003, 348:1201–1214.
Tefferi A, Gilliland DG: Oncogenes in myeloproliferative disorders. Cell Cycle 2007, 6:550–566.
Pardanani A, Ketterling RP, Li CY, et al.: FIP1L1-PDGFRA in eosinophilic disorders: prevalence in routine clinical practice, long-term experience with imatinib therapy, and a critical review of the literature. Leuk Res 2006, 30:965–970.
Jovanovic JV, Score J, Waghorn K, et al.: Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1-PDGFRA-positive chronic eosinophilic leukemia. Blood 2007, 109:4635–4640.
von Bubnoff N, Sandherr M, Schlimok G, et al.: Myeloid blast crisis evolving during imatinib treatment of an FIP1L1-PDGFR alpha-positive chronic myeloproliferative disease with prominent eosinophilia. Leukemia 2005, 19:286–287.
Ohnishi H, Kandabashi K, Maeda Y, et al.: Chronic eosinophilic leukaemia with FIP1L1-PDGFRA fusion and T674I mutation that evolved from Langerhans cell histiocytosis with eosinophilia after chemotherapy. Br J Haematol 2006, 134:547–549.
Cools J, Stover EH, Boulton CL, et al.: PKC412 overcomes resistance to imatinib in a murine model of FIP1L1-PDGFRalpha-induced myeloproliferative disease. Cancer Cell 2003, 3:459–469.
Lierman E, Folens C, Stover EH, et al.: Sorafenib is a potent inhibitor of FIP1L1-PDGFRalpha and the imatinib-resistant FIP1L1-PDGFRalpha T674I mutant. Blood 2006, 108:1374–1376.
Golub TR, Barker GF, Lovett M, Gilliland DG: Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 1994, 77:307–316.
Levine RL, Wadleigh M, Sternberg DW, et al.: KIAA1509 is a novel PDGFRB fusion partner in imatinib-responsive myeloproliferative disease associated with a t(5;14)(q33;q32). Leukemia 2005, 19:27–30.
Vizmanos JL, Novo FJ, Roman JP, et al.: NIN, a gene encoding a CEP110-like centrosomal protein, is fused to PDGFRB in a patient with a t(5;14)(q33;q24) and an imatinib-responsive myeloproliferative disorder. Cancer Res 2004, 64:2673–2676.
Wilkinson K, Velloso ER, Lopes LF, et al.: Cloning of the t(1;5)(q23;q33) in a myeloproliferative disorder associated with eosinophilia: involvement of PDGFRB and response to imatinib. Blood 2003, 102:4187–4190.
Apperley JF, Gardembas M, Melo JV, et al.: Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med 2002, 347:481–487.
Grand FH, Burgstaller S, Kuhr T, et al.: p53-Binding protein 1 is fused to the platelet-derived growth factor receptor beta in a patient with a t(5;15)(q33;q22) and an imatinib-responsive eosinophilic myeloproliferative disorder. Cancer Res 2004, 64:7216–7219.
Walz C, Metzgeroth G, Haferlach C, et al.: Characterization of three new imatinib-responsive fusion genes in chronic myeloproliferative disorders generated by disruption of the platelet-derived growth factor receptor beta gene. Haematologica 2007, 92:163–169.
Macdonald D, Reiter A, Cross NC: The 8p11 myeloproliferative syndrome: a distinct clinical entity caused by constitutive activation of FGFR1. Acta Haematol 2002, 107:101–107.
Raghavachar A, Fleischer S, Frickhofen N, et al.: T lymphocyte control of human eosinophilic granulopoiesis. Clonal analysis in an idiopathic hypereosinophilic syndrome. J Immunol 1987, 139:3753–3758.
Cogan E, Schandene L, Crusiaux A, et al.: Brief report: clonal proliferation of type 2 helper T cells in a man with the hypereosinophilic syndrome. N Engl J Med 1994, 330:535–538.
Roche-Lestienne C, Lepers S, Soenen-Cornu V, et al.: Molecular characterization of the idiopathic hypereosinophilic syndrome (HES) in 35 French patients with normal conventional cytogenetics. Leukemia 2005, 19:792–798.
Vaklavas C, Tefferi A, Butterfield J, et al.: “Idiopathic” eosinophilia with an occult T-cell clone: prevalence and clinical course. Leuk Res 2007, 31:691–694.
Roufosse F, Cogan E, Goldman M: Recent advances in pathogenesis and management of hypereosinophilic syndromes. Allergy 2004, 59:673–689.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pardanani, A., Tefferi, A. Primary eosinophilic disorders: A concise review. Curr Hematol Malig Rep 3, 37–43 (2008). https://doi.org/10.1007/s11899-008-0007-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-008-0007-9