Abstract
Purpose of Review
Pregnancy and exercise are systemic stressors that promote physiological growth of the heart in response to repetitive volume overload and maintenance of cardiac output. This type of remodeling is distinct from pathological hypertrophy and involves different metabolic mechanisms that facilitate growth; however, it remains unclear how metabolic changes in the heart facilitate growth and if these processes are similar in both pregnancy- and exercise-induced cardiac growth.
Recent Findings
The ability of the heart to metabolize a myriad of substrates balances cardiac demands for energy provision and anabolism. During pregnancy, coordination of hormonal status with cardiac reductions in glucose oxidation appears important for physiological growth. During exercise, a reduction in cardiac glucose oxidation also appears important for physiological growth, which could facilitate shuttling of glucose-derived carbons into biosynthetic pathways for growth. Understanding the metabolic underpinnings of physiological cardiac growth could provide insight to optimize cardiovascular health and prevent deleterious remodeling, such as that which occurs from postpartum cardiomyopathy and heart failure.
Summary
This short review highlights the metabolic mechanisms known to facilitate pregnancy-induced and exercise-induced cardiac growth, both of which require changes in cardiac glucose metabolism for the promotion of growth. In addition, we mention important similarities and differences of physiological cardiac growth in these models as well as discuss current limitations in our understanding of metabolic changes that facilitate growth.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Repetitive or sustained increases in cardiac workload promote hypertrophy of the adult heart, which begins as a compensatory growth process to reduce overall ventricular wall stress and maintain cardiac output. Interestingly, cardiac hypertrophy occurs in response to both pathological (e.g., hypertension) and physiological (e.g., pregnancy, exercise) stressors, with notable distinctions between the cellular and metabolic processes underlying the response to each type of stressor. Although there has been remarkable progress in the understanding of pathological cardiac hypertrophy and the stimuli which lead to harmful remodeling of the heart, much less is known regarding the mechanisms leading to physiological growth of the heart.
Both pregnancy and exercise training increase cardiac workload and drive physiological growth of the heart, which is a reversible phenomenon after parturition or prolonged cessation from exercise, respectively [1, 2]. We understand that these physiological stressors present differently in the heart than pathological stress, but recent studies support that not all physiological growth relies upon the same metabolic mechanisms for growth. By understanding the metabolic mechanisms underlying pregnancy- and exercise-induced cardiac growth, strategies can be developed to optimize cardiovascular health in response to these events and prevent adverse remodeling.
In this review, we address the following questions:
-
1.
What is physiological cardiac growth?
-
2.
What are the metabolic determinants of pregnancy-induced cardiac growth?
-
3.
What are the metabolic determinants of exercise-induced cardiac growth?
-
4.
What causes adverse cardiac events associated with pregnancy and exercise?
What Is Physiological Cardiac Growth?
Cardiac growth is simply defined as increased mass of the heart, and this typically occurs in response to elevated functional demand. Physiological growth is distinct from pathological hypertrophy in that physiological growth is associated with normal or enhanced cardiac function, increased capillary density, and no induction of the fetal gene program [1, 3]. Additionally, there are distinct metabolic events that distinguish physiological cardiac growth from pathological hypertrophy. One important distinction is that physiological cardiac growth results from intermittent or transient stress and is known to be reversible [2].
The heart is comprised of many cell types. While cardiomyocytes account for about one-third of the cells in the heart, they contribute to at least 70% of cardiac mass [4, 5]. In response to repetitive stimuli, cardiomyocyte growth is facilitated by the addition of sarcomeres, the contractile units of the heart, which form myofibrils and increase the length or width of cardiomyocytes. Parallel addition of sarcomeres increases cardiomyocyte width and leads to concentric cardiac growth—this form of growth is often associated with pressure overload from pathological stimuli such as sustained hypertension or from physiological stimuli such as weightlifting exercises. Serial addition of sarcomeres increases cardiomyocyte length and leads to eccentric cardiac growth—this form of growth is often associated with volume overload from pathological stimuli such as valvular disease or physiological stimuli such as pregnancy or aerobic exercise (reviewed in [6,7,8]). The focus of this review is on the metabolic mechanisms contributing to eccentric cardiac growth in response to pregnancy and aerobic exercise.
Several molecular events initiate and facilitate cardiac growth. Some studies have shown the requirement of signaling cascades mediated by insulin-like growth factor 1 (IGF-1) [9] and protein kinase B (AKT1) [10], while others imply the necessity of increased expression of genes such as Cbp/P300 interacting transactivator with GluAsp rich carboxy-terminal domain 4 (CITED4) [11] or microRNAs such as miR-222 [12] for physiological cardiac growth. Moreover, recent work highlights the contributions of long noncoding RNAs [13], calcium signaling [14], and lymphangiogenesis [15] in the progression of physiological cardiac growth. There are also mechano-sensing mechanisms and stretch-sensitive ion channels that are thought to coordinate overload of the heart with increased synthesis of proteins [16, 17] and could also be associated with synthesis of membrane components or nucleotides that are important for growing cells. Consistent with the requirement of increased macromolecule synthesis for growth, there are important changes in cardiac metabolism that are unique to physiological cardiac growth and instigated by pregnancy or exercise.
What Are Metabolic Determinants of Pregnancy-Induced Cardiac Growth?
Physiological cardiac growth occurs during pregnancy as a response to volume overload and is thought to compensate for increased circulatory demands. Many parallels exist between both pregnancy- and exercise-induced cardiac growth, such as both being reversible phenomena that are not associated with perturbations in cardiac function or fibrosis and both involving similar signaling mechanisms such as the activation of the phosphoinosotide 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathways [1, 18, 19]. Despite these similarities, pregnancy- and exercise-induced cardiac growth differs in the nature of cardiac overload and the mechanisms that lead to cardiomyocyte growth. These differences have been shown to occur at the transcriptomic level, where late-pregnant hearts had distinct transcriptional profiles compared with exercised hearts [20]. One reason for this difference is that exercise is an acute physiological stimulus, while pregnancy presents a chronic stimulus that is additionally affected by hormonal excursions. Of note, the rises in estrogen and progesterone throughout the course of pregnancy could alter mitochondrial function, as the binding of estrogen to its receptor has been associated with regulation of the metabolic transcription factor, nuclear respiratory factor-1 (NRF-1) (reviewed in [21]), while a recent study reports that progesterone binds a mitochondrial receptor and enhances beta-oxidation in H9C2 cells [22]. The long-term cardiac volume overload during pregnancy is associated with changes not only in hormonal status, but also in both systemic and cardiovascular metabolism.
Systemic metabolism changes dramatically during pregnancy. The early stages of pregnancy are associated with increased anabolic demands in the heart for generating macromolecules and biosynthetic precursors that are necessary to initiate and support maternal cardiac growth; however, in late pregnancy, systemic maternal metabolism shifts more to a catabolic state to divert these constituent building blocks to the fetus to support growth and development [23]. This metabolic switching is necessary to drive systemic adaptations during pregnancy. Glucose is the preferred substrate for fetal metabolism; thus, a reduction in systemic maternal glucose metabolism occurs during pregnancy. This reduction could be a result of the rise in insulin levels during pregnancy and subsequent development of peripheral insulin resistance in the mother [23,24,25]. The reduction in maternal glucose utilization might spare circulating glucose for fetal growth and also contribute to increased circulating levels of free fatty acids, triglycerides, lactate, and ketone bodies, which together sustain maternal metabolism (maternal systemic metabolism reviewed in [23]). In addition to changes in systemic metabolism, pregnancy is associated with significant cardiovascular adaptations such as increased blood volume, significant changes in hemodynamic parameters (e.g., increased cardiac output and reduced total peripheral resistance), alterations in systolic and diastolic blood pressures, and the development of reversible cardiac growth [26]. Even with this knowledge, little is known about the underlying metabolic mechanisms contributing to pregnancy-associated cardiac growth, likely due to the lack of inclusion of pregnant women and animals in clinical and preclinical studies, as well as the reduced awareness of pregnancy-associated cardiovascular adaptations and disease conditions.
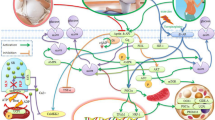
Despite the associations between changes in cardiac metabolism and ventricular remodeling, little is known about the extent of the metabolic changes in the maternal heart. Recent key metabolic findings in the maternal heart are shown in Fig. 1. Evidence suggests that reductions in cardiac glucose catabolism are essential for cardiac growth during pregnancy. Studies performed in the 1990s showed that changes in pyruvate oxidation occurred in the maternal heart [27], and, more recently, Liu et al. showed a progesterone-dependent increase in the expression of pyruvate dehydrogenase kinase 4 (Pdk4) in the late-pregnant heart [28]. In addition, those authors showed that inhibition of Pdk4 with dichloroacetate during late pregnancy inhibits cardiac growth, indicating that a reduction in cardiac glucose catabolism is required for pregnancy-induced cardiac growth. Recent findings corroborate these results, where Fulghum et al. showed a significant increase in Pdk4 expression and concomitant reduction in the phosphorylation of phosphofructokinase during late pregnancy [29••], similar to what has been observed in the heart following acute bouts of exercise [30]. In addition, increases in circulating ketone bodies and cardiac expression of Bdh1 were elevated in the maternal heart, seeming to follow the arc of pregnancy-induced cardiac growth [29••]. This is intriguing because ketone bodies are typically associated with metabolic remodeling during cardiac pathology [31, 32] but might play an important role in metabolic remodeling observed in the maternal heart during pregnancy. However, the extent to which ketone bodies influence growth of the maternal heart remains unknown. In addition, during pregnancy, the heart is thought to consume more lactate than glucose; however, the contribution of lactate to maternal cardiovascular adaptations has been vastly understudied. Therefore, it is necessary that future studies assess the contributions of these circulating metabolites to maternal systemic and cardiovascular adaptations.
Coordinated metabolic responses facilitate pregnancy-induced cardiac growth. Pregnancy-induced changes in circulating metabolite abundances lead to significant changes in the heart during late pregnancy when cardiac growth occurs. Increased Pdk4 and Bdh1 expression and reduced Pfkfb2 activity could facilitate the metabolic flexibility needed to promote anabolism in the heart and could be mediated by the rising estrogen, progesterone, and prolactin levels. Hormone levels represented in the figure follow trends from human data. Figure made using "http://biorender.com"
Increased glucose flux into ancillary biosynthetic pathways of glucose metabolism has been shown to support anabolic growth, but few studies have interrogated the full extent of metabolomic changes in the maternal heart. Recently, it was demonstrated that metabolites associated with these anabolic, pro-growth pathways, such as nucleotides, glycerophospholipids, and amino acids, were increased in the late pregnant murine heart and in 1-week post-birth murine hearts, which coincided with pregnancy-induced cardiac growth [29••]. Similar metabolite changes have been observed in newborn ovine hearts, suggesting a degree of metabolic coordination between the maternal-fetal unit. Of the amino acid metabolites identified, the urea cycle and polyamine metabolites were the most abundant in the hearts of late pregnant and post-birth mice. This included the urea cycle-associated metabolite, homoarginine, which dramatically increased during late pregnancy, similar to changes observed in the acute exercised female heart [33•], and is consistent with documented changes in nitric oxide during pregnancy [34]. This is of interest because the importance of the urea cycle has yet to be thoroughly interrogated in the heart. In addition, polyamines have been associated with cardiac hypertrophy, but few studies associate increased polyamines with cardiac growth during pregnancy. Therefore, the extent to which the urea cycle and polyamine metabolites contribute to the physiological and metabolic remodeling of the maternal heart currently warrants additional investigation. Despite this knowledge, studies have only shown a snapshot of static metabolite changes rather than information on specific metabolite flux, which would shed more light on the metabolic changes occurring in the heart during pregnancy.
What Are Metabolic Determinants of Exercise-Induced Cardiac Growth?
Regular exercise training is associated with numerous health benefits, including reductions in the risk for heart disease and adverse cardiovascular events [35]. Acute responses to exercise involve transient molecular and metabolic changes that facilitate augmented cardiac output and excitation-contraction coupling, while chronic responses to exercise involve sustained changes and structural adaptations that, like pregnancy-induced cardiac growth, reduce wall stress incurred during periods of elevated cardiac output [36]. While several molecular events are well-known to enable physiological growth of the heart, the metabolic underpinnings of physiological cardiac growth remain less evident. Current understanding of metabolic contributions to exercise-induced cardiac growth is highlighted in Fig. 2.
Coordinated metabolic responses facilitate exercise-induced cardiac growth. Exercise-induced elevations in circulating metabolites are taken up by the heart and utilized for both maintenance of contractile function as well as biosynthetic purposes. Reductions in cardiac PFK2FB2 activity influences glucose metabolism and may spare glucose-derived carbons for incorporation into biosynthetic pathways for promotion of cardiac growth. Elevations in fatty acids (FAs), branched-chain amino acids (BCAAs), glutamine, and ketone bodies (KBs) may work together to facilitate cardiac growth from routine exercise. Figure made using "http://biorender.com"
During exercise, higher rates of catecholamine-induced adipose tissue lipolysis and skeletal muscle contraction increase the concentration of fatty acids and lactate in circulation up to 2.4 mM [37] and nearly 10 mM [38, 39], respectively. As such, the heart increases its energetic reliance on these substrates while reducing the contribution of glucose oxidation to ATP production [7]. While at rest, fatty acids contribute up to 70% of oxidative metabolism in the heart [40, 41]. The contribution of fatty acids to energy production remains high during most bouts of exercise; however, during intense exercise, lactate may contribute up to 90% of total oxidative metabolism in the heart because of its high concentration in circulation [42]. Interestingly, it appears that fat oxidation and triacylglycerol turnover may increase in the presence of lactate [43, 44], which could implicate synergistic roles of lactate and fatty acids in the heart during exercise. Moreover, chronic exercise leads to elevations in cardiac expression of genes involved in fatty acid catabolism and transport [45], which increases cardiac fatty acid oxidation in the exercise-trained heart [46]. While acute exercise increases circulating lactate abundance and utilization by the heart, prolonged training appears to have minimal effects on lactate oxidation capacity or the abundance of mitochondria-associated lactate dehydrogenase [47].
During exercise, the increases in fatty acid and lactate oxidation in the heart are paired with a reduction in glucose oxidation [7]. This transient decrease in glucose utilization could be due to the heightened availability of competing substrates and may be critical for the adaptive response of the heart to exercise. During exercise, there appears to be a transient reduction in cardiac activity of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB2) activity; however, following exercise, cardiac PFKFB2 activity appears to increase. Expression of a kinase-deficient PFKFB transgene in the heart resulted in constitutive reduction in cardiac glycolysis and a physiological cardiac growth phenotype similar to that of an exercise-trained heart [30], suggesting that changes in cardiac glucose metabolism are sufficient to elicit physiological growth. The increase in cardiac glucose oxidation following exercise could be facilitated by increased translocation of GLUT4 to the cardiomyocyte membrane [48], and since less than 50% of glucose that enters the heart is fated for complete oxidation [49], increased glucose uptake may serve the purpose of shuttling glucose-sourced carbons into biosynthetic pathways. Interestingly, an increase in cardiac glycogen accumulation was observed immediately following exercise and simultaneously with reductions in glucose oxidation [30], which could facilitate a cycle of glucose storage and subsequent utilization [50] in the heart following exercise.
Ketone bodies, branched-chain amino acids (BCAAs), and glutamine contribute much less to cardiac energetics under normal conditions (<15% overall) than lactate, fatty acids, and glucose [7]. The ketone bodies β-hydroxybutyrate and acetoacetate have been shown to be important in heart failure by maintaining metabolic capacity in working heart models [32]. Although the failing heart relies upon ketone bodies, high circulating levels of ketone bodies in the absence of other substrates are associated with functional impairment of the heart [51, 52]. However, even in the presence of other substrates, ketone bodies have been shown to inhibit both glucose [53,54,55] and fatty acid oxidation [56, 57] in the heart. It is unknown if the heart uses ketone bodies solely for energy provision, as previous studies suggest ketones are anticatabolic in nature [58], and elevations in β-hydroxybutyrate reduce leucine oxidation and concomitantly increase skeletal muscle protein synthesis [59], which is possibly mediated by mechanisms that enhance mTOR signaling. Furthermore, β-hydroxybutyrate has been shown to inhibit histone deacetylases (HDACs) [60], which could activate transcription of genes that regulate glucose metabolism and cardiac adaptation to exercise. Additionally, metabolic pathway activity is influenced by the binding of β-hydroxybutyrate to hydroxycarboxylic acid receptor 2 (HCAR2) and free fatty acid receptor 3 (FFAR3). Binding of β-hydroxybutyrate to these G-protein coupled receptors has been associated with reductions in adipose lipolysis [61, 62], which further suggests that β-hydroxybutyrate could be an anti-catabolic metabolite and may implicate a temporal role in its major functions following exercise. It remains unknown, however, how these effects carry over to cardiac muscle following exercise.
One study showed that an acute bout of exercise increases circulating and cardiac abundance of β-hydroxybutyrate to a greater extent in female FVB/NJ mice than males [33•], which could indicate a sex-dependent response to cardiac utilization of ketone bodies. Other studies showed that ketogenic diets increase exercise performance in male, but not female rodents (reviewed in [63]). These findings could implicate a role of ketone body metabolism in exercise-induced cardiac growth since studies suggest that female C57BL6/J mice have greater cardiac growth responses to exercise training than males [64]; however, in humans, it appears that males have greater cardiac growth responses to exercise training than females [65]. Nevertheless, the role of cardiac ketone body metabolism in exercise-induced cardiac growth is unknown.
Branched-chain amino acids contribute minimally to myocardial oxygen consumption (<5%) but are important for protein synthesis and muscle growth [66]. In fact, a recent study indicates that myocardial accumulation of BCAAs is required for cardiomyocyte hypertrophy and is mediated in part by the coordination of glucose and BCAA utilization in the heart through Kruppel-like factor 15 (Klf15) to promote hypertrophic signaling [67•]. While circulating levels of BCAAs are dependent on exercise intensity [68, 69], myocardial abundance of BCAAs have been shown to increase following a bout of exercise but return to normal levels within 24 h [33•]. Interestingly, provision of a diet high in BCAAs for just 4 h at the end of the active phase leads to significant cardiac growth and hypertrophy of cardiomyocytes in mice [70•], which indicates that increases in cardiac BCAA abundance could be an important regulator of cardiac growth. That one high BCAA diet at the end of the active phase promotes cardiac growth implies metabolic responses may be dependent upon the time of day. Corroborating this notion is the finding that time of day significantly influences cardiac metabolite abundances following one bout of exercise. Of note, there were more changes in cardiac metabolite abundances following exercise in the early active phase (i.e., morning) compared with early rest phase (i.e., evening) exercise; however, early rest phase exercise increased cardiac abundance of corticosterone and 5-aminoimidazole-4-carboxyamide ribonucleotide (AICAR) [71•], the latter of which regulates the metabolic controller, AMP kinase (AMPK) [72]. The coordination of BCAA metabolism with other substrates in the heart may enhance hypertrophic signaling and spare glucose-sourced carbons for biosynthetic purposes; however, this hypothesis needs further examination.
While the contribution of glutamine to cardiac energetics is relatively low [7], its importance in anaplerosis and activation of biosynthetic pathways [73] might highlight its requirement for growth responses in the heart. In particular, glutamine has been shown to activate mTOR [74] and has been associated with supplying the Krebs cycle with metabolites via its interconversion to the amino acid glutamate [66] and then to α-ketoglutarate [75]. Furthermore, one group recently showed that L-glutamine supplementation reduces cardiac tissue injury following exercise [76], which may suggest an important, coordinated role of glutamine in tissue repair responses.
Taken together, exercise presents a transient stress response to the heart for maintenance of elevated cardiac output. While the heart responds via efficient oxidation of many substrates, it remains unclear how exercise influences substrate utilization to promote anabolism in the heart. Additionally, as metabolite abundances in circulation and in the heart change over time following exercise, it is important to consider temporal effects of cardiac substrate utilization during and following exercise to fully understand how exercise-induced changes in cardiac metabolism promote growth of the heart.
What Causes Adverse Cardiac Events Associated with Pregnancy and Exercise?
Although awareness of pregnancy-associated cardiovascular complications has increased in recent years, little advancement has been made in elucidating the molecular and metabolic mechanisms underpinning these diseases. This is likely due, in part, to a consequence of there being little information available regarding how these mechanisms change during normal pregnancies or, more broadly, in the female heart at all. Metabolic perturbations are seen in most non-obstetric cases of heart failure, so it seems reasonable to hypothesize that these also occur in the setting of pregnancy-associated cardiovascular complications. In support of this, it was recently shown that human iPSC-derived cardiomyocytes from patients with peripartum cardiomyopathy (PPCM) have altered lipid metabolism [77] compared with healthy hearts, while another study showed that mTOR dysregulation in the heart precipitates PPCM-like cardiac changes [78]. In addition, many preclinical models of pregnancy-associated cardiovascular diseases, such as pre-eclampsia and PPCM models, exhibit metabolic dysfunction. For example, cardiac-specific knockout of signal transducer and activator of transcription 3 (STAT3) or cardiac-specific knockout of peroxisome proliferator-activated receptor-gamma coactivator (PGC-1α) in mice not only results in a PPCM-like phenotype [79, 80] but also results in major metabolic changes in the heart. However, at this point, no studies are available that characterize metabolic flux and substrate flexibility in the maternal heart under these disease conditions. Nonetheless, many circulating biomarkers identified in pre-eclampsia patients, such as glutamate [81], tryptophan metabolites [82], and arachidonic acid metabolites [83], likely have a significant impact on metabolism and should be interrogated further. Despite these insights, the extent to which changes in cardiac metabolism play a role in pregnancy-associated diseases remains unclear.
Even though the cardiovascular complications of pregnancy might be much more prevalent than those associated with exercise, there are still important considerations for heart health when following an exercise training regime. While regular exercise has numerous benefits, both excessively prolonged and intense exercise may actually increase acute cardiac events and pathological remodeling of the heart (reviewed in [84, 85]). These types of exercise (i.e., ultramarathons) often lead to elevations in circulating cardiac troponins, with the intensity of exercise positively associated with troponin levels [86]. Furthermore, intense exercise can lead to 10-fold elevation in B-type natriuretic peptide, a marker of cardiac injury, with long-term strenuous exercise being additionally associated with myocardial fibrosis and even calcification of coronary arteries [84]. Although a single bout of strenuous exercise may depress cardiac function and mildly resemble cardiac injury [87, 88], the changes in cardiac function usually recover within a couple days following exercise [89]. Taken together, there appears to be a threshold of exercise duration and concomitant recovery that define physiological and pathological remodeling of the heart following exercise. While short-lived elevations in injury markers are observed to much lesser extent in typical exercise exertions, it remains unknown if and how these contribute to cardiac adaptation to exercise.
Conclusions
Physiological cardiac growth enables the heart to maintain elevated output during pregnancy or exercise with reduced wall stress. Coordination of myocardial substrate utilization balances substrate oxidation for provision of ATP with anabolic demands of growing cells. The mechanisms facilitating this type of growth are different than those responding to pathological stimuli; even more, the mechanisms by which pregnancy- and exercise-induced cardiac growth occur are also somewhat different. For example, both types of physiological cardiac growth appear to require reductions in glucose oxidation, which could divert glucose-sourced carbons into biosynthetic pathways, but this phenomenon might be regulated by Pdk4 in pregnancy-induced growth [28], whereas it might be regulated by PFKFB2 in exercise-induced growth [30]. Furthermore, while exercise is associated with changes in metabolite abundances in the heart [33•], the late-pregnant heart and postpartum heart are seemingly associated with a greater number of significantly changed metabolites that facilitate growth and maintenance of function [29••]. Interestingly, metabolite abundances in the female mouse heart following exercise change more than in the male heart, yet it remains unknown how the metabolic landscape of a pregnant heart might compare with the exercise-trained female heart. While several similarities seem to exist regarding physiological growth of the female heart, such as elevations in cardiac abundance of homoarginine and in circulating ketone bodies, the precise metabolic mechanisms facilitating cardiac growth in response to pregnancy or exercise remain unresolved. Since homoarginine and ketone bodies are likely liver-derived, it is important that future studies on physiological cardiac growth examine the liver-heart axis when assessing metabolism.
Currently, there are limitations to ascertaining mechanisms by which the heart grows in response to physiological stimuli or mechanisms by which the adapted heart reverts to normal size. Murine models of pregnancy are routinely used, but to date, none have examined the contribution of myocardial substrates to metabolite pools through isotope tracing, which would allow for flux analyses and would illuminate relationships between biosynthetic pathways. Similarly, in exercise, several studies perform untargeted metabolomics in the heart or use isotope tracers for short durations, which might not be adequate for deep network tracing of substrate contributions into major biosynthetic pathways. Additionally, studies on exercise-induced cardiac growth typically focus on aerobic exercise, with few studies examining the effects of resistance exercise on cardiac growth. One important reason may be the lack of consensus on murine models for weightlifting, as weightlifting maneuvers of humans are difficult to simulate in quadrupedal murine species; however, a recent study suggests there is a model that elicits similar skeletal muscle responses in mice as weightlifting does in humans [90], which could be a step forward in assessing metabolic determinants of resistance exercise-induced cardiac growth.
Most of our knowledge regarding physiological cardiac growth comes from the contribution of cardiomyocytes or whole-heart studies, but recent work (reviewed in [91]) highlights the important roles of metabolism and cell signaling in non-cardiomyocytes in the development of cardiac growth. We understand that a coordinated metabolic response facilitates growth of the heart, but future work is needed to integrate findings and to additionally elucidate the contribution of non-cardiomyocytes to cardiac growth.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chung E, Leinwand LA. Pregnancy as a cardiac stress model. Cardiovasc Res. 2014;101(4):561–70. https://doi.org/10.1093/cvr/cvu013.
Dorn GW 2nd. The fuzzy logic of physiological cardiac hypertrophy. Hypertension. 2007;49(5):962–70. https://doi.org/10.1161/HYPERTENSIONAHA.106.079426.
Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15(7):387–407. https://doi.org/10.1038/s41569-018-0007-y.
Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28(109):41–61.
Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D'Antoni ML, Debuque R, et al. Revisiting cardiac cellular composition. Circ Res. 2016;118(3):400–9. https://doi.org/10.1161/CIRCRESAHA.115.307778.
Fernandes T, Soci UP, Oliveira EM. Eccentric and concentric cardiac hypertrophy induced by exercise training: microRNAs and molecular determinants. Braz J Med Biol Res. 2011;44(9):836–47. https://doi.org/10.1590/s0100-879x2011007500112.
Fulghum K, Hill BG. Metabolic Mechanisms of exercise-induced cardiac remodeling. Front Cardiovasc Med. 2018;5:127. https://doi.org/10.3389/fcvm.2018.00127.
Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14(1):38–48. https://doi.org/10.1038/nrm3495.
Ikeda H, Shiojima I, Ozasa Y, Yoshida M, Holzenberger M, Kahn CR, et al. Interaction of myocardial insulin receptor and IGF receptor signaling in exercise-induced cardiac hypertrophy. J Mol Cell Cardiol. 2009;47(5):664–75. https://doi.org/10.1016/j.yjmcc.2009.08.028.
DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, et al. Akt1 is required for physiological cardiac growth. Circulation. 2006;113(17):2097–104. https://doi.org/10.1161/CIRCULATIONAHA.105.595231.
Bezzerides VJ, Platt C, Lerchenmuller C, Paruchuri K, Oh NL, Xiao C, et al. CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight. 2016;1(9) https://doi.org/10.1172/jci.insight.85904.
Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21(4):584–95. https://doi.org/10.1016/j.cmet.2015.02.014.
Li H, Trager LE, Liu X, Hastings MH, Xiao C, Guerra J, et al. lncExACT1 and DCHS2 regulate physiological and pathological cardiac growth. Circulation. 2022;145(16):1218–33. https://doi.org/10.1161/CIRCULATIONAHA.121.056850.
Ingrid M, Bonilla SB, Pokrass A, Mariangelo JIE, Kalyanasundaram A, Bogdanov V, Mezache L, Sakuta G, Beard CM, Belevych A, Tikunova S, Terentyeva R, Teretyev D, Davis J, Veeraraghavan R, Carnes CA, Gyorke S. STIM1 ablation impairs exercise-induced physiological cardiac hypertropohy and dysregulates autophagy in mouse hearts. J Appl Physiol. 2023; https://doi.org/10.1152/japplphysiol.00363.2022.
Bei Y, Huang Z, Feng X, Li L, Wei M, Zhu Y, et al. Lymphangiogenesis contributes to exercise-induced physiological cardiac growth. J Sport Health Sci. 2022;11(4):466–78. https://doi.org/10.1016/j.jshs.2022.02.005.
Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci U S A. 2010;107(15):7000–5. https://doi.org/10.1073/pnas.1001825107.
Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, et al. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105(10):1023–30. https://doi.org/10.1161/CIRCRESAHA.109.206581.
Li J, Umar S, Amjedi M, Iorga A, Sharma S, Nadadur RD, et al. New frontiers in heart hypertrophy during pregnancy. Am J Cardiovasc Dis. 2012;2(3):192–207.
Chung E, Yeung F, Leinwand LA. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl Physiol. 1985;112(9):1564–75. https://doi.org/10.1152/japplphysiol.00027.2012.
Chung E, Heimiller J, Leinwand LA. Distinct cardiac transcriptional profiles defining pregnancy and exercise. PLoS One. 2012;7(7):e42297. https://doi.org/10.1371/journal.pone.0042297.
Klinge CM. Estrogenic control of mitochondrial function. Redox Biol. 2020;31:101435. https://doi.org/10.1016/j.redox.2020.101435.
Dai Q, Likes CE 3rd, Luz AL, Mao L, Yeh JS, Wei Z, et al. A mitochondrial progesterone receptor increases cardiac beta-oxidation and remodeling. J Endocr Soc. 2019;3(2):446–67. https://doi.org/10.1210/js.2018-00219.
Liu LX, Arany Z. Maternal cardiac metabolism in pregnancy. Cardiovasc Res. 2014;101(4):545–53. https://doi.org/10.1093/cvr/cvu009.
King JC. Physiology of pregnancy and nutrient metabolism. Am J Clin Nutr. 2000;71(5 Suppl):1218S–25S. https://doi.org/10.1093/ajcn/71.5.1218s.
Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50(4):938–48. https://doi.org/10.1097/GRF.0b013e31815a5494.
Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003–8. https://doi.org/10.1161/CIRCULATIONAHA.114.009029.
Sugden MC, Holness MJ. Cardiac carbohydrate and lipid utilization during late pregnancy. Biochem Soc Trans. 1993;21(Pt 3):312S. https://doi.org/10.1042/bst021312s.
Liu LX, Rowe GC, Yang S, Li J, Damilano F, Chan MC, et al. PDK4 inhibits cardiac pyruvate oxidation in late pregnancy. Circ Res. 2017;121(12):1370–8. https://doi.org/10.1161/CIRCRESAHA.117.311456.
Fulghum KL, Smith JB, Chariker J, Garrett LF, Brittian KR, Lorkiewicz P, et al. Metabolic signatures of pregnancy-induced cardiac growth. Am J Physiol Heart Circ Physiol. 2022; https://doi.org/10.1152/ajpheart.00105.2022. This reference maps transcriptional, proteomic, and metabolic changes in the heart throughout the duration of pregnancy. The authors concluded that changes in mitochondrial subunit complex proteins as well as metabolic enzymes occur in late pregnancy and contribute to cardiac growth.
Gibb AA, Epstein PN, Uchida S, Zheng Y, McNally LA, Obal D, et al. Exercise-induced changes in glucose metabolism promote physiological cardiac growth. Circulation. 2017;136(22):2144–57. https://doi.org/10.1161/CIRCULATIONAHA.117.028274.
Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4(4) https://doi.org/10.1172/jci.insight.124079.
Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;133(8):698–705. https://doi.org/10.1161/CIRCULATIONAHA.115.017355.
Fulghum K, Collins HE, Jones SP, Hill BG. Influence of biological sex and exercise on murine cardiac metabolism. J Sport Health Sci. 2022; https://doi.org/10.1016/j.jshs.2022.06.001. This reference supports the importance of considering exercise intensity and biological sex and when assessing metabolic responses in the heart. The authors concluded that female FVB/NJ mice showed a greater number of metabolites changes in the heart than male mice following one bout of intense exercise.
Owusu Darkwa E, Djagbletey R, Sottie D, Owoo C, Vanderpuye NM, Essuman R, et al. Serum nitric oxide levels in healthy pregnant women: a case- control study in a tertiary facility in Ghana. Matern Health Neonatol Perinatol. 2018;4:3. https://doi.org/10.1186/s40748-017-0072-y.
Myers J. Exercise and Cardiovascular Health. Circulation. 2003;107:1. https://doi.org/10.1161/01.Cir.0000048890.59383.8d.
Lerchenmuller C, Rosenzweig A. Mechanisms of exercise-induced cardiac growth. Drug Discov Today. 2014;19(7):1003–9. https://doi.org/10.1016/j.drudis.2014.03.010.
Rodahl K, Miller HI, Issekutz B Jr. Plasma free fatty acids in exercise. J Appl Physiol. 1964;19:489–92. https://doi.org/10.1152/jappl.1964.19.3.489.
Kaijser L, Berglund B. Myocardial lactate extraction and release at rest and during heavy exercise in healthy men. Acta Physiol Scand. 1992;144(1):39–45. https://doi.org/10.1111/j.1748-1716.1992.tb09265.x.
Drake AJ, Haines JR, Noble MIM. Preferential uptake of lactate by the normal myocardium in dogs. Cardiovasc Res. 1980;14(2):65–72. https://doi.org/10.1093/cvr/14.2.65.
Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85(3):1093–129. https://doi.org/10.1152/physrev.00006.2004.
Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273(45):29530–9. https://doi.org/10.1074/jbc.273.45.29530.
Bertrand ME, Carre AG, Ginestet AP, Lefebvre JM, Desplanque LA, Lekieffre JP. Maximal exercise in normal subjects: changes in coronary sinus blood flow, contractility and myocardial extraction of FFA and lactate. Eur J Cardiol. 1977;5(6):481–91.
Goodwin GW, Taegtmeyer H. Improved energy homeostasis of the heart in the metabolic state of exercise. Am J Physiol Heart Circ Physiol. 2000;279(4):H1490–501. https://doi.org/10.1152/ajpheart.2000.279.4.H1490.
de Groot MJ, Coumans WA, Willemsen PH, van der Vusse GJ. Substrate-induced changes in the lipid content of ischemic and reperfused myocardium. Its relation to hemodynamic recovery. Circ Res. 1993;72(1):176–86. https://doi.org/10.1161/01.res.72.1.176.
Strom CC, Aplin M, Ploug T, Christoffersen TE, Langfort J, Viese M, et al. Expression profiling reveals differences in metabolic gene expression between exercise-induced cardiac effects and maladaptive cardiac hypertrophy. FEBS J. 2005;272(11):2684–95. https://doi.org/10.1111/j.1742-4658.2005.04684.x.
Riehle C, Wende AR, Zhu Y, Oliveira KJ, Pereira RO, Jaishy BP, et al. Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Mol Cell Biol. 2014;34(18):3450–60. https://doi.org/10.1128/MCB.00426-14.
Fulghum KL, Rood BR, Shang VO, McNally LA, Riggs DW, Zheng YT, et al. Mitochondria-associated lactate dehydrogenase is not a biologically significant contributor to bioenergetic function in murine striated muscle. Redox Biol. 2019;24:101177. https://doi.org/10.1016/j.redox.2019.101177.
Kolter T, Uphues I, Wichelhaus A, Reinauer H, Eckel J. Contraction-induced translocation of the glucose transporter Glut4 in isolated ventricular cardiomyocytes. Biochem Biophys Res Commun. 1992;189(2):1207–14. https://doi.org/10.1016/0006-291x(92)92333-s.
Gibb AA, Lorkiewicz PK, Zheng YT, Zhang X, Bhatnagar A, Jones SP, et al. Integration of flux measurements to resolve changes in anabolic and catabolic metabolism in cardiac myocytes. Biochem J. 2017;474(16):2785–801. https://doi.org/10.1042/BCJ20170474.
Reichelt ME, Mellor KM, Curl CL, Stapleton D, Delbridge LM. Myocardial glycophagy - a specific glycogen handling response to metabolic stress is accentuated in the female heart. J Mol Cell Cardiol. 2013;65:67–75. https://doi.org/10.1016/j.yjmcc.2013.09.014.
Russell RR 3rd, Taegtmeyer H. Changes in citric acid cycle flux and anaplerosis antedate the functional decline in isolated rat hearts utilizing acetoacetate. J Clin Invest. 1991;87(2):384–90. https://doi.org/10.1172/JCI115008.
Taegtmeyer H. On the inability of ketone bodies to serve as the only energy providing substrate for rat heart at physiological work load. Basic Res Cardiol. 1983;78(4):435–50. https://doi.org/10.1007/BF02070167.
Russell RR 3rd, Cline GW, Guthrie PH, Goodwin GW, Shulman GI, Taegtmeyer H. Regulation of exogenous and endogenous glucose metabolism by insulin and acetoacetate in the isolated working rat heart. A three tracer study of glycolysis, glycogen metabolism, and glucose oxidation. J Clin Invest. 1997;100(11):2892–9. https://doi.org/10.1172/JCI119838.
Williamson JR, Krebs HA. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J. 1961;80(3):540–7. https://doi.org/10.1042/bj0800540.
Hall LM. Preferential oxidation of acetoacetate by the perfused heart. Biochem Biophys Res Commun. 1961;6:177–9. https://doi.org/10.1016/0006-291x(61)90124-3.
Bassenge E, Wendt VE, Schollmeyer P, Bluemchen G, Gudbjarnason S, Bing RJ. Effect of Ketone Bodies on Cardiac Metabolism. Am J Physiol. 1965;208:162–8. https://doi.org/10.1152/ajplegacy.1965.208.1.162.
Little JR, Goto M, Spitzer JJ. Effect of ketones on metabolism of FFA by dog myocardium and skeletal muscle in vivo. Am J Physiol. 1970;219(5):1458–63. https://doi.org/10.1152/ajplegacy.1970.219.5.1458.
Thomsen HH, Rittig N, Johannsen M, Moller AB, Jorgensen JO, Jessen N, et al. Effects of 3-hydroxybutyrate and free fatty acids on muscle protein kinetics and signaling during LPS-induced inflammation in humans: anticatabolic impact of ketone bodies. Am J Clin Nutr. 2018;108(4):857–67. https://doi.org/10.1093/ajcn/nqy170.
Nair KS, Welle SL, Halliday D, Campbell RG. Effect of beta-hydroxybutyrate on whole-body leucine kinetics and fractional mixed skeletal muscle protein synthesis in humans. J Clin Invest. 1988;82(1):198–205. https://doi.org/10.1172/JCI113570.
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–4. https://doi.org/10.1126/science.1227166.
Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280(29):26649–52. https://doi.org/10.1074/jbc.C500213200.
Newman JC, Verdin E. beta-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr. 2017;37:51–76. https://doi.org/10.1146/annurev-nutr-071816-064916.
Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol. 2017;595(9):2857–71. https://doi.org/10.1113/JP273185.
Konhilas JP, Chen H, Luczak E, McKee LA, Regan J, Watson PA, et al. Diet and sex modify exercise and cardiac adaptation in the mouse. Am J Physiol Heart Circ Physiol. 2015;308(2):H135–45. https://doi.org/10.1152/ajpheart.00532.2014.
Marsh CE, Thomas HJ, Naylor LH, Dembo LG, Green DJ. Sex differences in cardiac adaptation to distinct modalities of exercise: a cardiac magnetic resonance study. Med Sci Sports Exerc. 2021;53(12):2543–52. https://doi.org/10.1249/MSS.0000000000002729.
Schwartz RG, Barrett EJ, Francis CK, Jacob R, Zaret BL. Regulation of myocardial amino acid balance in the conscious dog. J Clin Invest. 1985;75(4):1204–11. https://doi.org/10.1172/JCI111817.
Shao D, Villet O, Zhang Z, Choi SW, Yan J, Ritterhoff J, et al. Glucose promotes cell growth by suppressing branched-chain amino acid degradation. Nat Commun. 2018;9(1):2935. https://doi.org/10.1038/s41467-018-05362-7. This reference showed the relationship between BCAA and glucose metabolism in the heart, highlighting the importance of metabolite coordination in promoting growth.
Gawedzka A, Grandys M, Duda K, Zapart-Bukowska J, Zoladz JA, Majerczak J. Plasma BCAA concentrations during exercise of varied intensities in young healthy men-the impact of endurance training. PeerJ. 2020;8:e10491. https://doi.org/10.7717/peerj.10491.
Gumus Balikcioglu P, Ramaker ME, Mason KA, Huffman KM, Johnson JL, Ilkayeva O, et al. Branched-chain amino acid catabolism and cardiopulmonary function following acute maximal exercise testing in adolescents. Front Cardiovasc Med. 2021;8:721354. https://doi.org/10.3389/fcvm.2021.721354.
Latimer MN, Sonkar R, Mia S, Frayne IR, Carter KJ, Johnson CA, et al. Branched chain amino acids selectively promote cardiac growth at the end of the awake period. J Mol Cell Cardiol. 2021;157:31–44. https://doi.org/10.1016/j.yjmcc.2021.04.005. This reference highlights the importance of considering circadian rhythm when assessing cardiac metabolism and growth. The authors show that one 4 h feeding of a high BCAA diet at the end of the active phase promotes significant cardiomyocyte hypertrophy.
Sato S, Dyar KA, Treebak JT, Jepsen SL, Ehrlich AM, Ashcroft SP, et al. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab. 2022;34(2):329–45 e8. https://doi.org/10.1016/j.cmet.2021.12.016. This reference provides a comprehensive atlas of metabolite abundances in several tissues and in serum following exercise in early active or early rest phases. The study highlights the importance of considering time of day and its effects on tissue metabolism while demonstrating how exercise elicits a systemic, but integrated metabolic response.
Spaulding HR, Yan Z. AMPK and the adaptation to exercise. Annu Rev Physiol. 2022;84:209–27. https://doi.org/10.1146/annurev-physiol-060721-095517.
Lauzier B, Vaillant F, Merlen C, Gelinas R, Bouchard B, Rivard ME, et al. Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway. J Mol Cell Cardiol. 2013;55:92–100. https://doi.org/10.1016/j.yjmcc.2012.11.008.
Xia Y, Wen HY, Young ME, Guthrie PH, Taegtmeyer H, Kellems RE. Mammalian target of rapamycin and protein kinase A signaling mediate the cardiac transcriptional response to glutamine. J Biol Chem. 2003;278(15):13143–50. https://doi.org/10.1074/jbc.M208500200.
Gibb AA, Huynh AT, Gaspar RB, Ploesch TL, Lombardi AA, Lorkiewicz PK, et al. Glutamine uptake and catabolism is required for myofibroblast formation and persistence. J Mol Cell Cardiol. 2022;172:78–89. https://doi.org/10.1016/j.yjmcc.2022.08.002.
Lu CC, Ke CY, Wu WT, Lee RP. L-Glutamine is better for treatment than prevention in exhaustive exercise. Front Physiol. 2023;14:1172342. https://doi.org/10.3389/fphys.2023.1172342.
Hoes MF, Bomer N, Ricke-Hoch M, de Jong TV, Arevalo Gomez KF, Pietzsch S, et al. Human iPSC-derived cardiomyocytes of peripartum patients with cardiomyopathy reveal aberrant regulation of lipid metabolism. Circulation. 2020;142(23):2288–91. https://doi.org/10.1161/CIRCULATIONAHA.119.044962.
Kouzu H, Tatekoshi Y, Chang HC, Shapiro JS, McGee WA, De Jesus A, et al. ZFP36L2 suppresses mTORc1 through a P53-dependent pathway to prevent peripartum cardiomyopathy in mice. J Clin Invest. 2022;132(10) https://doi.org/10.1172/JCI154491.
Patten IS, Rana S, Shahul S, Rowe GC, Jang C, Liu L, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485(7398):333–8. https://doi.org/10.1038/nature11040.
Ricke-Hoch M, Bultmann I, Stapel B, Condorelli G, Rinas U, Sliwa K, et al. Opposing roles of Akt and STAT3 in the protection of the maternal heart from peripartum stress. Cardiovasc Res. 2014;101(4):587–96. https://doi.org/10.1093/cvr/cvu010.
Kenny LC, Broadhurst D, Brown M, Dunn WB, Redman CW, Kell DB, et al. Detection and identification of novel metabolomic biomarkers in preeclampsia. Reprod Sci. 2008;15(6):591–7. https://doi.org/10.1177/1933719108316908.
Zhao YJ, Zhou C, Wei YY, Li HH, Lei W, Boeldt DS, et al. Differential distribution of tryptophan-metabolites in fetal and maternal circulations during normotensive and preeclamptic pregnancies. Reprod Sci. 2022;29(4):1278–86. https://doi.org/10.1007/s43032-021-00759-0.
Plenty NL, Faulkner JL, Cotton J, Spencer SK, Wallace K, LaMarca B, et al. Arachidonic acid metabolites of CYP4A and CYP4F are altered in women with preeclampsia. Prostag Oth Lipid Mediat. 2018;136:15–22. https://doi.org/10.1016/j.prostaglandins.2018.03.001.
Eijsvogels TM, Fernandez AB, Thompson PD. Are there deleterious cardiac effects of acute and chronic endurance exercise? Physiol Rev. 2016;96(1):99–125. https://doi.org/10.1152/physrev.00029.2014.
Eijsvogels TM, Thompson PD. Exercise is medicine: at any dose? JAMA. 2015;314(18):1915–6. https://doi.org/10.1001/jama.2015.10858.
Eijsvogels TM, Hoogerwerf MD, Oudegeest-Sander MH, Hopman MT, Thijssen DH. The impact of exercise intensity on cardiac troponin I release. Int J Cardiol. 2014;171(1):e3–4. https://doi.org/10.1016/j.ijcard.2013.11.050.
Middleton N, Shave R, George K, Whyte G, Hart E, Atkinson G. Left ventricular function immediately following prolonged exercise: a meta-analysis. Med Sci Sports Exerc. 2006;38(4):681–7. https://doi.org/10.1249/01.mss.0000210203.10200.12.
Dawson E, George K, Shave R, Whyte G, Ball D. Does the human heart fatigue subsequent to prolonged exercise? Sports Med. 2003;33(5):365–80. https://doi.org/10.2165/00007256-200333050-00003.
McGavock JM, Warburton DE, Taylor D, Welsh RC, Quinney HA, Haykowsky MJ. The effects of prolonged strenuous exercise on left ventricular function: a brief review. Heart Lung. 2002;31(4):279–92; quiz 93-4. https://doi.org/10.1067/mhl.2002.126106.
Cui D, Drake JC, Wilson RJ, Shute RJ, Lewellen B, Zhang M, et al. A novel voluntary weightlifting model in mice promotes muscle adaptation and insulin sensitivity with simultaneous enhancement of autophagy and mTOR pathway. FASEB J. 2020;34(6):7330–44. https://doi.org/10.1096/fj.201903055R.
Trager LE, Lyons M, Kuznetsov A, Sheffield C, Roh K, Freeman R, et al. Beyond cardiomyocytes: Cellular diversity in the heart’s response to exercise. J Sport Health Sci. 2022; https://doi.org/10.1016/j.jshs.2022.12.011.
Acknowledgements
Figures 1 and 2 were made using "http://biorender.com".
Funding
The study is supported in part by grants from the NIH (HL147844, GM127607, HL154663, HL163003) and the Jewish Heritage Fund for Excellence Funding program (Louisville).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schulman-Geltzer, E.B., Collins, H.E., Hill, B.G. et al. Coordinated Metabolic Responses Facilitate Cardiac Growth in Pregnancy and Exercise. Curr Heart Fail Rep 20, 441–450 (2023). https://doi.org/10.1007/s11897-023-00622-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-023-00622-0