Abstract
Purpose of Review
To summarise the role of different imaging techniques for diagnosis and investigation of heart failure in women.
Recent Findings
Although sex differences in heart failure are well recognised, and the scope of imaging techniques is expanding, there are currently no specific guidelines for imaging of heart failure in women.
Summary
Diagnosis and stratification of heart failure is generally performed first line using transthoracic echocardiography. Understanding the aetiology of heart failure is central to ongoing management, and with non-ischaemic causes more common in women, a multimodality approach is generally required using advanced imaging techniques including cardiovascular magnetic resonance imaging, nuclear imaging techniques, and cardiac computed tomography. There are specific considerations for imaging in women including radiation risks and challenges during pregnancy, highlighting the clear unmet need for cardiology and imaging societies to provide imaging guidelines specifically for women with heart failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is increasingly recognised that there are sex differences in the epidemiology, aetiology, presentation, and outcomes of heart failure [1, 2], and that these should be reflected in patient management. Position statements from leading cardiovascular organisations now include recommendations specific for women [3•]; however, dedicated guidelines specific to cardiac imaging in heart failure are lacking. Imaging is required throughout the clinical pathway of patients with heart failure, from confirming diagnosis to understanding aetiology, monitoring disease progression and response to treatment and risk stratification, and generally there is no universal strategy that will be optimal for all patients.
This review paper aims to summarise the role of cardiac imaging in women with heart failure, and identify the best imaging tools to address specific clinical scenarios in order to optimise clinical outcomes.
Heart Failure in Women
Heart failure (HF) is an important cause of morbidity and mortality in women and it is estimated that 1 in 5 women will develop HF over the age of 40 years [4]. Women tend to develop HF at an older age in comparison to men, and non-ischaemic causes such as hypertension and valvular heart disease are more common.
HF in women is more commonly classified as heart failure with preserved (HFpEF) or mildly reduced ejection fraction (left ventricular ejection fraction 41–49%) than when compared to men, where left ventricular ejection fraction (LVEF) is more commonly reduced (LVEF ≤40%) [5]. Large registry studies have demonstrated that all patients with HF have high rates of 5-year mortality and rehospitalisation [6], and despite differences in baseline characteristics (females have more hypertension, anaemia, and depression, and less coronary artery disease (CAD), hyperlipidaemia, atrial fibrillation, and tobacco use) [7, 8], both men and women with HF with reduced ejection fraction (HFrEF) or HFpEF have similar rates of in-hospital mortality during an admission for acute decompensated heart failure [7].
Additionally, hospital admission rates for HF have decreased over time in men, but increased in women [9]. A recent meta-analysis of HF clinical trial data found that women with HFpEF were around 20% less likely to experience death or hospitalisation over a 4.5-year follow-up period, though this difference was less pronounced in the presence of atrial fibrillation, renal dysfunction, stable angina, or advanced NYHA symptoms [8]. This increase in survival appears to be offset by a decrease in quality of life, as women living with HF have higher self-reported psychological and physical disability scores [10].
The currently available HF guidelines do not address sex-specific recommendations in terms of diagnosis or outcomes, and have minimal content regarding the difference in underlying aetiologies [11, 12]. There is a sex-specific guideline relating to prevention of cardiovascular disease in women [13]; however, it relates primarily to risk factor stratification and treatment optimisation, and is now a decade out of date.
Imaging for Diagnosis of Heart Failure
HF is a clinical syndrome resulting from structural and/or functional cardiac abnormalities leading to inadequate cardiac output at rest or on exercise or lead to increased intracardiac pressures [14]. Diagnosis is clinical, based on characteristic signs and symptoms; however, confirmation and classification requires quantification of LVEF, which also aids patient management and risk stratification. Echocardiography is generally the first-line imaging modality used for LVEF; however, alternative strategies are available and may have specific advantages in women where acoustic windows may be challenging (Table 1).
Imaging for Heart Failure Aetiology and Prognosis
Following the diagnosis of heart failure, further imaging may be required to understand the aetiology in order to guide subsequent clinical management. Table 2 summarises the imaging characteristics of the varying imaging modalities discussed in this review and used in different causes of heart failure in women. Figure 1 provides exemplar images of these varying imaging modalities.
Varying imaging modalities and causes of heart failure in women. TTE, (transthoracic echocardiogram) — images A–C. Patient with non-ischaemic cardiomyopathy. Apical 4-chamber view in diastole (A) and systole (B) showing severely impaired systolic function (LVEF 20%) by Simpson’s Biplane. Strain map showing globally reduced longitudinal strain (C). CCT, cardiovascular computed tomography — images D–G. Coronary artery calcium scoring (D), curved reformats showing mixed calcified and non-calcified atheroma (E) and calcified plaque (F), and short axis cine (G). CMR, cardiovascular magnetic resonance — images H–K. 4ch cine of dilated heart (H), subendocardial infarct on LGE images (I), mid-wall inflammation of myocarditis seen on T1 map (J), global subendocardial perfusion defect on short axis perfusion map (K). PET, positron emission tomography — images L–N. Focal intense FDG uptake in the left ventricle (D–E) and affecting the basal inferoseptum, inferior, inferolateral, and lateral walls (E). Axial slice showing FDG avidity in the mediastinal lymph nodes (F) in a patient with a history of systemic and cardiac sarcoidosis
Coronary Artery Disease
Although non-ischaemic aetiologies predominate in women with HFrEF, CAD remains important and diagnosis is commonly late due to both atypical presenting symptoms and reduced diagnostic accuracy of standard imaging investigations. Women are at increased risk of developing heart failure post-ST-elevation myocardial infarction, and outcomes are worse.
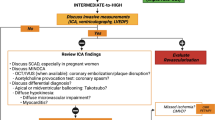
A 2014 consensus statement from the American Heart Association for non-invasive imaging of suspected CAD in women recommended exercise electrocardiograph (ECG) for low-to-intermediate risk women, cardiac computed tomography (CCT) for intermediate risk women, and stress imaging (myocardial perfusion imaging, echocardiography, cardiovascular magnetic resonance (CMR)) for intermediate-to-high risk women [15], although these guidelines were not specific for diagnosing CAD in heart failure. There are, however, different challenges with many of these imaging methods. Exercise ECG is known to be less sensitive and specific in women [16], and nuclear imaging can yield false positive results with perfusion defects in the left ventricular anterior wall due to breast attenuation [17]. Breast irradiation also requires consideration with both nuclear imaging and CCT. The latest European Society of Cardiology heart failure guidelines make no sex-specific recommendations, and propose CCT in patients with low to intermediate risk of CAD and invasive coronary angiography for symptomatic patients with angina despite medical therapy [14]. Advanced imaging has further demonstrated that women have a higher prevalence of non-obstructive CAD than obstructive CAD, despite having more risk factors [18], leading to worse cardiovascular outcomes [19, 20].
Microvascular Disease
Although epicardial coronary disease is less common, there is growing evidence that coronary microvascular disease or dysfunction (CMD) plays a significant role in the pathophysiology of CAD in women [21]. CMD is defined as impaired vasodilatation of arterioles resulting in a blunted increase in blood flow from rest to stress. CMD appears to be more prevalent in women compared to men [22], and has been shown to be an important prognostic marker linked to increased cardiovascular events in women [21]. It is also thought to play a key role in the pathophysiology of HFpEF, with abnormal coronary flow reserve (CFR) found in HFpEF patients undergoing invasive coronary physiological testing [23] and thought to result in increased myocardial fibrosis [24], driving clinical HFpEF. Further larger studies are required to confirm these findings and to evaluate the prognostic significance in heart failure.
Although conventionally diagnosed via invasive coronary physiological measurements, non-invasive imaging techniques (positron emission tomography [PET] and CMR) are now able to quantify myocardial blood flow (MBF) at rest and with hyperaemia following adenosine or regadenoson, and hence calculate CFR [25, 26]. PET-CT can help to differentiate CMD (reduced CFR and normal epicardial coronary anatomy) from obstructive CAD (reduced CFR and epicardial stenosis). PET is now considered a non-invasive alternative to invasive methods, and the characterisation of CMD by PET has been shown to be clinically prognostic [27, 28]; however, the risk of exposure to ionising radiation should be considered.
Recent technological advances in CMR have permitted automated quantitative measurement of MBF using myocardial perfusion mapping [29] at rest and following adenosine stress, enabling calculation of MBF. The absence of a regional perfusion defect and detection of reduced global stress MBF (<2.25 ml/g/min) has been shown to accurately detect CMD when compared against the standard assessment using invasive measures of index of microcirculatory resistance [30], and may be of benefit in women with HF, particularly with symptoms of chest pain but no epicardial coronary disease.
Cancer Treatment–Related Cardiac Dysfunction
There is increased recognition of the importance of healthy survivorship in oncology, with prioritisation of early detection and management of treatment-related complications within clinical guidelines [31]. Many cancer therapies including those used to treat breast cancer (anthracyclines and HER-2 therapies) can lead to cardiac complications including HF, hence the introduction of serial surveillance imaging for cancer therapy-related cardiac dysfunction (CTRCD). CTRCD is defined as a decrease in LVEF by 10 percentage points, to a value less than 50% using echocardiography [14, 32]. Sex differences in incidence and mortality are well established across many different cancer types, and many of the malignancies with female preponderance (notably breast) are treated with potentially cardiotoxic treatments making surveillance screening especially important.
Echocardiography is the recommended first line for assessment of cardiotoxicity in all published oncology and cardiology guidelines [31,32,33•] due to its wide availability, safety profile, lack of ionising radiation, patient tolerability, and cost effectiveness. However, 2D echocardiography depends on good-quality acoustic windows which is commonly challenging, particularly for breast cancer patients following mastectomy or reconstructive implants. Reproducibility of LVEF by 2D echocardiography is around 10% [34], the same threshold for diagnosis of CTRCD, leading to concerns regarding the use of 2D echocardiography for serial surveillance screening. Whilst 3D echocardiography provides superior accuracy and precision due to the lack of geometric assumptions, it may not be feasible in all patients as it too depends on quality acoustic windows [34].
Echocardiography-derived global longitudinal strain (GLS) has been increasingly adopted by guidelines as an adjunctive early biomarker for diagnosis of CTRCD. Recent data from a study of strain-guided management of potentially cardiotoxic chemotherapy [35•] (94% female participants) found that patients following a GLS (as compared to LVEF)–guided pathway for administration of cardioprotective medications had less cardiotoxicity, although LVEF reductions were similar in the two groups. Further data is needed, to more clearly determine the role of GLS in this context.
CMR overcomes the reliance on acoustic windows and is currently recommended for CTRCD surveillance where echocardiographic images are suboptimal or conflicting, or where discontinuation of chemotherapy is considered [32, 35•]. CMR-derived LVEF demonstrates superior reproducibility with a minimum detectable difference of 5.9% [36] and is therefore of significant benefit in such patients. Higher operational costs with more limited availability however preclude it from more widespread use in this context. CMR may also provide additional insights into the underlying mechanisms of cardiotoxicity given its tissue characterisation techniques; late gadolinium enhancement (LGE) imaging and parametric mapping methods (T1, T2, and ECV mapping) for identifying and quantifying myocardial injury and oedema. LGE is not commonly found with CTRCD secondary to anthracyclines and/or trastuzumab [37]; therefore, the absence of LGE could help distinguish anthracycline- and/or trastuzumab-related cardiomyopathy from unrelated cardiomyopathies. CMR with T1, T2, and ECV mapping has shown acutely elevated values in those with acute toxicity, although studies have been small thus far [38].
Of note, multi-gated acquisition scans were historically used first line to monitor for anthracycline toxicity; however, the associated radiation exposure and inability to interrogate the wider cardiovascular structures or measure strain mean that echocardiography should be used in preference [39].
Peripartum Cardiomyopathy
Peripartum cardiomyopathy (PPCM) is a form of HFrEF which develops either during the last trimester or early within the postpartum period [40•]. PPCM is generally a diagnosis of exclusion but a detailed clinical history and relevant clinical tests are required to rule out other important differential diagnoses of HF in this context. Echocardiography is the first-line imaging modality, with LVEF <45% used for diagnosis [41]. CMR is commonly requested for tissue characterisation where the aetiology remains unclear, and to rule out alternative diagnoses [42]. In one multicentre study involving 34 patients, 71% of patients had a non-specific LGE pattern [43], and whilst there was no typical LGE patterns specific to PPCM, its use can help to determine other differentials such as myocarditis. Both CMR and contrast echocardiography can also be important for assessing the complications of PPCM such as LV thrombus formation, which can occur in 10–17% of cases [44].
Sarcoidosis
Sarcoidosis is a multi-organ, systematic granulomatous disorder of unknown cause which has a slightly higher prevalence in females [45]. The characteristic features of sarcoidosis on imaging generally include bilateral hilar lymphadenopathy, peri-lymphatic nodules (CT), osteolytic bone changes (CT/MRI), and parotid uptake on nuclear imaging [46].
The prevalence of cardiac sarcoidosis has increased significantly over the past decades, and HF at presentation is noted to carry a particularly poor outcome [47]. Of those with cardiac sarcoidosis, isolated cardiac sarcoidosis has been reported in cases ranging from 27 to 54%, and these patients with isolated cardiac involvement were found to have worse LV systolic function at presentation compared to those with systemic sarcoidosis [48]. Generally, echocardiography is used for suspected sarcoidosis, with commonly described findings including impaired right or left ventricular systolic or diastolic function, regional wall motion abnormalities, aneurysms, focal wall thinning, and impaired GLS [49]. Nuclear imaging using SPECT may identify focal perfusion defects which may correspond to granulomatous replacement of myocardium, and FDG-PET can be useful to detect active cardiac sarcoidosis via increased FDG uptake [49] suggestive of inflammation. Focal perfusion defects on cardiac PET have been shown to correlate with higher risk of cardiac death or ventricular tachycardia [50], which can be helpful prognostically. Finally, CMR has a high sensitivity and specificity for detecting cardiac involvement in sarcoidosis, where scarring may be extensive and detected via LGE imaging, and commonly seen in the basal anteroseptum. Notably, LV dysfunction in sarcoidosis is generally accompanied by scar, and alternative diagnoses should be considered if LV impairment is seen without LGE on CMR. Alongside scar assessment for diagnosis, disease activity can be monitored with CMR via assessment of oedema and inflammation detectable with T2-weighted imaging, with quantification via T1 and T2 parametric mapping. This can be used to identify areas for endomyocardial biopsy which can increase the sensitivity of tissue diagnosis as well as monitoring response to therapy [49].The presence of high burden LGE on CMR in patients cardiac sarcoidosis has additionally been shown to be a marker of poor prognosis with patients being at increased risk of major adverse cardiac events [51•].
Takotsubo Cardiomyopathy
Takotsubo or stress-induced cardiomyopathy (TTC) has a clear female preponderance with 80–90% of cases found in women [52, 53]. Triggers have more commonly been shown to be emotional in females and physical in males [53, 54]. Initial diagnosis is often via echocardiography, although obstructive CAD generally should be excluded using invasive angiography or urgent CCT if available. The majority of TTC patients display a classical pattern of regional wall motion abnormalities with circumferential hypokinesia/akinesia of the apical LV segments, with normal or hyper-dynamic contraction of the basal segments, giving the appearance of LV apical ballooning. There are also other recognised phenotypes of TTC, including the midventricular-variant characterised by akinesia of the midventricular LV with hyper-dynamic basal and apical contraction, and the reverse variant, which demonstrates basal LV akinesia with hyper-dynamic apical LV contraction, as well as right ventricular involvement with akinesia.
CMR can provide additional information beyond echocardiography in TTC. CMR may demonstrate increases in T2 signal intensity and native T1, T2, and ECV values co-located to the wall motion abnormality [55], which can persist after function normalises. LGE is not typically a feature of TTC; however, recent data has emerged showing that small amounts of LGE may be identified acutely in 10–40% of patients [56]. This LGE is usually less bright (“low-intensity LGE”) compared to the LGE associated with myocardial infarction and myocarditis, and is reversible.
Complications can occur in TTC that can be identified on imaging. These include pleural and pericardial effusions, LV thrombus, and LV outflow tract obstruction with systolic anterior motion of the mitral valve due to the hyper-dynamic basal contraction. Echocardiographic features are similar between the sexes, although an increased rate of LV thrombus has been observed in males [52].
Autoimmune Diseases
Autoimmune rheumatic diseases (ARDs) have a sex bias towards women of approximately 2:1, and may be higher (7:1 for systemic lupus erythematosus (SLE)) [57]. The risk of incident cardiovascular disease is significantly higher in patients with rheumatoid arthritis [58], SLE [59], and systemic sclerosis [60] and related coronary disease outcomes in these populations are worse [61]. Alongside ischaemia (which is often poorly diagnosed and treated), causes of heart failure in patients with ARDs include myocarditis (with or without myocardial fibrosis), and valvular disease [62•]. Whilst the majority of disease-modifying anti-rheumatic drugs have no effect on major adverse cardiac events [63], some anti-rheumatic drugs have also been demonstrated to have adverse cardiovascular effects, which rarely have been reported to cause restrictive cardiomyopathy during prolonged use (chloroquine) and to precipitate episodes of acute congestive HF (cyclophosphamide) or worsen existing heart failure (TNF-alpha inhibitors) [64, 65].
Echocardiography and CMR are the two most commonly utilised modalities to assess ARD-related heart failure, with CMR favoured for tissue characterisation to assess for active inflammation and guide risk stratification and therapy in the future [66]. Current guidelines on the use of CMR in rheumatology patients with HF note the usual pattern of diastolic dysfunction with a low prevalence of systolic abnormalities seen related to ARDs, and highlight the technique’s ability to make relevant findings relating to fibrosis and inflammation that may relate to ARD activity [67]. In addition to its high diagnostic accuracy for CAD [68], stress CMR provides a functional imaging option that avoids radiation exposure for younger females — particularly those who are unable to exercise to an adequate level due to arthritis or other musculoskeletal limitations.
PET/SPECT can be useful in the diagnosis of ARD-related myocarditis or vasculitis by demonstrating increased metabolic activity in the myocardium or circumferentially in a region of affected vessel walls [69]. PET can be useful for monitoring response to treatment, with metabolic changes being identifiable before any anatomical changes that would be identifiable by CCT or MRI [69].
Valvular Heart Disease Including Aortic Stenosis
Several recent studies have demonstrated that the pathophysiology and clinical presentation in valvular heart disease may be different between women and men — particularly in aortic stenosis, with increasing recognition of the impact of myocardial remodelling and fibrosis on both symptoms and outcome. For example, despite having a higher LVEF at presentation, women are more likely to have paradoxical low-flow low-gradient aortic stenosis compared to men and this may be contributory to the pathophysiology of aortic stenosis in women and later referrals for intervention, despite paradoxical low-flow low-gradient aortic stenosis carrying a worse prognosis [70].
Early diagnosis is of paramount importance to improve clinical outcomes in women with aortic stenosis, and echocardiography is recommended first line [71] and is gold standard for non-invasive haemodynamic assessment. Recent data has, however, shown added value of other modalities for better phenotyping of patients with aortic stenosis and further interrogating sex differences in the pathophysiology. At a valvular level, women reach a similar haemodynamic degree of stenosis severity with lower levels of valvular calcification compared to men, necessitating different thresholds for determining severe stenosis using multi-detector computed tomography; current suggested thresholds are 1200 Agatston units in women and 2000 Agatston units in men [71, 72]. Explanted stenotic valves show differing fibrosis scores, adding further evidence to sex-related differences in underlying pathophysiology [73]. At a ventricular level, myocardial remodelling patterns with severe aortic stenosis differ between sex based on evidence from CMR that is not apparent using 2-dimensional echocardiography. One study [74] included 168 patients (45% female) undergoing surgical intervention for severe AS, and showed women were significantly more likely to have normal LV geometry or concentric remodelling and increased LVEF compared to men. Men, however, more commonly displayed concentric hypertrophy or eccentric hypertrophy with larger indexed volumes and a maladaptive phenotype which resulted in a lower LVEF, higher cardiac blood biomarkers (NT-proBNP and hsTnT), and more focal and diffuse fibrosis. The relatively lower myocardial fibrosis seen with aortic stenosis in females has also been demonstrated in a multicentre CMR study with LGE imaging, which showed that the presence of LGE was associated with adverse prognosis in both sexes [75•].
Special Considerations: Radiation and Pregnancy
Cardiovascular disease is an important cause of morbidity and mortality during pregnancy [76], and therefore, cardiovascular assessment is commonly required. There are unique challenges to overcome when imaging in pregnancy including maternal and foetal radiation, exposure to magnetic fields during MR scans, and foetal exposure to contrast agents. Therefore, the risks and benefits must be carefully weighed and non-ionising imaging modalities such as echocardiography and MRI should be used first line where possible.
Radiation Exposure
Radiation exposure has the potential for both stochastic effects and deterministic effects on the developing foetus and the risks are highest between 3rd and 8th weeks’ gestation [77]. Stochastic effects (such as inducing malignancy) are the result of cellular damage at DNA level and the radiation dose-effect relationship is unpredictable. Deterministic effects, in contrast, are predictable effects once threshold radiation doses have been exceeded and these effects are due to multicellular damage. Theoretical risks depending on timing of radiation exposure include malformations and spontaneous death.
With regard to stochastic effects, an exposure of 50 mGy is considered to double the relative risk of childhood cancer from 0.1 to 0.2% and traditionally a threshold of 150mGy is used for deterministic effects [77]. However, if the benefit outweighs the risk, then an informed discussion between the patient and clinician is required prior to the use of ionising radiation. It is important to note that the dose of radiation to the foetus from cardiac imaging is generally low if the foetus can be kept out of the direct X-ray beam, and shielding used where appropriate. The dose to a foetus from a prospectively gated CCT is usually around 1 mGy, 5–17mGy for radionuclide SPECT, 2 mGy for PET, and 0.074 mGy for an invasive coronary angiogram [78]. Iodinated contrast agents are known to cross the human placenta; however, teratogenic effects have not been detected clinically after the administration of these media, despite a theoretical potential to induce foetal hypothyroidism. The American College of Radiology therefore recommends that iodinated contrast should therefore not be withheld if indicated during pregnancy. Foetal radiation exposure may be higher with nuclear imaging due to the distribution of radiopharmaceuticals which may concentrate in the maternal bladder with proximity to the placenta. Despite this, doses from typical diagnostic nuclear medicine and PET agents are not expected to approach exposures exceeding 50 mGy [78]. Doses should be kept as low as possible, and the mother should be encouraged to keep hydrated in order to encourage frequent voiding.
Radiation exposure to breast tissue is also of concern. It has been predicted that a CCT could result in a lifetime excess relative risk for breast cancer of 1.4–2.6% and 0.2–0.4% in women aged 25 and 55 years respectively [79]. It is also important to note that lactating breast is more radiosensitive than in the non-pregnant state, and the principles of “as low as reasonably achievable” should be stringently applied. Radiation reduction techniques including cranial breast displacement have been shown to reduce the breast skin entrance dose during CCT [80].
MRI
There has been no evidence to date to suggest that MRI (up to 3T) causes harm to the baby in utero [81]; therefore, CMR can be performed safely in pregnancy, and both the American College of Radiology and the European Society of Cardiology recommend that diagnosis of complex cardiac disease should use MRI where other basic modalities (principally echocardiography) are inadequate. It is however generally recommended to wait until after 12 weeks gestation where possible and to scan at the lowest possible field strength. However, the use of gadolinium contrast agents has been associated with infiltrative skin conditions, rheumatological conditions, and an increased risk of stillbirth or neonatal death, and hence is best avoided [81]. In the post-partum period, there is no evidence to suggest harm to the baby from gadolinium during breastfeeding [82]. The yield from CMR in pregnancy is high — in the largest series of its kind, Herrey et al. showed in data from 84 patients that CMR changed management in 35% and in 50% of patients who received contrast, of whom almost half were undergoing scans for cardiomyopathy/myocarditis [83•].
Conclusions
Diagnosis and stratification of HF is generally performed first line using transthoracic echocardiography. Understanding the aetiology of heart failure is central to ongoing management, with non-ischaemic causes more commonly found in women. This generally involves use of one or more advanced imaging techniques including CMR or PET for tissue characterisation, and CCT or nuclear myocardial perfusion imaging for coronary assessment. There are additional specific considerations for imaging in women including radiation risks and challenges with imaging during pregnancy. There is now a clear unmet need for cardiology and imaging societies to provide imaging specific guidelines for women with heart failure.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Motiejunaite J, Akiyama E, Cohen-Solal A, Maggioni AP, Mueller C, Choi DJ, et al. The association of long-term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur Heart J. 2020;41(13):1357–64.
Lawson CA, Zaccardi F, Squire I, Ling S, Davies MJ, Lam CSP, et al. 20-year trends in cause-specific heart failure outcomes by sex, socioeconomic status, and place of diagnosis: a population-based study. Lancet Public Health. 2019;4(8):e406–e20.
• Ordovas KG, Baldassarre LA, Bucciarelli-Ducci C, Carr J, Fernandes JL, Ferreira VM, et al. Cardiovascular magnetic resonance in women with cardiovascular disease: position statement from the Society for Cardiovascular Magnetic Resonance (SCMR). J Cardiovasc Magn Reson. 2021;23(1):52 This paper is an important position statement regarding CMR and cardiovascular disease in women.
Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–72.
Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43(3):317–27.
Shah Kevin S, Xu H, Matsouaka Roland A, Bhatt Deepak L, Heidenreich Paul A, Hernandez Adrian F, et al. Heart failure with preserved, borderline, and reduced ejection fraction. J Am Coll Cardiol. 2017;70(20):2476–86.
Hsich EM, Grau-Sepulveda MV, Hernandez AF, Peterson ED, Schwamm LH, Bhatt DL, et al. Sex differences in in-hospital mortality in acute decompensated heart failure with reduced and preserved ejection fraction. Am Heart J. 2012;163(3):430–7.e3.
Dewan P, Rørth R, Raparelli V, Campbell Ross T, Shen L, Jhund Pardeep S, et al. Sex-related differences in heart failure with preserved ejection fraction. Circ Heart Fail. 2019;12(12):e006539.
Sun LY, Tu JV, Coutinho T, Turek M, Rubens FD, McDonnell L, et al. Sex differences in outcomes of heart failure in an ambulatory, population-based cohort from 2009 to 2013. CMAJ. 2018;190(28):E848–E54.
Dewan P, Rørth R, Jhund Pardeep S, Shen L, Raparelli V, Petrie Mark C, et al. Differential impact of heart failure with reduced ejection fraction on men and women. J Am Coll Cardiol. 2019;73(1):29–40.
Yancy Clyde W, Jessup M, Bozkurt B, Butler J, Casey Donald E, Drazner Mark H, et al. 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2013;62(16):e147–239.
Yancy Clyde W, Jessup M, Bozkurt B, Butler J, Casey Donald E, Colvin Monica M, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol. 2017;70(6):776–803.
Mosca L, Benjamin Emelia J, Berra K, Bezanson Judy L, Dolor Rowena J, Lloyd-Jones Donald M, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update. J Am Coll Cardiol. 2011;57(12):1404–23.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Mieres Jennifer H, Gulati M, Bairey Merz N, Berman Daniel S, Gerber Thomas C, Hayes Sharonne N, et al. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease. Circulation. 2014;130(4):350–79.
Morise AP, Diamond GA. Comparison of the sensitivity and specificity of exercise electrocardiography in biased and unbiased populations of men and women. Am Heart J. 1995;130(4):741–7.
Burrell S, MacDonald A. Artifacts and pitfalls in myocardial perfusion imaging. J Nucl Med Technol. 2006;34(4):193–211 quiz 2-4.
Lansky AJ, Ng VG, Maehara A, Weisz G, Lerman A, Mintz GS, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5(3, Supplement):S62–72.
Shaw Leslee J, Shaw Richard E, Merz CNB, Brindis Ralph G, Klein Lloyd W, Nallamothu B, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology–National Cardiovascular Data Registry. Circulation. 2008;117(14):1787–801.
Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women's Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009;169(9):843–50.
Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135(6):566–77.
Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv. 2015;8(11):1445–53.
Yang JH, Obokata M, Reddy YNV, Redfield MM, Lerman A, Borlaug BA. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22(3):432–41.
Loffler AI, Pan JA, Balfour PC Jr, Shaw PW, Yang Y, Nasir M, et al. Frequency of coronary microvascular dysfunction and diffuse myocardial fibrosis (measured by cardiovascular magnetic resonance) in patients with heart failure and preserved left ventricular ejection fraction. Am J Cardiol. 2019;124(10):1584–9.
Feher A, Sinusas AJ. Quantitative assessment of coronary microvascular function: dynamic single-photon emission computed tomography, positron emission tomography, ultrasound, computed tomography, and magnetic resonance imaging. Circ Cardiovasc Imaging. 2017;10(8).
Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3(6):623–40.
Schindler TH, Nitzsche EU, Schelbert HR, Olschewski M, Sayre J, Mix M, et al. Positron emission tomography-measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol. 2005;45(9):1505–12.
Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129(24):2518–27.
Kellman P, Hansen MS, Nielles-Vallespin S, Nickander J, Themudo R, Ugander M, et al. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson. 2017;19(1):43.
Kotecha T, Martinez-Naharro A, Boldrini M, Knight D, Hawkins P, Kalra S, et al. Automated pixel-wise quantitative myocardial perfusion mapping by CMR to detect obstructive coronary artery disease and coronary microvascular dysfunction: validation against invasive coronary physiology. JACC Cardiovasc Imaging. 2019;12(10):1958–69.
Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO clinical practice guidelines. Ann Oncol. 2012;23(Suppl 7):vii155–66.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39.
• Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017;19(1):9–42 This paper provides a summary of the role of imaging in cancer treatment related cardiac dysfunction.
Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61(1):77–84.
• Thavendiranathan P, Negishi T, Somerset E, Negishi K, Penicka M, Lemieux J, et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol. 2021;77(4):392–401 This paper provides a summary of the role of strain in cancer treatment related cardiac dysfunction.
Lambert J, Lamacie M, Thampinathan B, Altaha MA, Esmaeilzadeh M, Nolan M, et al. Variability in echocardiography and MRI for detection of cancer therapy cardiotoxicity. Heart. 2020;106(11):817–23.
Modi K, Joppa S, Chen KA, Athwal PSS, Okasha O, Velangi PS, et al. Myocardial damage assessed by late gadolinium enhancement on cardiovascular magnetic resonance imaging in cancer patients treated with anthracyclines and/or trastuzumab. Eur Heart J Cardiovasc Imaging. 2021;22(4):427–34.
Thavendiranathan P, Wintersperger BJ, Flamm SD, Marwick TH. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: a systematic review. Circ Cardiovasc Imaging. 2013;6(6):1080–91.
Huang H, Nijjar PS, Misialek JR, Blaes A, Derrico NP, Kazmirczak F, et al. Accuracy of left ventricular ejection fraction by contemporary multiple gated acquisition scanning in patients with cancer: comparison with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017;19(1):34.
• Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(2):207–21 This paper provides an excellent summary of peripartum cardiomyopathy and the role of imaging.
Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–78.
Pennell DJ. Cardiovascular magnetic resonance. Circulation. 2010;121(5):692–705.
Haghikia A, Rontgen P, Vogel-Claussen J, Schwab J, Westenfeld R, Ehlermann P, et al. Prognostic implication of right ventricular involvement in peripartum cardiomyopathy: a cardiovascular magnetic resonance study. ESC Heart Fail. 2015;2(4):139–49.
Cooper LT, Mather PJ, Alexis JD, Pauly DF, Torre-Amione G, Wittstein IS, et al. Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail. 2012;18(1):28–33.
Cozier YC, Berman JS, Palmer JR, Boggs DA, Serlin DM, Rosenberg L. Sarcoidosis in black women in the United States: data from the Black Women's Health Study. Chest. 2011;139(1):144–50.
Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, et al. Diagnosis and detection of sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;201(8):e26–51.
Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, et al. Cardiac sarcoidosis. Circulation. 2015;131(7):624–32.
Okada DR, Bravo PE, Vita T, Agarwal V, Osborne MT, Taqueti VR, et al. Isolated cardiac sarcoidosis: a focused review of an under-recognized entity. J Nucl Cardiol. 2018;25(4):1136–46.
Ramirez R, Trivieri M, Fayad ZA, Ahmadi A, Narula J, Argulian E. Advanced imaging in cardiac sarcoidosis. J Nucl Med. 2019;60(7):892.
Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63(4):329–36.
• Franke KB, Marshall H, Kennewell P, Pham HD, Tully PJ, Rattanakosit T, et al. Risk and predictors of sudden death in cardiac sarcoidosis: a systematic review and meta-analysis. Int J Cardiol. 2021;328:130–40 This study shows the role of imaging and risk prediction in cardiac sarcoidosis.
Murakami T, Yoshikawa T, Maekawa Y, Ueda T, Isogai T, Sakata K, et al. Gender differences in patients with takotsubo cardiomyopathy: multi-center registry from Tokyo CCU Network. PLoS One. 2015;10(8):e0136655-e.
Schneider B, Athanasiadis A, Stöllberger C, Pistner W, Schwab J, Gottwald U, et al. Gender differences in the manifestation of tako-tsubo cardiomyopathy. Int J Cardiol. 2013;166(3):584–8.
Agdamag AC, Patel H, Chandra S, Rao A, Suboc TM, Marinescu K, et al. Sex differences in takotsubo syndrome: a narrative review. J Women's Health. 2019;29(8):1122–30.
Dabir D, Luetkens J, Kuetting DLR, Feisst A, Isaak A, Schild HH, et al. Cardiac magnetic resonance including parametric mapping in acute takotsubo syndrome: preliminary findings. Eur J Radiol. 2019;113:217–24.
Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306(3):277–86.
Angum F, Khan T, Kaler J, Siddiqui L, Hussain A. The prevalence of autoimmune disorders in women: a narrative review. Cureus. 2020;12(5):e8094-e.
Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71(9):1524.
Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145(5):408–15.
Au K, Singh MK, Bodukam V, Bae S, Maranian P, Ogawa R, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum. 2011;63(7):2078–90.
Mohamed MO, Roddy E, Ya’qoub L, Myint PK, Al Alasnag M, Alraies C, et al. Acute myocardial infarction in autoimmune rheumatologic disease: a nationwide analysis of clinical outcomes and predictors of management strategy. Mayo Clin Proc. 2021;96(2):388–99.
• Mavrogeni SI, Markousis-Mavrogenis G, Koutsogeorgopoulou L, Dimitroulas T, Vartela V, Rigopoulos A, et al. Pathophysiology and imaging of heart failure in women with autoimmune rheumatic diseases. Heart Fail Rev. 2019;24(4):489–98 This paper provides an excellent summary of heart failure, imaging and autoimmune diseases.
Authors/Task Force m, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(39):2733–79.
Gasparyan AY, Ayvazyan L, Cocco G, Kitas GD. Adverse cardiovascular effects of antirheumatic drugs: implications for clinical practice and research. Curr Pharm Des. 2012;18(11):1543–55.
Lee KS, Kronbichler A, Eisenhut M, Lee KH, Shin JI. Cardiovascular involvement in systemic rheumatic diseases: an integrated view for the treating physicians. Autoimmun Rev. 2018;17(3):201–14.
Mavrogeni SI, Sfikakis PP, Dimitroulas T, Koutsogeorgopoulou L, Katsifis G, Markousis-Mavrogenis G, et al. Can cardiovascular magnetic resonance prompt early cardiovascular/rheumatic treatment in autoimmune rheumatic diseases? Current practice and future perspectives. Rheumatol Int. 2018;38(6):949–58.
Mavrogeni SI, Kitas GD, Dimitroulas T, Sfikakis PP, Seo P, Gabriel S, et al. Cardiovascular magnetic resonance in rheumatology: current status and recommendations for use. Int J Cardiol. 2016;217:135–48.
Greenwood JP, Maredia N, Younger JF, Brown JM, Nixon J, Everett CC, et al. Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of coronary heart disease (CE-MARC): a prospective trial. Lancet. 2012;379(9814):453–60.
James OG, Christensen JD, Wong TZ, Borges-Neto S, Koweek LM. Utility of FDG PET/CT in inflammatory cardiovascular disease. Radiographics. 2011;31(5):1271–86.
Clavel MA, Dumesnil JG, Capoulade R, Mathieu P, Senechal M, Pibarot P. Outcome of patients with aortic stenosis, small valve area, and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60(14):1259–67.
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739–91.
Clavel MA, Pibarot P, Messika-Zeitoun D, Capoulade R, Malouf J, Aggarval S, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol. 2014;64(12):1202–13.
Simard L, Cote N, Dagenais F, Mathieu P, Couture C, Trahan S, et al. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: is valvular fibrosis the explanation? Circ Res. 2017;120(4):681–91.
Treibel TA, Kozor R, Fontana M, Torlasco C, Reant P, Badiani S, et al. Sex dimorphism in the myocardial response to aortic stenosis. JACC Cardiovasc Imaging. 2018;11(7):962–73.
• Singh A, Musa TA, Treibel TA, Vassiliou VS, Captur G, Chin C, et al. Sex differences in left ventricular remodelling, myocardial fibrosis and mortality after aortic valve replacement. Heart. 2019;105(23):1818–24 This study shows the sex differences in aortic stenosis.
American College of O, Gynecologists' Presidential Task Force on P, Heart D, Committee on Practice B-O. ACOG Practice Bulletin No. 212: Pregnancy and Heart Disease. Obstet Gynecol. 2019;133(5):e320–e56.
Wieseler KM, Bhargava P, Kanal KM, Vaidya S, Stewart BK, Dighe MK. Imaging in pregnant patients: examination appropriateness. Radiographics. 2010;30(5):1215–29 discussion 30-3.
Colletti PM, Lee KH, Elkayam U. Cardiovascular imaging of the pregnant patient. AJR Am J Roentgenol. 2013;200(3):515–21.
Hurwitz LM, Reiman RE, Yoshizumi TT, Goodman PC, Toncheva G, Nguyen G, et al. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: implications for cancer induction. Radiology. 2007;245(3):742–50.
Foley SJ, McEntee MF, Achenbach S, Brennan PC, Rainford LS, Dodd JD. Breast surface radiation dose during coronary CT angiography: reduction by breast displacement and lead shielding. AJR Am J Roentgenol. 2011;197(2):367–73.
Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316(9):952–61.
Rofsky NM, Weinreb JC, Litt AW. Quantitative analysis of gadopentetate dimeglumine excreted in breast milk. J Magn Reson Imaging. 1993;3(1):131–2.
• Herrey AS, Francis JM, Hughes M, Ntusi NAB. Cardiovascular magnetic resonance can be undertaken in pregnancy and guide clinical decision-making in this patient population. Eur Heart J Cardiovasc Imaging. 2019;20(3):291–7 This study provides an excellent summary of use of cardiovascular magnetic resonance in pregnancy.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Sex and Gender Aspects in Heart Failure
The editors would like to thank Dr. Christiane Angermann for taking the time to review this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kozor, R., Abiodun, A., Kott, K. et al. Non-invasive Imaging in Women With Heart Failure — Diagnosis and Insights Into Disease Mechanisms. Curr Heart Fail Rep 19, 114–125 (2022). https://doi.org/10.1007/s11897-022-00545-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-022-00545-2