Abstract
Purpose of Review
Growing evidence supports the contribution of age in the composition and function of the gut microbiome, with specific findings associated with health in old age and longevity.
Recent Findings
Current studies have associated certain microbiota, such as Butyricimonas, Akkermansia, and Odoribacter, with healthy aging and the ability to survive into extreme old age. Furthermore, emerging clinical and pre-clinical research have shown promising mechanisms for restoring a healthy microbiome in elderly populations through various interventions such as fecal microbiota transplant (FMT), dietary interventions, and exercise programs.
Summary
Despite several conceptually exciting interventional studies, the field of microbiome research in the elderly remains limited. Specifically, large longitudinal studies are needed to better understand causative relationships between the microbiome and healthy aging. Additionally, individualized approaches to microbiome interventions based on patients’ co-morbidities and the underlying functional capacity of their microbiomes are needed to achieve optimal results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past several decades, scientists have established an extensive relationship between the gastrointestinal (GI) microbiome and host health. For example, commensal microbiota contribute to host immune system development and function [1], with disruptions potentially contributing to immune-mediated diseases such as systemic lupus erythematosus (SLE) and inflammatory bowel disease (IBD) [2, 3]. Microbiome composition and function further influence the metabolism of nutrients and drugs [4, 5]. Growing research also suggests an important role for microbes in the gut-brain axis that modulates neuropsychological and sensory disorders, such as autism and irritable bowel syndrome (IBS) [6, 7].
Scientists have yet to identify one specific healthy microbiome, and it is generally agreed upon that there is no singular “normal” composition [8]. This is likely due to the vast spectrum of factors that influence the gut microbiome, including variations in diet [9], genetics [10], and environment [11]. As such, changes in the microbiome also occur as part of the natural aging process. Microbiome development begins at birth as soon as newborns exit the vaginal canal and encounter the mother’s vaginal fluids [12], or alternatively through the skin and the environment for babies delivered by Cesarean section [13]. For breast-fed infants, breastmilk contains several prebiotics (e.g., human milk oligosaccharides) which selectively support the growth of beneficial bacteria in the GI tract [13]. As children transition to solid foods, they encounter new dietary components such as starches and cell wall polysaccharides; their microbiomes must then shift to select for bacteria capable of metabolizing these nutrients [14]. While changes in infant and early childhood microbiomes have been studied extensively, there is less information regarding alterations in the gut microbiome of the elderly. Accordingly, there is growing interest in understanding the effect of aging on the host microbiome and whether aging and its associated features, such as frailty and declined cognition, can be modulated by gut bacteria. According to the United Nations, the number of people over the age of 65 worldwide in 2021 was 761 million, with that number expected to rise to 1.6 billion by 2050 [15]. A greater understanding of this population’s microbiome is thus of growing relevance in addressing human health and disease.
Increasing research suggests that natural, or “healthy”, aging leads to specific changes in gut microbiome composition, such as the loss of certain commensal genera, including Prevotella, Faecalibacterium, and Bifidobacterium, and the species Eubacterium rectale [16, 17]. These taxa are instead replaced at older age by other commensal organisms, such as Butyricimonas, Akkermansia, and Odoribacter [12, 16,17,18]. Akkermansia muciniphila, in particular, has been widely studied in aging and disease and is known to contribute to mucin degradation in the intestines [19]. Some have speculated that Akkermansia levels can indicate health status, with an increased relative abundance (above that seen in healthy aging) associated with excellent health among centenarians and a decreased relative abundance associated with thinning of the gut mucus layer and decreased acylglycerol, [18, 20] an endocannabinoid that regulates gut permeability and decreases intestinal inflammation [18, 20].
Scientists have also identified several pathobionts, or conditionally pathogenic microorganisms, that are increased in “unhealthy” aging [17, 18], a process characterized by rapid physical and mental decline and associated with disease progression and physical frailty. Some of these pathobionts include Eggerthella, Actinomyces, and Enterobacteriaceae, the presence and quantity of which may help physicians predict lifespan and disease outcomes [17, 18].
One challenge in conducting and interpreting microbiome studies in the elderly is distinguishing results attributable solely to age from those due to different states of health. Unique subjects by which to study these questions are those who are of “extreme” old age, such as centenarians (≥ 100 years old) and supercentenarians (≥ 110 years old). Microbiome features of these extremely aged individuals presumably confer longevity rather than any deleterious aspects of aging. For example, bacterial strains that are often decreased in the elderly, such as Christensenella and Bifidobacterium, are actually increased in semi-supercentenarians (i.e., 105–109 years old) [18], suggesting their beneficial effect. Additionally, the highly studied Akkermansia taxon, which is abundant in healthy aging, is even more dramatically increased in extreme aging [18].
To further clarify the independent contributions of age and health, Wilmanski et al. cross-sectionally evaluated gut microbiome compositions by decade of life in 3653 U.S. adults, aged 18–87 years old. The authors found that starting around 40–60 years old, individuals become more “unique” in their microbiomes as measured by the Bray–Curtis dissimilarity matrix, in which individuals’ microbiomes were compared to the one most similar from the remaining subjects [21••]. More microbiome dissimilarity was seen with each passing decade, a finding that was consistent regardless of sex, body mass index (BMI), and alpha diversity (i.e., within-sample diversity). Samples in this analysis were evaluated in two separate groups (n = 2539 and n = 1114) as there was a change in microbiome vendors and sample processing during the study; nevertheless, the finding of greater microbiome dissimilarity with increasing age was present in each cohort, lending further strength to the results. In the same publication, the investigators analyzed a different cohort of only men, aged > 78 yo (n = 599 discovery cohort and n = 308 validation cohort). Again, microbiome uniqueness, as measured by Bray–Curtis dissimilarity, was positively correlated with age. Notably, the strength of this correlation increased among healthy participants, as determined by medication use, self-perceived health, life-space score (LSC) [22], and walking speed. When sub-analyzing the community dwellers of this male cohort (i.e., those who did not reside in nursing homes, assisted living, or were hospitalized in the past 12 months; n = 706), investigators identified a correlation between the relative abundance of Bacteroides and all-cause mortality, independent of multiple potential confounders (e.g., age, BMI, and self-perceived health). This association between Bacteroides abundance and mortality was even stronger among community dwelling subjects in this cohort 85 + years old, suggesting the potential use of decreased Bacteroides as a longevity biomarker [21••]. In contrast, a large 2023 cohort study (n = 1575, including 297 centenarians) found a relative abundance of Bacteroides in centenarians and furthermore identified several other “youth-associated” features, such as decreased pathobionts, in centenarians [23]. Thus, the role of Bacteroides spp. and their utility as a gut microbial predictor of long life requires additional study. Another growing area of research is exploring the centenarian virome, with early results suggesting increased viral diversity and unique genera enrichment that distinguish centenarians from other older adults (> 60) [24•].
The logical next steps in this line of research will be longitudinal studies of the microbiome to determine whether microbes associated with longevity are present earlier in life (suggesting their role in predicting or promoting long life) or if their increase occurs only upon old age, suggesting that these changes are relevant only in older age or are secondary to other factors of extreme age.
Geriatric Health and the Microbiome
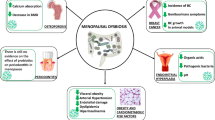
Although we know that microbiome compositions shift throughout the aging process, the exact mechanisms for this are unclear. Below, we explore some common life changes and medical conditions among the elderly in which intestinal microbiomes are altered (Fig. 1).
Inflamm-aging
Studies from as early as the 1960’s have indicated a decrease in immune function in aging adults [25]. This process, now known as immunosenescence, is associated with a decline in immune system function that leads to an accumulation of pro-inflammatory cytokines. The increased inflammatory state in elderly populations is now commonly referred to as “inflamm-aging” [26]. Pro-inflammatory states place patients at higher risk for a variety of conditions such as autoimmune and cardiovascular diseases, as well as infections [27,28,29].
Within the GI tract, the maintenance of functioning epithelial and mucus barriers is essential for protection against infection and disease [30]. Increased intestinal permeability can lead to translocation of microbes into host circulation, exacerbating a pro-inflammatory state [30]. A study in wildtype C57BL/6 mice showed that age-associated disruption of the small intestine mucosal barrier led to increased interaction between gut microbiota and the host immune system, as determined by fluorescent in situ hybridization using the bacterial probe EUB338-Alexa Fluor 488, as well as enlargement of solitary intestinal lymphoid tissue (SILT), which are hypertrophied upon interaction with gut microbes [31, 32]. Barrier defects were also associated with relative decreases in Akkermansia [31].
Experiments in germ-free mouse models by Thevaranjan et al. suggest that it is the changing microbiome itself in aging populations that leads to a pro-inflammatory state, with germ-free mice living much longer than their conventional counterparts [33]. Furthermore, young, germ-free mice gavaged with the microbiome of older mice developed greater intestinal permeability and circulating TNF than mice gavaged with microbiomes of other young mice [33]. However, additional research, especially from longitudinal studies in humans, will be necessary to confirm a causal relationship between the gut microbiome and inflamm-aging.
Diet and Environment
Elderly individuals requiring greater assistance with activities of daily living (ADLs) may transition from community living to long-term care facilities. This relocation has been shown to produce microbiome shifts due to presumed changes in environmental, dietary, and medical factors [34]. For instance, in general adult population studies, the microbes residing on household surfaces correlate with gut microbiome composition, which bears consideration in the transition to long-term care environments. Furthermore, both aging and exposure to healthcare facilities, such as long-term care facilities, are associated with an increased risk for Clostridioides difficile infection (CDI), a major cause of healthcare-associated, inflammatory diarrhea [35].
Regardless of age, there is strong evidence to suggest that specific diets can cause unique alterations in the microbiome [36, 37], as well as corresponding serum and fecal metabolites [38]. A well-controlled study by Tanes et al. followed 30 subjects who were randomized to vegan (high fiber), omnivore (intermediate fiber) and formula-based (no fiber) diets [39]. After 6 days, the subjects were given a “gut purge” using a combination of oral antibiotics and polyethylene glycol. Researchers found that the microbiome of vegan subjects recovered more rapidly after the “purge” compared to the other groups, regaining greater diversity in a shorter time span [39]. Subjects adhering to the formula-based diet, on the other hand, had the most prolonged recovery phase. In another cross-sectional study, the gut microbiomes of previously uncontacted Yanomami Amerindians, who live in the High Orinoco state of Venezuela and eat a largely plant-based, high-fiber diet, were compared to microbiomes of individuals residing in the United States and semi-transculturated populations, such as Guahibo Amerindians and Malawians. The Yanomami were noted to have a markedly higher gut microbiome diversity compared to those in the United States, with the semi-transculturated populations having an intermediate level of diversity. It is noted, however, that other social and medical factors, rather than diet alone, could also have contributed to this increased diversity [40].
Nevertheless, a component of age-related changes in the microbiome appears definitively related to diet and eating, particularly as the elderly are at increased risk for poor dentition or chewing difficulties, decreased appetite, and lack of social support in obtaining nutritious foods [41]. For example, one of the most dramatic diet changes that has been shown to cause microbiome alterations is the move from independent, community living to assisted living within a long-term care facility. This transition often leads to a change from a high-fiber, low-fat diet to a low-fiber, high-fat diet, which has been associated with a shift to a lower diversity microbiome in long-term care residents compared to community dwelling counterparts [34]. Of note, the disparity between these long-term care residents and community dwellers correlated with the amount of time spent in long-term care. During digestion, fiber is metabolized into short chain fatty acids (SCFAs), which provide many benefits to the GI tract by serving as an energy source for protective microbiota, assisting with anti-inflammatory responses, and maintaining gut barrier integrity [42]. Thus, SCFA deficiencies caused by dietary changes when moving to long term care facility can indirectly contribute to intestinal dysfunction.
Co-morbidities
An area of growing interest is the study of the gut-brain axis via microbes that may influence cognitive function (Table 1). The topic is of particular relevance as mild cognitive impairment (MCI) is highly prevalent in the elderly, affecting approximately 10% of those aged 70–74 yo and 25% of those 80–84 yo [43]. Furthermore, patients with MCI are far more likely to progress to dementia [43]. Pharmacologic treatments to date can only slow the progression of MCI, but not reverse it [44]. While there is still disagreement on whether microbiome alterations influence cognitive function and vice versa [45, 46], ongoing long-term projects such as MOTION (Microbiome Of the ageing gut and its effect on human gut health and cogniTION), which studies cognitive and microbiome changes of healthy aging [47], provide hope that these interactions will soon be clarified.
A 2019 study of shotgun metagenomic sequences, comparing 57 nursing home residents with dementia, including Alzheimer’s disease (AD), with 51 elderly individuals without AD or other forms of dementia, revealed higher levels of pro-inflammatory gut bacteria in those with dementia [61]. The authors also noted a decrease in butyrate-synthesizing bacterial species, such as those in the genera Butyrivibrio and Eubacteria, in the AD group when compared to both subjects without dementia and subjects with other dementias besides AD [61]. A subsequent systematic review and metanalysis similarly found decreased alpha diversity in the gut microbiomes of AD patients compared to healthy controls, but not between those with mild cognitive impairment (MCI) and healthy controls. Differences in microbiome compositions between AD, MCI, and healthy samples (i.e., beta diversity) were not consistently altered [62]. One challenge in studying the gut microbiome as it relates to dementia is the lack of clear, objective, and non-invasive tests to conclusively determine diagnosis and disease stage, thus further complicating the interpretation of study results. While beyond the scope of the gut microbiome, we note with interest that post-mortem studies of AD brain tissue have identified the presence of microbes within the brain, suggesting the presence of a brain microbiome associated with neurodegenerative disease [63].
Furthermore, a large genome wide association study identified several microbiome genera associated with high risk alleles of the apolipoprotein E ε4 (APOE ε4) gene, a well-established risk factor for AD [64•]. Some of the most significant findings of this study included a strong correlation between the pro-inflammatory genus Collinsella and APOE risk alleles, as well as a proposed protective role for the genus Eubacterium fissicatena [64•].
Parkinson’s disease (PD) is another neurological disorder that is more common in the elderly and for which there is growing interest in the gut microbiome as a biomarker or therapy. A 2020 meta-analysis of 16S sequencing data from Japan, the United States, Finland, Russia, and Germany found that patients with PD have relatively decreased Roseburia and Faecalibacterium – both important producers of the SCFA butyrate [65]. A 2022 shotgun sequencing study of 490 PD and 234 healthy controls confirmed these findings and also identified several other genera that are altered in PD patients, such as an increase in pathogenic species of Prevotella [66•]. Interestingly, multiple studies have noted an increase in the Akkermansia genus [65] among those with PD. This is surprising considering Akkermansia is generally associated with healthy aging and is particularly abundant in supercentenarians [18]. Some scientists have speculated that Akkermansia is an important component of healthy aging, but that increased abundance puts patients at risk for neurocognitive disease [67]. We further hypothesize that changes in Akkermansia abundance may be secondary to the development of constipation, a common gastrointestinal complication of PD and a condition that has independently been associated with increased Akkermansia in multiple other studies [68]. As the link between PD and Akkermansia is an inconsistent finding [66•], further research is needed to determine the precise role of this genus in PD and in aging more broadly. In a PD mouse model that overexpresses α-synuclein aggregates, a common finding in the brains of PD patients, mice colonized with the gut microbiome of 6 human PD patients had increased physical motor impairments and constipation compared to mice colonized with healthy donor microbiota [69]. Building on these early findings of altered microbiota in PD, a pilot randomized control trial found that stool from healthy donors, given as lyophilized pills twice a week for 12 weeks, could improve constipation and gut motility as well as transiently improve objective motor skills among patients with mild to moderate PD [70•]. While significant translational and clinical data development are still needed, these initial findings maintain the promise that gut microbiome modulation may improve gastrointestinal and/or neurological symptoms of PD and provide deeper insight into disease pathophysiology.
Several early-stage studies have also been conducted on the relationship between the gut microbiome and sarcopenia, the progressive deterioration of muscle mass that occurs with aging and that leads to physical frailty. While these studies have yielded conflicting results about which bacterial species are increased or decreased in the condition, study findings have consistently demonstrated no change in overall microbial diversity between frail and non-frail elderly individuals [71, 72]. Pre-clinical experiments have also suggested a role for gut bacteria in skeletal health, although details of how these effects are mediated have been unclear [73, 74]. In correlating human subject research, a relatively large 16S study (i.e., 60 individuals with osteoporosis and 60 age- and gender-matched controls with normal bone mineral density) found a relative abundance of Actinomyces, Clostridium XIVa, Eggerthella, and Lactobacillus and a relative decrease in Veillonella in those with osteoporosis. There were, however, no changes in overall microbiome alpha diversity between groups [75].
Interventions to Delay or Reverse Aging
While a more thorough understanding is required of the microbial changes that can be isolated to age specifically, studies have already begun evaluating how to restore a healthy microbiome in aging populations to promote health and longevity.
FMT
Fecal microbiota transplant (FMT) is a therapy that has been growingly incorporated into the treatment of recurrent CDI [76] and has furthermore been studied for inflammatory bowel disease [77] and post-antibiotic dysbiosis [78]. During FMT, stool from a healthy donor is transplanted into a recipient via colonoscopy, naso- or oro-enteric tubes, enema, or capsule, with the goal of transferring the corresponding intestinal microbes, as well as their contained functions and metabolic products. This has led some to speculate whether the microbiome from a young, healthy donor can be transplanted into an elderly individual to reverse some of the effects of unhealthy aging (Table 2).
A study by Parker et. al demonstrated that transfer of an “aged” microbiome from elderly mice to younger mice caused several age-associated phenotypes including advanced central nervous system deterioration and vision deficits [79•]. Importantly, in a set of correlating experiments, age-related changes improved in elderly mice after microbiome transplantation with stool of younger mice [79•]. This work provides strong pre-clinical evidence that microbiome profiles between young and aged mice are not only different, but that the associated physiological effects of these microbiomes are transferrable. These and similar findings have been reproduced by other investigators [59], including D’Amato et al., who demonstrated that transferring the microbiome of elderly mice to young ones can lead to cognitive deficits [80].
Progeria is a particularly unique disease by which to study microbiome and senescence, as affected individuals carry a mutation in the gene encoding lamin A which leads to rapid aging. Despite a normal appearance at birth, affected individuals typically develop fatal complications of their disease, predominantly cardiovascular disease, in their teens or early adulthood [89]. Using a mouse model of progeria, Bárcena et al. showed that certain bacterial strains enriched in human centenarians such as Akkermansia muciniphila can be transplanted to increase mouse lifespan and to reverse intestinal mucosal thinning [81]. Although these findings are still in the preclinical phase, they hold exciting promise for the use of FMT from young donors, or its therapeutic components, to reverse certain aspects of unhealthy aging.
Diet and Probiotics
As discussed previously, elderly populations often have variations in diet as they age, which contribute to microbiome changes. One of the most studied dietary changes associated with aging is reduced fiber intake; however clinical trials supplementing fiber have yielded conflicting results regarding shifts in microbiota composition and inflammatory status [90, 91], with some researchers hypothesizing that the efficacy of dietary interventions and supplements may depend on the host’s initial microbiome profile. For example, in a double-blind, crossover trial of 21 healthy volunteers over 60 years old who were given supplemental wheat bran-derived arabinoxylan-oligosaccharide found that resulting microbiome compositions varied based on subjects’ initial Prevotella abundance [92]. Although limited, these findings suggest that an individualized approach is required to manipulate the microbiome, with screening of patients’ initial microbiomes necessary to tailor the intervention needed for the desired outcome.
In addition to specific supplements, certain diets have been associated with gut health. The Mediterranean diet, consisting of plant-based foods, whole grains, and healthy fats, has been shown to prevent cardiovascular disease in all age ranges [93], with the effects of this diet potentially mediated by the gut microbiome. For example, a 2020 study by Ghosh et al. found that adherence to the Mediterranean diet for at least one year corresponded to a relative increase in intestinal F. prausnitzii, R. hominis, E. rectale, E. eligens, E. xylanophilum, B. thetaiotaomicron, P. copri and A. hadrus [48••]. Adherence to the diet furthermore correlated with improved cognitive function, as measured by the BabCock Memory Score and Constructional Praxis, as well as decreased systemic inflammatory markers such as high-sensitivity C reactive protein (hsCRP) and interleukin 17 (IL-17) levels. Mouse studies have also demonstrated that a Western diet, which is high in fat and sodium, leads to an increased “predicted age” of the gut microbiome based on a Bayesian model trained on male C57BL/6 J mice whose microbiomes were characterized from week 9 to week 112 of life. These microbiome disturbances reversed once the mice returned to a standard diet [94]. Interventional diet studies evaluating both the gut microbiota and clinical outcomes in elderly, human cohorts are therefore of particular interest given these individuals’ susceptibility to cognitive decline and unhealthy aging.
Probiotic interventions have been specifically studied in the aged. Unfortunately, similar to studies in the general population, the generation of clinically actionable data has been dampened by the great heterogeneity of studied products and outcomes as well as the multitude of underpowered studies [95]. While no singular or combination of probiotic organisms have been identified to definitively improve or reverse signs of aging [96], a growing number of studies are evaluating specific microbial strains and their impact on objective physiological effects. For example, in a double blind, placebo controlled study, L. reuteri ATCC PTA 6475 supplementation in elderly women with low bone mineral density improved tibia total volumetric BMD (vBMD) [97, 98]. Furthermore, in the Senescence Accelerated Mouse-Prone 8 (SAMP8) mouse model, probiotic Lactobacillus casei Shirota administration reduced age-related muscle deterioration and mitochondrial dysfunction [99]. In humans, some small, but double-blinded randomized controlled trials have identified specific probiotics that appear to improve cognitive function in elderly adults, especially probiotics that include Bifidobacterium and Lactobacillus spp. [49, 50]. Thus, as a more rigorous understanding between microbiome manipulation and objective health measures develops, probiotic therapies may entail customized cocktails of microorganisms to target specific deficiencies or conditions in a personalized approach to care.
Exercise
Multiple studies have reported an alteration in the gut microbiome following the implementation of an exercise program [100], with early results suggesting that this is true in elderly populations as well [101,102,103]. A 2020 study by Zhu et al. utilized fecal specimens from the American Gut Project, which also included patient-reported information on BMI and exercise habits [102]. The study included samples from 1,589 adults (aged 18–60 years) with a healthy BMI (18.5 ≤ BMI ≤ 25) and 897 elderly patients (aged > 60), who were further stratified by BMI into normal weight (n = 462), overweight (BMI > 25, n = 413) and underweight (BMI < 18.5, n = 22), as well as by exercise frequency. Investigators found that as the reported frequency of exercise increased in elderly patients, the microbiome of the elderly patients more closely resembled that of the healthy BMI adults based on the relative abundance of specific taxa and common pathways. For example, the relative abundance of Actinobacteria in exercising elderly adults increased compared to non-exercising elderly adults and approached the levels seen in healthy BMI adults. Furthermore, the relative abundance of Cyanobacteria, decreased in exercising elderly patients, again approaching levels seen in healthy BMI adults. (Of note, however, Cyanobacteria produce toxins such as β-N-Methylamino-l-alanine (BMAA) have been implicated in neurodegenerative diseases such as AD and ALS [104, 105].) In a smaller study by Erlandson et al., 15 sedentary elderly patients (aged 50–75) were recruited for a supervised 24 week, thrice-weekly cardiovascular and resistance exercise program. Stool samples were collected before and after the intervention for 16S sequencing [103]. Researchers observed an increased relative abundance of Bifidobacterium after 24 weeks of the exercise program, as well as increased butyrate levels. Considering the speculated role of Bifidobacterium in extreme aging and improved cognitive function, these findings suggest that the health benefits related to exercise may also be mediated through the gut microbiome.
Despite these results, there is significant interpersonal variation in the reported microbiome changes that occur with exercise [106]. Additionally, many of the current studies do not have a control arm, lack rigor, and/or have small sample sizes. Future studies are needed to identify if there is in fact a relationship between exercise and healthy aging microbiota, as well as the type of physical activity that can influence gut microbiomes.
Conclusions
Microbiome research in the elderly is an exciting, rapidly growing field; however, a major gap in the literature is the lack of longitudinal data by which to distinguish between causative and correlative relationships given the many concomitant changes that occur with age, including altered dentition, diet, sleep, and lifestyle patterns. A challenge in interpreting currently available data is the difference in sequencing methodologies utilized. For instance, 16S study results can differ depending on the portion of the variable region within the 16S gene that is being sequenced [107], as well as due to variation in 16S copy number between bacterial species [107, 108]. Comparing 16S data across studies is also challenging as the results provide only the relative abundances of taxa in a group [109]; thus, abundances of one group may appear to change but only because of changes in the abundance of other taxa [110]. Furthermore, as taxonomy does not necessarily inform microbial function [111], it is likely that a future shift in focus to metagenomic function may better clarify the mechanisms by which gut microbes influence their host.
Additional challenges specific to clinical microbiome studies in the elderly and the extremely elderly include difficulties with mobility, the tendency for increased medical co-morbidities, as well as difficulties determining capacity for decision-making, and finding a proxy for subjects who may not be able to provide their own consent to participate in research.
Nonetheless, early findings suggest that there is the potential to reverse microbiome aging with interventions such as FMT, exercise, and dietary modifications. Several large, longitudinal cohort studies are now underway that aim to characterize microbiome changes throughout aging, including the MOTION study and a new branch of the Wisconsin Longitudinal study (WLS) [47, 112]. As mentioned previously, the MOTION study is a longitudinal, prospective cohort study of 360 healthy individuals over the age of 60, that is specifically interested in the relationship between cognitive function and gut microbiome in elderly populations [47]. The WLS, a longitudinal study of one-third of the Wisconsin high-school graduates in 1957, recently incorporated a microbiota branch to the project through the collection of 429 stool specimens (74% response rate), which will analyze the gut microbiome as it relates to environmental conditions and disease development [112].
As the field of medicine becomes more individualized with the growth of genetics, epigenetics, and other biomarkers, we must also consider the importance of a unique microbiome profile in diagnosis and treatment. A common theme throughout much of the research is the significance of individualized care, with treatments based on the initial host microbiome composition. Although many of these studies are still in the early stages and require additional evidence to confirm a true causative relationship between illness and dysbiosis, elucidation of a unique microbiome disease profile opens the door to new avenues of treatment for these diseases.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. https://doi.org/10.1038/s41422-020-0332-7.
Ma Y, Xu X, Li M, Cai J, Wei Q, Niu H. Gut microbiota promote the inflammatory response in the pathogenesis of systemic lupus erythematosus. Mol Med. 2019;25(1):35. https://doi.org/10.1186/s10020-019-0102-5.
Chassaing B, Darfeuille-Michaud A. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1720–8. https://doi.org/10.1053/j.gastro.2011.01.054.
Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–81. https://doi.org/10.1038/nature18646.
Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019;570(7762):462–7. https://doi.org/10.1038/s41586-019-1291-3.
Kang DW, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. 2017;5(1):10. https://doi.org/10.1186/s40168-016-0225-7.
Jacobs JP, Gupta A, Bhatt RR, Brawer J, Gao K, Tillisch K, et al. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome. 2021;9(1):236. https://doi.org/10.1186/s40168-021-01188-6.
Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016;8(1):51. https://doi.org/10.1186/s13073-016-0307-y.
Rinninella E, Cintoni M, Raoul P, Lopetuso LR, Scaldaferri F, Pulcini G, et al. Food components and dietary habits: keys for a healthy gut microbiota composition. Nutrients. 2019;11(10). https://doi.org/10.3390/nu11102393.
Hall AB, Tolonen AC, Xavier RJ. Human genetic variation and the gut microbiome in disease. Nat Rev Genet. 2017;18(11):690–9. https://doi.org/10.1038/nrg.2017.63.
Ahn J, Hayes RB. Environmental Influences on the Human Microbiome and Implications for Noncommunicable Disease. Annu Rev Public Health. 2021;42:277–92. https://doi.org/10.1146/annurev-publhealth-012420-105020.
Song SJ, Wang J, Martino C, Jiang L, Thompson WK, Shenhav L, et al. Naturalization of the microbiota developmental trajectory of Cesarean-born neonates after vaginal seeding. Med (N Y). 2021;2(8):951-64.e5. https://doi.org/10.1016/j.medj.2021.05.003.
Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185(5):385–94. https://doi.org/10.1503/cmaj.121189.
Dominguez-Bello MG, Godoy-Vitorino F, Knight R, Blaser MJ. Role of the microbiome in human development. Gut. 2019;68(6):1108–14. https://doi.org/10.1136/gutjnl-2018-317503.
Affairs UNDoEaS. Leaving No One Behind In An Ageing World. World Social Report. 2023;2023:17–34. https://doi.org/10.18356/9789210019682.
Jeffery IB, Lynch DB, O’Toole PW. Composition and temporal stability of the gut microbiota in older persons. Isme J. 2016;10(1):170–82. https://doi.org/10.1038/ismej.2015.88.
Ghosh TS, Das M, Jeffery IB, O’Toole PW. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. eLife. 2020;9:e50240. https://doi.org/10.7554/eLife.50240.
Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, et al. Gut microbiota and extreme longevity. Curr Biol. 2016;26(11):1480–5. https://doi.org/10.1016/j.cub.2016.04.016.
Cani PD, Depommier C, Derrien M, Everard A, de Vos WM. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatol. 2022;19(10):625–37. https://doi.org/10.1038/s41575-022-00631-9.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–71. https://doi.org/10.1073/pnas.1219451110.
Wilmanski T, Diener C, Rappaport N, Patwardhan S, Wiedrick J, Lapidus J, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021;3(2):274–86. https://doi.org/10.1038/s42255-021-00348-0. Microbiome uniqueness, as measured by Bray-Curtis dissimilarity, is positively correlated with age.
Peel C, Sawyer Baker P, Roth DL, Brown CJ, Brodner EV, Allman RM. Assessing mobility in older adults: the UAB Study of Aging Life-Space Assessment. Phys Ther. 2005;85(10):1008–119.
Pang S, Chen X, Lu Z, Meng L, Huang Y, Yu X, et al. Longevity of centenarians is reflected by the gut microbiome with youth-associated signatures. Nat Aging. 2023. https://doi.org/10.1038/s43587-023-00389-y.
Johansen J, Atarashi K, Arai Y, Hirose N, Sørensen SJ, Vatanen T, et al. Centenarians have a diverse gut virome with the potential to modulate metabolism and promote healthy lifespan. Nat Microbiol. 2023;8(6):1064–78. https://doi.org/10.1038/s41564-023-01370-6. Early work suggests that like the microbiome, the centenarian virome may also have distinct features compared to aging adults such as increased diveristy and unique genera.
Walford RL. The immunologic theory of aging1. Gerontologist. 1964;4(4):195–7. https://doi.org/10.1093/geront/4.4.195.
Franceschi C, BonafÈ M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908(1):244–54. https://doi.org/10.1111/j.1749-6632.2000.tb06651.x.
Jaiswal S, Libby P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat Rev Cardiol. 2020;17(3):137–44. https://doi.org/10.1038/s41569-019-0247-5.
Zhao TV, Sato Y, Goronzy JJ, Weyand CM. T-Cell aging-associated phenotypes in autoimmune disease. Front Aging. 2022;3:867950. https://doi.org/10.3389/fragi.2022.867950.
Goronzy JJ, Weyand CM. Mechanisms underlying T cell ageing. Nat Rev Immunol. 2019;19(9):573–83. https://doi.org/10.1038/s41577-019-0180-1.
Choi W, Yeruva S, Turner JR. Contributions of intestinal epithelial barriers to health and disease. Exp Cell Res. 2017;358(1):71–7. https://doi.org/10.1016/j.yexcr.2017.03.036.
Sovran B, Hugenholtz F, Elderman M, Van Beek AA, Graversen K, Huijskes M, et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci Rep. 2019;9(1):1437. https://doi.org/10.1038/s41598-018-35228-3.
Pabst O, Herbrand H, Friedrichsen M, Velaga S, Dorsch M, Berhardt G, et al. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol. 2006;177(10):6824–32. https://doi.org/10.4049/jimmunol.177.10.6824.
Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21(4):455-66.e4. https://doi.org/10.1016/j.chom.2017.03.002.
Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488(7410):178–84. https://doi.org/10.1038/nature11319.
Asempa TE, Nicolau DP. Clostridium difficile infection in the elderly: an update on management. Clin Interv Aging. 2017;12:1799–809. https://doi.org/10.2147/cia.S149089.
Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184(16):4137-53.e14. https://doi.org/10.1016/j.cell.2021.06.019.
Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8. https://doi.org/10.1126/science.1208344.
Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65(1):63–72. https://doi.org/10.1136/gutjnl-2014-308209.
Tanes C, Bittinger K, Gao Y, Friedman ES, Nessel L, Paladhi UR, et al. Role of dietary fiber in the recovery of the human gut microbiome and its metabolome. Cell Host Microbe. 2021;29(3):394-407.e5. https://doi.org/10.1016/j.chom.2020.12.012.
Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, et al. The microbiome of uncontacted Amerindians. Sci Adv. 2015;1(3). https://doi.org/10.1126/sciadv.1500183.
Salazar N, Valdés-Varela L, González S, Gueimonde M, de los Reyes-Gavilán CG. Nutrition and the gut microbiome in the elderly. Gut Microbes. 2017;8(2):82–97. https://doi.org/10.1080/19490976.2016.1256525.
Ragonnaud E, Biragyn A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun Ageing. 2021;18(1):2. https://doi.org/10.1186/s12979-020-00213-w.
Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American academy of neurology. Neurology. 2018;90(3):126–35. https://doi.org/10.1212/wnl.0000000000004826.
Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev. 2012;2012(9):Cd009132. https://doi.org/10.1002/14651858.CD009132.pub2.
van Soest APM, Hermes GDA, Berendsen AAM, van de Rest O, Zoetendal EG, Fuentes S, et al. Associations between Pro- and Anti-Inflammatory gastro-intestinal microbiota, diet, and cognitive functioning in Dutch Healthy older adults: The NU-AGE study. Nutrients. 2020;12(11). https://doi.org/10.3390/nu12113471.
Fam J, Sun Y, Qi P, Lau RC, Feng L, Kua EH, et al. Mindfulness practice alters brain connectivity in community-living elders with mild cognitive impairment. Psychiatr Clin Neurosci. 2020;74(4):257–62. https://doi.org/10.1111/pcn.12972.
Phillips S, Watt R, Atkinson T, Rajan S, Hayhoe A, Savva GM, et al. A protocol paper for the MOTION Study-A longitudinal study in a cohort aged 60 years and older to obtain mechanistic knowledge of the role of the gut microbiome during normal healthy ageing in order to develop strategies that will improve lifelong health and wellbeing. PLoS ONE. 2022;17(11):e0276118. https://doi.org/10.1371/journal.pone.0276118.
Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. 2020;69(7):1218–28. https://doi.org/10.1136/gutjnl-2019-319654. Elderly patient adherence to a Mediterranean diet for one year led to a decrease in frailty and inflammation, with an increase in cognitive function.
Kim C-S, Cha L, Sim M, Jung S, Chun WY, Baik HW, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol: Ser A. 2021;76(1):32–40. https://doi.org/10.1093/gerona/glaa090.
Hwang Y-H, Park S, Paik J-W, Chae S-W, Kim D-H, Jeong D-G, et al. Efficacy and safety of lactobacillus plantarum c29-fermented soybean (DW2009) in individuals with mild cognitive impairment: A 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients. 2019. https://doi.org/10.3390/nu11020305.
Asaoka D, Xiao J, Takeda T, Yanagisawa N, Yamazaki T, Matsubara Y, et al. Effect of Probiotic Bifidobacterium breve in improving cognitive function and preventing brain atrophy in older patients with suspected mild cognitive impairment: results of a 24-week randomized, double-blind. Placebo-Controlled Trial J Alzheimers Dis. 2022;88(1):75–95. https://doi.org/10.3233/jad-220148.
Komanduri M, Savage K, Lea A, McPhee G, Nolidin K, Deleuil S, et al. The relationship between gut microbiome and cognition in older Australians. Nutrients. 2021;14(1). https://doi.org/10.3390/nu14010064.
Pan Q, Li YQ, Guo K, Xue M, Gan Y, Wang K, et al. Elderly patients with mild cognitive impairment exhibit altered gut microbiota profiles. J Immunol Res. 2021;2021:5578958. https://doi.org/10.1155/2021/5578958.
Zhang X, Wang Y, Liu W, Wang T, Wang L, Hao L, et al. Diet quality, gut microbiota, and microRNAs associated with mild cognitive impairment in middle-aged and elderly Chinese population. Am J Clin Nutr. 2021;114(2):429–40. https://doi.org/10.1093/ajcn/nqab078.
Li B, He Y, Ma J, Huang P, Du J, Cao L, et al. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement. 2019;15(10):1357–66. https://doi.org/10.1016/j.jalz.2019.07.002.
Park SH, Lee JH, Kim JS, Kim TJ, Shin J, Im JH, et al. Fecal microbiota transplantation can improve cognition in patients with cognitive decline and Clostridioides difficile infection. Aging (Albany NY). 2022;14(16):6449–66. https://doi.org/10.18632/aging.204230.
Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019;80:633–43. https://doi.org/10.1016/j.bbi.2019.05.008.
Ueda A, Shinkai S, Shiroma H, Taniguchi Y, Tsuchida S, Kariya T, et al. Identification of Faecalibacterium prausnitzii strains for gut microbiome-based intervention in Alzheimer’s-type dementia. Cell Rep Med. 2021;2(9):100398. https://doi.org/10.1016/j.xcrm.2021.100398.
Lee J, Venna VR, Durgan DJ, Shi H, Hudobenko J, Putluri N, et al. Young versus aged microbiota transplants to germ-free mice: increased short-chain fatty acids and improved cognitive performance. Gut Microbes. 2020;12(1):1–14. https://doi.org/10.1080/19490976.2020.1814107.
Yang X, Yu D, Xue L, Li H, Du J. Probiotics modulate the microbiota-gut-brain axis and improve memory deficits in aged SAMP8 mice. Acta Pharm Sin B. 2020;10(3):475–87. https://doi.org/10.1016/j.apsb.2019.07.001.
Haran JP, Bhattarai SK, Foley SE, Dutta P, Ward DV, Bucci V, et al. Alzheimer's disease microbiome is associated with dysregulation of the anti-inflammatory P-Glycoprotein Pathway. mBio. 2019;10(3). https://doi.org/10.1128/mBio.00632-19.
Hung CC, Chang CC, Huang CW, Nouchi R, Cheng CH. Gut microbiota in patients with Alzheimer’s disease spectrum: a systematic review and meta-analysis. Aging (Albany NY). 2022;14(1):477–96. https://doi.org/10.18632/aging.203826.
Westfall S, Dinh DM, Pasinetti GM. Investigation of potential brain microbiome in Alzheimer’s disease: implications of study bias. J Alzheimers Dis. 2020;75(2):559–70. https://doi.org/10.3233/jad-191328.
Cammann D, Lu Y, Cummings MJ, Zhang ML, Cue JM, Do J, et al. Genetic correlations between Alzheimer’s disease and gut microbiome genera. Sci Rep. 2023;13(1):5258. https://doi.org/10.1038/s41598-023-31730-5. Distinct microbiome profiles are associated with high-risk alleles of the apolipoprotein E ε4 (APOE ε4) gene, a well-established risk factor for AD.
Nishiwaki H, Ito M, Ishida T, Hamaguchi T, Maeda T, Kashihara K, et al. Meta-analysis of gut dysbiosis in Parkinson’s disease. Mov Disord. 2020;35(9):1626–35. https://doi.org/10.1002/mds.28119.
Wallen ZD, Demirkan A, Twa G, Cohen G, Dean MN, Standaert DG, et al. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat Commun. 2022;13(1):6958. https://doi.org/10.1038/s41467-022-34667-x. Researchers identified altered microbiome signatures in PD patients, such as an increase in pathogenic Prevotella species.
Zhang N, Zhang Y, Wang Z, Pan F, Ren R, Li Z, et al. Regular fecal microbiota transplantation to Senescence Accelerated Mouse-Prone 8 (SAMP8) mice delayed the aging of locomotor and exploration ability by rejuvenating the gut microbiota. Front Aging Neurosci. 2022;14. https://doi.org/10.3389/fnagi.2022.991157.
Cao H, Liu X, An Y, Zhou G, Liu Y, Xu M, et al. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci Rep. 2017;7(1):10322. https://doi.org/10.1038/s41598-017-10835-8.
Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. 2016;167(6):1469-80.e12. https://doi.org/10.1016/j.cell.2016.11.018.
DuPont HL, Suescun J, Jiang ZD, Brown EL, Essigmann HT, Alexander AS, et al. Fecal microbiota transplantation in Parkinson’s disease-A randomized repeat-dose, placebo-controlled clinical pilot study. Front Neurol. 2023;14:1104759. https://doi.org/10.3389/fneur.2023.1104759. Early research suggest FMT could decrease symptoms in PD patients.
Picca A, Ponziani FR, Calvani R, Marini F, Biancolillo A, Coelho-Júnior HJ, et al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the BIOSPHERE Study. Nutrients. 2020. https://doi.org/10.3390/nu12010065.
Almeida HM, Sardeli AV, Conway J, Duggal NA, Cavaglieri CR. Comparison between frail and non-frail older adults’ gut microbiota: A systematic review and meta-analysis. Ageing Res Rev. 2022;82:101773. https://doi.org/10.1016/j.arr.2022.101773.
Quach D, Collins F, Parameswaran N, McCabe L, Britton RA. Microbiota reconstitution does not cause bone loss in Germ-Free Mice. mSphere. 2018;3(1). https://doi.org/10.1128/mSphereDirect.00545-17.
Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci U S A. 2016;113(47):E7554–63. https://doi.org/10.1073/pnas.1607235113.
Das M, Cronin O, Keohane DM, Cormac EM, Nugent H, Nugent M, et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology. 2019;58(12):2295–304. https://doi.org/10.1093/rheumatology/kez302.
Kelly CR, Yen EF, Grinspan AM, Kahn SA, Atreja A, Lewis JD, et al. Fecal microbiota transplantation is highly effective in real-world practice: initial results from the FMT national registry. Gastroenterology. 2021;160(1):183-92.e3. https://doi.org/10.1053/j.gastro.2020.09.038.
Kedia S, Virmani S, Vuyyuru SK, Kumar P, Kante B, Sahu P, et al. Faecal microbiota transplantation with anti-inflammatory diet (FMT-AID) followed by anti-inflammatory diet alone is effective in inducing and maintaining remission over 1 year in mild to moderate ulcerative colitis: a randomised controlled trial. Gut. 2022;71(12):2401. https://doi.org/10.1136/gutjnl-2022-327811.
Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by Autologous FMT. Cell. 2018;174(6):1406-23.e16. https://doi.org/10.1016/j.cell.2018.08.047.
Parker A, Romano S, Ansorge R, Aboelnour A, Le Gall G, Savva GM, et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. 2022;10(1):68. https://doi.org/10.1186/s40168-022-01243-w. Animal models have demonstrated that the physiological effects of young and aging microbiomes on cognitive function are transferable.
D’Amato A, Di Cesare ML, Lucarini E, Man AL, Le Gall G, Branca JJV, et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome. 2020;8(1):140. https://doi.org/10.1186/s40168-020-00914-w.
Bárcena C, Valdés-Mas R, Mayoral P, Garabaya C, Durand S, Rodríguez F, et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med. 2019;25(8):1234–42. https://doi.org/10.1038/s41591-019-0504-5.
Xu L, Zhang Q, Dou X, Wang Y, Wang J, Zhou Y, et al. Fecal microbiota transplantation from young donor mice improves ovarian function in aged mice. J Genet Genomics. 2022;49(11):1042–52. https://doi.org/10.1016/j.jgg.2022.05.006.
Kim KH, Chung Y, Huh JW, Park DJ, Cho Y, Oh Y, et al. Gut microbiota of the young ameliorates physical fitness of the aged in mice. Microbiome. 2022;10(1):238. https://doi.org/10.1186/s40168-022-01386-w.
Valeri F, Dos Santos Guilherme M, He F, Stoye NM, Schwiertz A, Endres K. Impact of the age of cecal material transfer donors on Alzheimer’s disease pathology in 5xFAD Mice. Microorganisms. 2021;9(12). https://doi.org/10.3390/microorganisms9122548.
Stebegg M, Silva-Cayetano A, Innocentin S, Jenkins TP, Cantacessi C, Gilbert C, et al. Heterochronic faecal transplantation boosts gut germinal centres in aged mice. Nat Commun. 2019;10(1):2443. https://doi.org/10.1038/s41467-019-10430-7.
Zeng X, Li X, Li X, Wei C, Shi C, Hu K, et al. Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation. Blood. 2023. https://doi.org/10.1182/blood.2022017514.
Chen Y, Zhang S, Zeng B, et al. Transplant of microbiota from long-living people to mice reduces aging-related indices and transfers beneficial bacteria. Aging (Albany NY). 2020;12(6):4778–93. https://doi.org/10.18632/aging.102872.
Hu C, Liu M, Sun B, Tang L, Zhou X, Chen L. Young fecal transplantation mitigates the toxicity of perfluorobutanesulfonate and potently refreshes the reproductive endocrine system in aged recipients. Environ Int. 2022;167:107418. https://doi.org/10.1016/j.envint.2022.107418.
Talukder P, Saha A, Roy S, Ghosh G, Dutta Roy D, Barua S. Progeria—a rare genetic condition with accelerated ageing process. Appl Biochem Biotechnol. 2023;195(4):2587–96. https://doi.org/10.1007/s12010-021-03514-y.
Ganda Mall J-P, Fart F, Sabet JA, Lindqvist CM, Nestestog R, Hegge FT, et al. Effects of dietary fibres on acute indomethacin-induced intestinal hyperpermeability in the elderly: a randomised placebo controlled parallel clinical trial. Nutrients. 2020. https://doi.org/10.3390/nu12071954.
Tran TTT, Cousin FJ, Lynch DB, Menon R, Brulc J, Brown JR, et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome. 2019;7(1):39. https://doi.org/10.1186/s40168-019-0654-1.
Chung WSF, Walker AW, Bosscher D, Garcia-Campayo V, Wagner J, Parkhill J, et al. Relative abundance of the Prevotella genus within the human gut microbiota of elderly volunteers determines the inter-individual responses to dietary supplementation with wheat bran arabinoxylan-oligosaccharides. BMC Microbiol. 2020;20(1):283. https://doi.org/10.1186/s12866-020-01968-4.
Delgado-Lista J, Alcala-Diaz JF, Torres-Peña JD, Quintana-Navarro GM, Fuentes F, Garcia-Rios A, et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. 2022;399(10338):1876–85. https://doi.org/10.1016/S0140-6736(22)00122-2.
Low A, Soh M, Miyake S, Seedorf H. Host age prediction from fecal microbiota composition in male C57BL/6J Mice. Microbiol Spectr. 2022;10(3):e0073522. https://doi.org/10.1128/spectrum.00735-22.
Chen LA, Sears CL. 3 - Prebiotics, Probiotics, and Synbiotics. In: Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Philadelphia: W.B. Saunders; 2015. p. 19- 25.e1.
Chenhuichen C, Cabello-Olmo M, Barajas M, Izquierdo M, Ramírez-Vélez R, Zambom-Ferraresi F, et al. Impact of probiotics and prebiotics in the modulation of the major events of the aging process: A systematic review of randomized controlled trials. Exp Gerontol. 2022;164:111809. https://doi.org/10.1016/j.exger.2022.111809.
Li P, Ji B, Luo H, Sundh D, Lorentzon M, Nielsen J. One-year supplementation with Lactobacillus reuteri ATCC PTA 6475 counteracts a degradation of gut microbiota in older women with low bone mineral density. npj Biofilms Microbiomes. 2022;8(1):84. https://doi.org/10.1038/s41522-022-00348-2.
Nilsson AG, Sundh D, Bäckhed F, Lorentzon M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: a randomized, placebo-controlled, double-blind, clinical trial. J Intern Med. 2018;284(3):307–17. https://doi.org/10.1111/joim.12805.
Chen LH, Chang SS, Chang HY, Wu CH, Pan CH, Chang CC, et al. Probiotic supplementation attenuates age-related sarcopenia via the gut-muscle axis in SAMP8 mice. J Cachexia Sarcopenia Muscle. 2022;13(1):515–31. https://doi.org/10.1002/jcsm.12849.
Dalton A, Mermier C, Zuhl M. Exercise influence on the microbiome-gut-brain axis. Gut Microbes. 2019;10(5):555–68. https://doi.org/10.1080/19490976.2018.1562268.
Zhong F, Wen X, Yang M, Lai HY, Momma H, Cheng L, et al. Effect of an 8-week exercise training on gut microbiota in physically inactive older women. Int J Sports Med. 2021;42(7):610–23. https://doi.org/10.1055/a-1301-7011.
Zhu Q, Jiang S, Du G. Effects of exercise frequency on the gut microbiota in elderly individuals. MicrobiologyOpen. 2020;9(8):e1053. https://doi.org/10.1002/mbo3.1053.
Erlandson KM, Liu J, Johnson R, Dillon S, Jankowski CM, Kroehl M, et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther Adv Infect Dis. 2021;8:20499361211027068. https://doi.org/10.1177/20499361211027067.
Cox PA, Kostrzewa RM, Guillemin GJ. BMAA and Neurodegenerative Illness. Neurotox Res. 2018;33(1):178–83. https://doi.org/10.1007/s12640-017-9753-6.
Silva DF, Candeias E, Esteves AR, Magalhães JD, Ferreira IL, Nunes-Costa D, et al. Microbial BMAA elicits mitochondrial dysfunction, innate immunity activation, and Alzheimer’s disease features in cortical neurons. J Neuroinflammation. 2020;17(1):332. https://doi.org/10.1186/s12974-020-02004-y.
Ramos C, Gibson GR, Walton GE, Magistro D, Kinnear W, Hunter K. Systematic review of the effects of exercise and physical activity on the gut microbiome of older adults. Nutrients. 2022;14(3). https://doi.org/10.3390/nu14030674.
Abellan-Schneyder I, Matchado MS, Reitmeier S, Sommer A, Sewald Z, Baumbach J, et al. Primer, Pipelines, Parameters: Issues in 16S rRNA Gene Sequencing. mSphere. 2021;6(1). https://doi.org/10.1128/mSphere.01202-20.
Kembel SW, Wu M, Eisen JA, Green JL. Incorporating 16S gene copy number information improves estimates of microbial diversity and abundance. PLoS Comput Biol. 2012;8(10):e1002743. https://doi.org/10.1371/journal.pcbi.1002743.
Jo J-H, Kennedy EA, Kong HH. Research techniques made simple: bacterial 16s ribosomal RNA gene sequencing in cutaneous research. J Investig Dermatol. 2016;136(3):e23–7. https://doi.org/10.1016/j.jid.2016.01.005.
Weiss S, Xu ZZ, Peddada S, Amir A, Bittinger K, Gonzalez A, et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome. 2017;5(1):27. https://doi.org/10.1186/s40168-017-0237-y.
Inkpen SA, Douglas GM, Brunet TDP, Leuschen K, Doolittle WF, Langille MGI. The coupling of taxonomy and function in microbiomes. Biol Philos. 2017;32(6):1225–43. https://doi.org/10.1007/s10539-017-9602-2.
Herd P, Schaeffer NC, DiLoreto K, Jacques K, Stevenson J, Rey F, et al. The influence of social conditions across the life course on the human gut microbiota: a pilot project with the wisconsin longitudinal study. J Gerontol B Psychol Sci Soc Sci. 2017;73(1):124–33. https://doi.org/10.1093/geronb/gbx029.
Acknowledgements
Akshata Shukla assisted with manuscript review.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
LA.C. and K.B. wrote the main manuscript text and prepared accompanying figures and tables.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, L.A., Boyle, K. The Role of the Gut Microbiome in Health and Disease in the Elderly. Curr Gastroenterol Rep (2024). https://doi.org/10.1007/s11894-024-00932-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s11894-024-00932-w