Abstract
Purpose of Review
CoronaVirus Disease of 2019 (COVID-19) has negatively influenced the management of multiple conditions in regards to the gastroenterology patient. An equivalent change in the management of Helicobacter pylori (H. pylori)-related diseases was reported, as practically no eradication treatment was offered during most of the pandemic. Given the scarcity of published data, we performed a literature review trying to elucidate the effect of COVID-19 on H. pylori treatment.
Recent Findings
COVID-19 has produced more questions than answers as to the outcome of COVID-19 in H. Pylori infected patients, post-COVID-19 patients treated for H. pylori, acid suppression and COVID-19 incidence and outcomes, and H. pylori eradication treatment in patients having recovered from COVID-19.
Summary
We strongly believe that this scientific uncertainty produced by the COVID-19 pandemic has set up the stage for an incremental change in H. pylori treatment as COVID-19 has offered us the chance to speed up how we will, in the near future, approach patients with a possible Η. pylori infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CoronaVirus Disease of 2019 (COVID-19) has negatively influenced the prevention and management of multiple conditions in regards to the gastroenterology patient as planned outpatient consultations and colorectal cancer (CRC) screening strategies were put on halt for roughly 2 years, mostly due to a concern about the risk (although low) of COVID-19 transmission during gastrointestinal (GI) endoscopy [1]. In the United States of America (USA), endoscopic procedures and inpatient consults were reduced by more than half (53.9% and 51.7%, respectively), while, in the United Kingdom (UK), a 96% reduction in trainee procedures was reported. Similar data were reported from Asia and Australia [2,3,4]. As things were not complicated enough, numerous studies (mostly retrospective) and meta-analyses confirmed the GI involvement in COVID-19 [5,6,7].
It is no wonder that an equivalent change in the management of H. pylori-related diseases was reported, as practically no eradication treatment was offered for the same time, although a cross-sectional study from China reported that the majority (76.95%) of participants over 40 years old were willing to undergo gastroscopy for early gastric cancer screening, with individuals with known H. pylori infection being more likely to undergo gastroscopy [8]. Given that H. pylori prevalence estimates are high, with approximately 4.4 billion individuals infected worldwide in 2015 [9], and that higher rates of inflammation, intestinal metaplasia (IM) and H. pylori infection were recorded in the COVID-19 period than in the pre-pandemic period [10], researchers have tried to report on associations between H. pylori status and treatment and COVID-19.
What was the Outcome of COVID-19 in H. Pylori Infected Patients?
H. pylori has been pathophysiologically linked in the pathogenesis of diseases by (a) increasing the expression of angiotensin-converting enzyme-2 (ACE-2) receptors in the GI tract, (b) having a direct association with infection duration and severity, and (c) using its virulent factors to promote immune dysregulation [11]. Even though H. pylori infection does not seem to affect the clinical outcome of COVID-19 [12], other researchers have implied that H. pylori infection history and IM may increase predisposition for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), implying a possible co-pathogenicity [13]; when sucralfate or hydrotalcite was administered in a small cohort of COVID-19 patients with upper GI symptoms, rates of lower GI involvement (diarrhea) were reduced (raising the concept of possible viral load reduction and transmission blockade cranially to caudally via the use of a mucosal protective agent) [14].
However, caution is needed when putting the blame on H. pylori as other diseases could partially be held responsible for increasing ACE-2 receptor expression. Furthermore, we have limited knowledge of any symptoms reported before H. pylori or after COVID-19 infection. Many data reported from studies cannot be interpreted safely as a control group of non-COVID-19 patients was missing or studies were significantly underpowered. Last but not least, other non-H. pylori factors could be responsible for reported symptoms [15,16,17].
Antibiotic Use During the COVID-19 Pandemic
Another aspect that needs to be considered was the use of multiple antibiotics (taken off-label), especially in the early COVID-19 period, for two main reasons. Firstly, and taken into consideration the early necessity for treating a novel disease with increased transmittance and mortality rates accompanied by the scientific obscurity surrounding the nature and mechanism of SARS-CoV-2 infection, repurposing of an already approved drug by regulatory mechanisms, such as the US Food and Drug Administration (FDA) and the European Medical Agency (EMA), was promoted over discovery of new pharmaceutical agents de novo due to established drug efficiency and cost-effectiveness. Even though bacterial co-infection was identified in 3.5% and secondary bacterial infection in 15.5% of COVID-19 patients, the end result was the empirical use of broad-spectrum antibiotics in 72% of hospitalized patients in an attempt to treat respiratory symptoms (the same symptoms seen in patients with community-acquired pneumonia) [18,19,20,21]. Secondly, the rationale behind prescribing some antibiotics was based on their basic in vitro antiviral and anti-inflammatory/immunomodulatory properties [22, 23].
On one hand azithromycin (a macrolide) was prescribed all too often in the early stages of the COVID-19 pandemic. Macrolide antibiotics interfere with protein synthesis by binding to the 23s ribosomal ribonucleic acid (rRNA) of the 50s ribosomal subunit. However, cross-resistance between azithromycin and clarithromycin (one of the most important antibiotics in current first-line H. pylori eradication treatment worldwide) is a well-known fact; the clinically significant mechanism by which H. pylori evades clarithromycin is a point mutation in domain V of the 23 S rRNA gene [24, 25]. Meta-analyses do not support the use of azithromycin in the management of COVID-19 [26]. On the other, respiratory fluoroquinolones were recommended in the treatment of community-acquired pneumonia in COVID-19 patients. Levofloxacin is a fluoroquinolone used mainly in the second-line treatment of H. pylori eradication. Quinolones inhibit the bacterial type II topoisomerase [deoxyribonucleic acid (DNA) gyrase] and topoisomerase IV, therefore, hindering deoxyribonucleic acid (DNA) synthesis. However, bacterial evasion is also evident after quinolone administration through a mutation of DNA gyrase or topoisomerase IV [22].
What About Post-COVID-19 Patients Treated for H. Pylori?
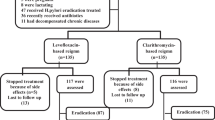
According to our knowledge, up to now, only one randomized controlled trial (RCT) in Egypt has reported on previously treated as having confirmed or suspected COVID-19 patients with newly diagnosed H. pylori infection. A total of 270 naïve H. pylori-infected patients with previous treatment for COVID-19 were randomized to either a clarithromycin-based (clarithromycin 500 mg/12 h, amoxicillin 1 gr/12 h, esomeprazole 40 mg/12 h) or a levofloxacin-based regimen (levofloxacin 500 mg/24 h, amoxicillin 1 mg/12 h, esomeprazole 40 mg/12 h) [27]. Although not statistically significant, based on the per-protocol (PP) and intention-to-treat (ITT) analyses, higher treatment response was observed among patients under the levofloxacin-based regimen versus the clarithromycin-based regimen [74.36% and 64.44% (p = 0.11) versus 64.66% and 55.56% (p = 0.14), respectively]. Interestingly enough, in Egypt, a country with > 50% resistance rates to clarithromycin and no official data on levofloxacin resistance [28], clarithromycin-based triple therapy is still considered a drug of choice to treat H. pylori infection, in discordance with the Maastricht VI/Florence consensus report [29, 30]. The authors arbitrarily attributed their even lesser levofloxacin-based regimen eradication rates to an even more increase in levofloxacin resistance in the Egyptian community after the COVID-19 pandemic (aftermath of levofloxacin misuse in the management of COVID-19) [27]. However, one should not forget that both eradication treatments underperformed significantly compared to eradication rates of at least 90% as the desired outcome, thereby rendering their findings of low scientific value [31].
Acid Suppression and COVID-19 Incidence and Outcomes
Based on the pathophysiological concept that gastric acidity can act as a barrier preventing microorganisms from reaching the intestine [32] and given the true fact that proton pump inhibitors (PPIs) are one of the most common prescribed medicines worldwide [33, 34] associated with increased risk of pneumonia [35], data on PPI use [and other antisecretory drugs like histamine H2 receptor antagonists (H2RA)] and COVID-19 incidence and severity were searched for, both in basic research and in clinical settings [36, 37]. A recent meta-analysis among 18,109 COVID-19 patients under PPIs revealed that PPI use was significantly associated with severe outcomes of COVID-19 [hazard ratio (HR) = 1.53; 95% confidence interval (CI): 1.20–1.95] but not with COVID-19 incidence; H2RA use was significantly associated with decreased COVID-19 incidence (HR = 0.86, 95% CI: 0.76–0.97). Although extreme caution is warranted when trying to interpret studies of substantial heterogeneity and plausible protopathic bias, results seem to favour H2Ras [38]. Based on the aforementioned data, H. pylori eradication treatment of a COVID-19-infected patient seems to be justified only if the patient had a previously known peptic ulcer or GI bleeding or before starting anticoagulant treatment but should be put on hold after COVID-19 resolution.
Pediatric COVID-19 Populations and H. Pylori
In accordance with the adult population, elective diagnostic endoscopy in children was substantially suspended (except for emergency cases) during the COVID-19 pandemic [39, 40]. Since the “test and treat” strategy for H. pylori infection in children is not warranted but the initial diagnosis of H. pylori infection should be limited to invasive gastric biopsy-based methods [41], pediatricians and gastroenterologists failed to identify H. pylori-infected children during COVID-19 lockdown. In view of this, experts suggested that diagnosis-wise, in children likely having H. pylori-associated gastritis or peptic ulcer disease (based on their presenting symptoms), non-invasive tests [13C-urea breath test (UBT) or a stool monoclonal H. pylori antigen test], could be employed. Treatment-wise, an empiric eradication strategy could be adopted [42].
How did H. Pylori Eradication Treatment Affect Patients Having Recovered from COVID-19?
Up to now, a few months after the World Health Organization (WHO) declared the end of the 3 years COVID-19 pandemic, with SARS-CoV-2 no longer constituting a public health emergency of international concern (PHEIC) [43], we practically have no concrete knowledge regarding H. pylori eradication treatment rates in patients having recovered from a previous COVID-19 infection; no RCT or even preliminary data have been reported.
Conclusions
While waiting for research to shed some light, editorials and expert opinions highlight a possible future infection control through vaccination control for the benefit of all mankind [44]. Based on the fact that a chronic untreated H. pylori infection may induce systemic effects and contribute to tissue damage in distant sites from the stomach, such as the lungs (the respiratory and the digestive tract share a common embryological origin), researchers believe that more efforts are necessary to understand the pathophysiological pathways involved [45]. To make things even more complicated, many epidemiological studies have reported on a possible pathogenetical role of H. pylori in extragastric diseases via a potential relation to chronic systemic inflammation and molecular mimicry [46]. Furthermore, the recent context of COVID-19 has renewed the interest of pathologists in diseases of infectious origin [47].
As data on the long COVID-19 syndrome are starting to emerge, we will begin gradually to see the full extent of the long-term GI involvement after COVID-19 resolution [48]. Furthermore, all data suggest that antibiotic resistance has increased over the years of the pandemic (the end result of self-antibiotic and empirical antibiotic medication, and antibiotics prescribed by treating physicians) [49, 50]. New H. pylori prevalence studies accompanied by resistance rates with geographical criteria are necessary to establish a new baseline. Furthermore, H. pylori societies worldwide should endorse properly designed RCTs, based on non-inferiority methodology, focusing on tailored treatment that should be effective (eradication rates of at least 90%) for the local populations both for the treatment regimen under study as well as for the regimen against which they are compared (the critical comparator is how close the cure rate comes to 100%) [51••,52]. Patient-based H. pylori therapy does not mean susceptibility-guided treatment alone; patient-based H. pylori therapy includes personalized treatment in regards to dosing and duration [31, 53].
If H. pylori infection is, by definition, an infectious disease, then it should be governed by the laws of antimicrobial stewardship (as all infectious diseases do) so as to achieve acceptable eradication rates, reduce unnecessary antibiotic prescription and limit the emergence of antibiotic resistance worldwide [52]. The Maastricht VI/Florence consensus report on the management of H. pylori infection has already advocated susceptibility testing through polymerase chain reaction (PCR) or next-generation sequencing (NGS) on biological materials (gastric biopsies or stools), recognizing the need for respecting the principles of antibiotic stewardship [30]. The only footnote is whether a molecular-based susceptibility-guided strategy can be used in routine clinical practice. However, antimicrobial susceptibility testing for H. pylori is now available and can be used for initial therapy [54, 55]. Profiling H. pylori antibiotic resistance by NGS from stool samples provides rapid results highly comparable to those obtained from gastric biopsies [56]. As more and more major diagnostic laboratories in the USA have not only shown interest but now offer practical, rapid, and non-invasive susceptibility testing, through stool PCR or NGS, this may reduce the need for upper GI endoscopies providing, at the same time, reliable antibiotic susceptibility rates. Kits for PCR-based testing for clarithromycin are available and have been approved for clinical use by European regulatory agencies [57]. The respective increase in clinical use will, by causality, lead to price decreases, making them affordable for more and more patients.
We strongly believe that the COVID-19 pandemic has set up the stage for such an incremental change in H. pylori treatment (Fig. 1). The SARS-CoV-2 infection, amidst its obvious health-related problems, has offered us the chance to speed up, even more, how we will, in the near future, approach patients with a possible Η. pylori infection. Antibiotic misuse, common during the COVID-19 era, will no longer pause a threat to H. pylori eradication with post-COVID-19 patients (and their treating physicians) not having to worry about worsened local eradication rates or previous antisecretory treatment. Taking into perspective that every cloud (COVID-19) has a silver lining (molecular susceptibility testing), the future for H. pylori treatment is now.
Data Availability
No datasets were generated or analysed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Repici A, Aragona G, Cengia G, Cantù P, Spadaccini M, Maselli R, et al. Low risk of COVID-19 transmission in GI endoscopy. Gut. 2020;69:1925–7. https://doi.org/10.1136/gutjnl-2020-321341.
Zorniak M, Sirtl S, Mahajan UM, Stubbe HC, Chapula M, Wosiewicz P, et al. Influence of COVID-19 pandemic on endoscopic procedures in two European large-capacity Endoscopy Units: keep Calm, keep safe and scope on? Dig Dis. 2021;39:540–8. https://doi.org/10.1159/000511076.
Conlon C, Campion J, Farrelly NM, Ring E, Dunne T, Gorman D, et al. Endoscopy training through the COVID-19 pandemic: maintaining procedural volumes and key performance standards. Frontline Gastroenterol. 2022;14:38–44. https://doi.org/10.1136/flgastro-2021-102069.
Ekmektzoglou K, Tziatzios G, Siau K, Pawlak KM, Rokkas T, Triantafyllou K, et al. Covid-19: exploring the new normal in gastroenterology training. Acta Gastroenterol Belg. 2021;84:627–35. https://doi.org/10.51821/84.4.014.
Skourtis A, Ekmektzoglou K, Xanthos T, Stouraitou S, Iacovidou N. Non-typical clinical presentation of COVID-19 patients in Association with Disease severity and length of Hospital Stay. J Pers Med. 2023;13:132. https://doi.org/10.3390/jpm13010132.
Tsibouris P, Ekmektzoglou K, Agorogianni A, Kalantzis C, Theofanopoulou A, Toumbelis K, et al. Gastrointestinal involvement in COVID-19 patients: a retrospective study from a Greek COVID-19 referral hospital. Ann Gastroenterol. 2020;33:465–72. https://doi.org/10.20524/aog.2020.0514.
Rokkas T. Gastrointestinal involvement in COVID-19: a systematic review and meta-analysis. Ann Gastroenterol. 2020;33:355–65. https://doi.org/10.20524/aog.2020.0506.
Ma K, Chen X, Xiang X, Mao X, Zhu N, Wang T, et al. Willingness to undergo gastroscopy for early gastric cancer screening and its Associated factors during the COVID-19 pandemic - A Nationwide Cross-sectional Study in China. Patient Prefer Adherence. 2023;17:505–16. https://doi.org/10.2147/PPA.S400908.
Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and Meta-analysis. Gastroenterology. 2017;153:420–9. https://doi.org/10.1053/j.gastro.2017.04.022.
Bozdağ E, Gülmez S, Yücesoy FS, Özcan A, Yırgın H, Somuncu E, et al. Analysis of upper gastrointestinal endoscopy results during the Covid-19 pandemic. A high-volume single-center experience. Ann Ital Chir. 2022;93:391–7.
Sugimoto M, Yamaoka Y, Shirai N, Furuta T. Role of renin-angiotensin system in gastric oncogenesis. J Gastroenterol Hepatol. 2012;27:442–51. https://doi.org/10.1111/j.1440-1746.2011.06964.x.
Balamtekin N, Artuk C, Arslan M, Gülşen M. The Effect of Helicobacter pylori on the presentation and clinical course of Coronavirus Disease 2019 infection. Pediatr Gastroenterol Nutr. 2021;72:511–3. https://doi.org/10.1097/MPG.0000000000003005.
Gasbarrini G, Termite F, Simeoni S, Bonvicini F. SARS-CoV-2 and Helicobacter Pylori: can they become Co-pathogens? J Emerg Intern Med. 2022;6:33.
Zhang M, Feng C, Zhang X, Hu S, Zhan Y, Min M, et al. Susceptibility factors of stomach for SARS-CoV-2 and Treatment Implication of Mucosal Protective Agent in COVID-19. Front Med (Lausanne). 2021;7:597967. https://doi.org/10.3389/fmed.2020.597967.
Gkogkou E, Barnasas G, Vougas K, Trougakos IP. Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators. Redox Biol. 2020;36:101615. https://doi.org/10.1016/j.redox.2020.101615.
Enko D, Kriegshäuser G. Functional 13C-urea and glucose hydrogen/methane breath tests reveal significant association of small intestinal bacterial overgrowth in individuals with active Helicobacter pylori infection. Clin Biochem. 2017;50:46–9. https://doi.org/10.1016/j.clinbiochem.2016.08.017.
Rao SSC, Bhagatwala J. Small intestinal bacterial overgrowth: clinical features and therapeutic management. Clin Transl Gastroenterol. 2019;10:e00078. https://doi.org/10.14309/ctg.0000000000000078.
Ng YL, Salim CK, Chu JJH. Drug repurposing for COVID-19: approaches, challenges and promising candidates. Pharmacol Ther. 2021;228:107930. https://doi.org/10.1016/j.pharmthera.2021.107930.
Garg SK. Antibiotic misuse during COVID-19 pandemic: a recipe for disaster. Indian J Crit Care Med. 2021;25:617–9. https://doi.org/10.5005/jp-journals-10071-23862.
Li J, Wang J, Yang Y, Cai P, Cao J, Cai X, et al. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: a retrospective analysis. Antimicrob Resist Infect Control. 2020;9:153. https://doi.org/10.1186/s13756-020-00819-1.
Fu Y, Yang Q, Xu M, Kong H, Chen H, Fu Y, et al. Secondary bacterial infections in critical Ill patients with Coronavirus Disease 2019. Open Forum Infect Dis. 2020;7:ofaa220. https://doi.org/10.1093/ofid/ofaa220.
Karampela I, Dalamaga M. Could respiratory fluoroquinolones, levofloxacin and Moxifloxacin, prove to be beneficial as an Adjunct Treatment in COVID-19? Arch Med Res. 2020;51:741–2. https://doi.org/10.1016/j.arcmed.2020.06.004.
Min JY, Jang YJ. Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012;2012:649570. https://doi.org/10.1155/2012/649570.
Fass RJ. Erythromycin, clarithromycin, and azithromycin: use of frequency distribution curves, scattergrams, and regression analyses to compare in vitro activities and describe cross-resistance. Antimicrob Agents Chemother. 1993;37:2080–6. https://doi.org/10.1128/AAC.37.10.2080.
Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2021;18:613–29. https://doi.org/10.1038/s41575-021-00449-x.
Ayerbe L, Risco-Risco C, Forgnone I, Pérez-Piñar M, Ayis S. Azithromycin in patients with COVID-19: a systematic review and meta-analysis. J Antimicrob Chemother. 2022;77:303–9. https://doi.org/10.1093/jac/dkab404.
Kamal A, Ghazy RM, Sherief D, Ismail A, Ellakany WI. Helicobacter pylori eradication rates using clarithromycin and levofloxacin-based regimens in patients with previous COVID-19 treatment: a randomized clinical trial. BMC Infect Dis. 2023;23:36. https://doi.org/10.1186/s12879-023-07993-8.
Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: a systematic review and Meta-analysis in World Health Organization regions. Gastroenterology. 2018;155:1372–1382e17. https://doi.org/10.1053/j.gastro.2018.07.007.
Alboraie M, Elhossary W, Aly OA, Abbas B, Abdelsalam L, Ghaith D, et al. Egyptian recommendations for management of Helicobacter pylori infection: 2018 report. Arab J Gastroenterol. 2019;20:175–9. https://doi.org/10.1016/j.ajg.2019.09.001.
Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut 2022;gutjnl-2022-327745. https://doi.org/10.1136/gutjnl-2022-327745.
Rokkas T, Ekmektzoglou K, Graham DY. Current role of tailored therapy in treating Helicobacter pylori infections. A systematic review, meta-analysis and critical analysis. Helicobacter. 2023;28:e12936. https://doi.org/10.1111/hel.12936.
Martinsen TC, Bergh K, Waldum HL. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol. 2005;96:94–102. https://doi.org/10.1111/j.1742-7843.2005.pto960202.x.
Hálfdánarson OO, Pottegård A, Björnsson ES, Lund SH, Ogmundsdottir MH, Steingrímsson E, et al. Proton-pump inhibitors among adults: a nationwide drug-utilization study. Th Adv Gastroenterol. 2018;11:1756284818777943. https://doi.org/10.1177/1756284818777943.
Lassalle M, Le Tri T, Bardou M, Biour M, Kirchgesner J, Rouby F, et al. Use of proton pump inhibitors in adults in France: a nationwide drug utilization study. Eur J Clin Pharmacol. 2020;76:449–57. https://doi.org/10.1007/s00228-019-02810-1.
Wang CH, Li CH, Hsieh R, Fan CY, Hsu TC, Chang WC, et al. Proton pump inhibitors therapy and the risk of pneumonia: a systematic review and meta-analysis of randomized controlled trials and observational studies. Expert Opin Drug Saf. 2019;18:163–72. https://doi.org/10.1080/14740338.2019.1577820.
Yuan S, Wang R, Chan JF, Zhang AJ, Cheng T, Chik KKH, et al. Metallodrug ranitidine bismuth citrate suppresses SARS-CoV-2 replication and relieves virus-associated pneumonia in Syrian hamsters. Nat Microbiol. 2020;5:1439–48. https://doi.org/10.1038/s41564-020-00802-x.
Freedberg DE, Conigliaro J, Wang TC, Tracey KJ, Callahan MV, Abrams JA, et al. Famotidine Use is Associated with Improved Clinical outcomes in hospitalized COVID-19 patients: a propensity score matched Retrospective Cohort Study. Gastroenterology. 2020;159:1129–31. https://doi.org/10.1053/j.gastro.2020.05.053.
Kim HB, Kim JH, Wolf BJ. Acid suppressant use in association with incidence and severe outcomes of COVID-19: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2022;78:383–91. https://doi.org/10.1007/s00228-021-03255-1.
Gralnek IM, Hassan C, Beilenhoff U, Antonelli G, Ebigbo A, Pellisè M, et al. ESGE and ESGENA position Statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy. 2020;52:483–90. https://doi.org/10.1055/a-1155-6229.
Ruan W, Fishman DS, Lerner DG, Engevik MA, Elmunzer BJ, Walsh CM, et al. Changes in Pediatric Endoscopic Practice during the Coronavirus Disease 2019 pandemic: results from an International Survey. Gastroenterology. 2020;159:1547–50. https://doi.org/10.1053/j.gastro.2020.05.068.
Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, Casswall T, et al. Joint ESPGHAN/NASPGHAN guidelines for the management of Helicobacter pylori in Children and adolescents (Update 2016). J Pediatr Gastroenterol Nutr. 2017;64:991–1003. https://doi.org/10.1097/MPG.0000000000001594.
Monzani A, Lionetti P, Rabbone I, Lionetti E. The best is the enemy of the good: time for a biopsy-sparing approach for Helicobacter pylori diagnosis and treatment in children in the COVID-19 era? Helicobacter 2021;26:e12826. 26. https://doi.org/10.1111/hel.12826.
World Health Organization. Statement on the fifteenth meeting of the IHR. (2005) Emergency Committee on the COVID-19 pandemic. Available from: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. [Assessed on 10 June, 2023].
Marshall BJ. COVID-19 has triggered a new century of vaccination and infection control for the benefit of all mankind. Precis Clin Med. 2021;4:77–9. https://doi.org/10.1093/pcmedi/pbab010.
Gonzalez I, Lindner C, Schneider I, Morales MA, Rojas A. Inflammation at the crossroads of Helicobacter pylori and COVID-19. Future Microbiol. 2022;17:77–80. https://doi.org/10.2217/fmb-2021-0250.
He J, Liu Y, Ouyan Q, Li R, Li J, Chen W, et al. Helicobacter pylori and unignorable extragastric diseases: mechanism and implications. Front Microbiol. 2022;13:972777. https://doi.org/10.3389/fmicb.2022.972777.
Guenzi É, Chen R, Sannier A, Drabent P, Pote N, Couvelard A. [Digestive infectious pathology: diagnoses not to be missed]. Ann Pathol. 2023;43:222–35. https://doi.org/10.1016/j.annpat.2023.02.007.
Fernández-de-Las-Peñas C, Torres-Macho J, Guijarro C, Martín-Guerrero JD, Pellicer-Valero OJ, Plaza-Manzano G. Trajectory of Gastrointestinal Symptoms in Previously Hospitalized COVID-19 Survivors: The Long COVID Experience Multicenter Study. Viruses. 2023;15:1134. https://doi.org/10.3390/v15051134.
Sulayyim HJA, Ismail R, Hamid AA, Ghafar NA. Antibiotic resistance during COVID-19: a systematic review. Int J Environ Res Public Health. 2022;19:11931. https://doi.org/10.3390/ijerph191911931.
Lai CC, Chen SY, Ko WC, Hsueh PR. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents. 2021;57:106324. https://doi.org/10.1016/j.ijantimicag.2021.106324.
•• Graham DY, Hernaez R, Rokkas T. Cross-roads for meta-analysis and network meta-analysis of H. Pylori therapy. Gut. 2022;71:643–50. https://doi.org/10.1136/gutjnl-2021-326170. (Network meta-analysis indicating the poor quality of the clinical trials using unoptimized H. pylori regimens and incomparable comparisons related to marked geographic and ethnic genotypic and phenotypic heterogeneity and comparator regimens often consisting of invalid strawman comparisons).
Rokkas T, Graham DY. How widespread and convenient H. pylori susceptibility testing will result in pharmacological opportunities. Expert Rev Gastroenterol Hepatol. 2023;17:1–7. https://doi.org/10.1080/17474124.2023.2162502.
Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8:79–8. https://doi.org/10.1038/nrgastro.2010.210.
Graham DY, Moss SF. Antimicrobial susceptibility testing for Helicobacter pylori is now widely available: when, how, why. Am J Gastroenterol. 2022;117:524–8. https://doi.org/10.14309/ajg.0000000000001659.
Graham DY. Implications of the paradigm shift in management of Helicobacter pylori infections. Th Adv Gastroenterol. 2023;16:17562848231160858. https://doi.org/10.1177/17562848231160858.
Moss S, Atsawarungruangkit A, Dang L, Chua David, Zhou Y, Chong ZZ, et al. Rapid Prediction of H. Pylori Antibiotic Resistance using Next Generation sequencing of Stool samples compared to gastric biopsies. Am J Gastroenterol. 2021;116:634.
Dore MP, Graham DY. Modern approach to the diagnosis of Helicobacter pylori infection. Aliment Pharmacol Ther. 2022;55:14–S21. https://doi.org/10.1111/apt.16566.
Funding
Open access funding provided by the Cyprus Libraries Consortium (CLC).
Author information
Authors and Affiliations
Contributions
K.E. conceptualized, supervised, revised and edited the manuscript. T.R. supervised, revised and edited the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ekmektzoglou, K., Rokkas, T. H. Pylori Treatment in the COVID-19 Era. What Have We Learned So Far?. Curr Gastroenterol Rep 26, 86–91 (2024). https://doi.org/10.1007/s11894-024-00922-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-024-00922-y