Abstract
Purpose of Review
Gastroparesis is a chronic disorder characterized by a constellation of foregut symptoms, including postprandial nausea, vomiting, distension, epigastric pain, and regurgitation in the absence of gastric outlet obstruction. Despite considerable research over the past decades, there remains to be only nominal understanding of disease classification, diagnostic criteria, pathogenesis, and preferred therapy.
Recent Findings
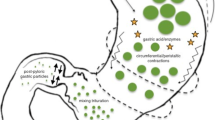
We critically reassess current approaches for disease identification and stratification, theories of causation, and treatment for gastroparesis. Gastric scintigraphy, long considered a diagnostic standard, has been re-evaluated in light of evidence showing low sensitivity, whereas newer testing modalities are incompletely validated. Present concepts of pathogenesis do not provide a unified model linking biological impairments with clinical manifestations, whereas available pharmacological and anatomical treatments lack explicit selection criteria or evidence for sustained effectiveness. We propose a disease model that embodies the re-programming of distributed neuro-immune interactions in the gastric wall by inflammatory perturbants. These interactions, combined with effects on the foregut hormonal milieu and brain-gut axis, are postulated to generate the syndromic attributes characteristically linked with gastroparesis.
Summary
Research linking models of immunopathogenesis with diagnostic and therapeutic paradigms will lead to reclassifications of gastroparesis that guide future trials and technological developments.
Key points
• The term gastroparesis embodies a heterogenous array of symptoms and clinical findings based on a complex assimilation of afferent and efferent mechanisms, gastrointestinal locations, and pathologies.
• There currently exists no single test or group of tests with sufficient capacity to be termed a definitional standard for gastroparesis.
• Present research regarding pathogenesis suggests the importance of immune regulation of intrinsic oscillatory activity involving myenteric nerves, interstitial cells of Cajal, and smooth muscle cells.
• Prokinetic pharmaceuticals remain the mainstay of management, although novel treatments are being studied that are directed to alternative muscle/nerve receptors, electromodulation of the brain-gut axis, and anatomical (endoscopic, surgical) interventions.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Camilleri M, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37. quiz 38. https://doi.org/10.1038/ajg.2012.373.
Kassander P. Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum). Ann Intern Med. 1958;48:797–812. https://doi.org/10.7326/0003-4819-48-4-797.
•• Revicki DA, et al. Gastroparesis cardinal symptom index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res. 2004;13:833–844. https://doi.org/10.1023/B:QURE.0000021689.86296.e4. This study provides the first demonstration of a validated, reliable, and quantitative patient-reported measure of symptom severity in the setting of gastroparesis, which has since become the standard measure used in foregut disease related clinical outcomes research.
Revicki DA, et al. Development and content validity of a gastroparesis cardinal symptom index daily diary. Aliment Pharmacol Ther. 2009;30:670–80. https://doi.org/10.1111/j.1365-2036.2009.04078.x.
• Pasricha PJ, et al. Outcomes and factors associated with reduced symptoms in patients with gastroparesis. Gastroenterology. 2015;149:1762–1774 e1764. https://doi.org/10.1053/j.gastro.2015.08.008. This study assessed 262 patients with gastroparesis at 7 centers associated with the NIH Gastroparesis Clinical Research Consortium Registry over 4 years and determined that only 28% experienced significant symptom reduction, thereby confirming chronicity of the disease, and that, when symptom reduction occurred, it was limited to several specific associations.
Parkman HP, et al. Early satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying, and water load testing. Neurogastroenterol Motil. 2017;29:e12981. https://doi.org/10.1111/nmo.12981.
Parkman HP, et al. Nausea and vomiting in gastroparesis: similarities and differences in idiopathic and diabetic gastroparesis. Neurogastroenterol Motil. 2016;28:1902–14. https://doi.org/10.1111/nmo.12893.
Koch KL, et al. Baseline features and differences in 48 week clinical outcomes in patients with gastroparesis and type 1 vs type 2 diabetes. Neurogastroenterol Motil. 2016;28:1001–15. https://doi.org/10.1111/nmo.12800.
•• Jung HK, et al. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–1233. https://doi.org/10.1053/j.gastro.2008.12.047. This study represents the most extensive assessment of the epidemiological features of gastroparesis to date, defining three categories of disease (definate, probable, and possible) based on symptoms, endoscopy, and gastric scintigraphy, and portrayed age-adjusted incidence, prevalence, and survival.
Cogliandro RF, et al. Is gastroparesis a gastric disease. Neurogastroenterol Motil. 2019;31(5):e13562. https://doi.org/10.1111/nmo.13562.
Pasricha PJ, et al. Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathologic features. Gastroenterology. 2021;160:2006–17. https://doi.org/10.1053/j.gastro.2021.01.230.
Cangemi DJ, Lacy BE. Gastroparesis and functional dyspepsia: different diseases or different ends of the spectrum? Curr Opin Gastroenterol. 2020;36:509–17. https://doi.org/10.1097/MOG.0000000000000677.
• Tack J, Talley NJ. Functional dyspepsia--symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. 2013;10:134–141. https://doi.org/10.1038/nrgastro.2013.14. This paper employed the Rome III consensus criteria to define functional dyspepsia, and proposed a subdivision of the disease into two entities, namely the postprandial distress syndrome and the epigastric pain syndrome.
Drossman DA, Hasler WL. Rome IV-functional GI disorders: Disorders of gut-brain interaction. Gastroenterology. 2016;150:1257–61. https://doi.org/10.1053/j.gastro.2016.03.035.
• Schol J, et al. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on gastroparesis. Neurogastroenterol Motil. 2021;33:e14237. This paper represented a Delphi consensus by 40 individuals from 19 European countries, and confirmed a general symptom profile for patients with gastroparesis, preferred diagnostic testing, current pharmacotherapy, and impact on quality of life and health care costs, but did not reach a consensus in relation to pathophysiology.
• Abell TL, et al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36:44–54. This paper provided a consensus recommendation regarding a standardized method for measuring gastric emptying (GE) by scintigraphy, suggesting that imaging be performed at 0, 1, 2, and 4 h after test meal ingestion.
Rao SS, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23:8–23. https://doi.org/10.1111/j.1365-2982.2010.01612.x.
Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Wise JL, et al. Gastric emptying scans: Poor adherence to national guidelines. Dig Dis Sci. 2021;66:2897–906. https://doi.org/10.1007/s10620-020-06314-2.
Jones KL, et al. A longitudinal study of gastric emptying and upper gastrointestinal symptoms in patients with diabetes mellitus. Am J Med. 2002;113:449–55. https://doi.org/10.1016/s0002-9343(02)01228-7.
Jalleh R, Marathe CS, Rayner CK, Jones KL, Horowitz M. Diabetic gastroparesis and glycaemic control. Curr Diab Rep. 2019;19:153. https://doi.org/10.1007/s11892-019-1281-8.
• Wang XJ, Burton DD, Breen-Lyles M, Camilleri M. Gastric accommodation influences proximal gastric and total gastric emptying in concurrent measurements conducted in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2021320:G759–G767. https://doi.org/10.1152/ajpgi.00008.2021. This study derived associations beween proximal gastric volumes and gastric emptying throrugh the assessment of a labled mixed solid and liquid meal, and demonstrated that increased gastric accomodation is associated with prolongation of gastric emptying.
Ang D. Measurement of gastric accommodation: a reappraisal of conventional and emerging modalities. Neurogastroenterol Motil. 2011;23:287–91. https://doi.org/10.1111/j.1365-2982.2011.01690.x.
Tack J. Functional dyspepsia: Impaired fundic accommodation. Curr Treat Options Gastroenterol. 2000;3:287–94. https://doi.org/10.1007/s11938-000-0042-7.
Gryback P, Jacobsson H, Neuger L, Hellstrom PM. Gastroparesis versus dyspepsia by intragastric meal distribution: new diagnostics and definitions ahead. Scand J Gastroenterol. 2020;55:251–5. https://doi.org/10.1080/00365521.2019.1710244.
Steinsvik EK, et al. Gastric function in diabetic gastroparesis assessed by ultrasound and scintigraphy. Neurogastroenterol Motil. 2022;34:e14235. https://doi.org/10.1111/nmo.14235.
Jacob D, et al. Effects of NK1 receptors on gastric motor functions and satiation in healthy humans: results from a controlled trial with the NK1 antagonist aprepitant. Am J Physiol Gastrointest Liver Physiol. 2017;313:G505–10. https://doi.org/10.1152/ajpgi.00197.2017.
• Banerjee S, Pal A, Fox M. Volume and position change of the stomach during gastric accommodation and emptying: A detailed three-dimensional morphological analysis based on MRI. Neurogastroenterol Motil. 2020;32:e13865. https://doi.org/10.1111/nmo.13865. This study employed dynamic anatomic MRI methods to infer 3D gastric morphology in 7 segmented regions during gastric accommodation and emptying in response to a liquid meal, and demonstratred the location and properties of accommodation in relation to distal stomach empyting.
Parthasarathy G, et al. Effect of neostigmine on gastroduodenal motility in patients with suspected gastrointestinal motility disorders. Neurogastroenterol Motil. 2015;27:1736–46. https://doi.org/10.1111/nmo.12669.
• Desprez C, et al. Assessment of pyloric sphincter distensibility and pressure in patients with diabetic gastroparesis. Neurogastroenterol Motil. 2021;33:e14064. https://doi.org/10.1111/nmo.14064. This study employed the EndoFLIP system to assess pyloric distensibility in patients with diabetic gastroparesis, idiopathic gastroparesis, and normal volunteers, and determined that distensibility was reduced in both disease groups compared with normals.
Jehangir A, Malik Z, Petrov RV, Parkman HP. EndoFLIP and pyloric dilation for gastroparesis symptoms refractory to pyloromyotomy/pyloroplasty. Dig Dis Sci. 2021;66:2682–90. https://doi.org/10.1007/s10620-020-06510-0.
Desprez C, et al. Pyloric distensibility measurement predicts symptomatic response to intrapyloric botulinum toxin injection. Gastrointest Endosc. 2019;90:754-760 e751. https://doi.org/10.1016/j.gie.2019.04.228.
Kloetzer L, et al. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol Motil. 2010;22:527–33, e117. https://doi.org/10.1111/j.1365-2982.2010.01468.x.
Rouphael C, et al. Role of wireless motility capsule in the assessment and management of gastrointestinal dysmotility in patients with diabetes mellitus. Neurogastroenterol Motil. 2017;29:e13087. https://doi.org/10.1111/nmo.13087.
Lee AA, et al. Validation of diagnostic and performance characteristics of the wireless motility capsule in patients with suspected gastroparesis. Clin Gastroenterol Hepatol. 2019;17:1770-1779 e1772. https://doi.org/10.1016/j.cgh.2018.11.063.
Stein E, et al. Wireless motility capsule versus other diagnostic technologies for evaluating gastroparesis and constipation: a comparative effectiveness review. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 May. Report No.: 13-EHC060-EF.
Hasler WL, et al. Relating gastric scintigraphy and symptoms to motility capsule transit and pressure findings in suspected gastroparesis. Neurogastroenterol Motil. 2018;30:e13196. https://doi.org/10.1111/nmo.13196.
Shafi MA, Pasricha PJ. Post-surgical and obstructive gastroparesis. Curr Gastroenterol Rep. 2007;9:280–5. https://doi.org/10.1007/s11894-007-0031-2.
He CL, et al. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–34. https://doi.org/10.1053/gast.2001.26264.
• Horiguchi K, Komuro T. Ultrastructural observations of fibroblast-like cells forming gap junctions in the W/Wν Mouse small intestine. J Autonomic Nervous Syst. 2000;80:142–147. This study determined the ultrastructure of fibroblast-like cells distributed in the myenteric plexus, and observed that these cells formed gap junctions with smooth muscle cells of circular/longitudinal muscle that may underlie the abillity of fibroblast-like cells to transduce molecular or electrical signals to smooth muscle cells.
Ishikawa K, Komuro T, Hirota S, Kitamura Y. Ultrastructural identification of the c-kit-expressing interstitial cells in the rat stomach: a comparison of control and Ws/Ws mutant rats. Cell Tissue Res. 1997;289:137–43. https://doi.org/10.1007/s004410050859.
• Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev. 2014;94:859–907. https://doi.org/10.1152/physrev.00037.2013. This work provides a substantial review of the molecular, structural and physiological role of the cells that constitute the neuromotor syncytium in the wall of the GI tract, notably the interstitial cells of Cajal (ICC), PDGFRα(+) cells presumed to be multifunctional fibroblasts, and smooth muscle cells, and proposes a biological basis for their physiological and pathophysiological roles.
Nakayama S, et al. Pacemaker phase shift in the absence of neural activity in guinea-pig stomach: a microelectrode array study. J Physiol. 2006;576:727–38. https://doi.org/10.1113/jphysiol.2006.118893.
Watkins CC, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Investig. 2000;106:373–84.
Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–44. https://doi.org/10.1053/gast.1997.v113.pm9352855.
Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725-733. https://doi.org/10.1152/ajpgi.00406.2006.
Ordog T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–9. https://doi.org/10.2337/diabetes.49.10.1731.
Choi KM, et al. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil. 2007;19:585–95. https://doi.org/10.1111/j.1365-2982.2007.00936.x.
Dishy V, et al. The effect of sildenafil on gastric emptying in patients with end-stage renal failure and symptoms of gastroparesis. Clin Pharmacol Ther. 2004;76:281–6. https://doi.org/10.1016/j.clpt.2004.04.012.
Pasricha PJ, et al. Changes in the gastric enteric nervous system and muscle: a case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. https://doi.org/10.1186/1471-230X-8-21.
Grover M, et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140:1575-1585 e1578. https://doi.org/10.1053/j.gastro.2011.01.046.
Choi KM, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138:2399-2409. e2391.
Korenaga K, Micci MA, Taglialatela G, Pasricha PJ. Suppression of nNOS expression in rat enteric neurons by the receptor for advanced glycation end-products. Neurogastroenterol Motil. 2006;18:392–400. https://doi.org/10.1111/j.1365-2982.2006.00774.x.
Heckert J, Thomas RM, Parkman HP. Gastric neuromuscular histology in patients with refractory gastroparesis: Relationships to etiology, gastric emptying, and response to gastric electric stimulation. Neurogastroenterol Motil. 2017;29:e13068. https://doi.org/10.1111/nmo.13068.
Park KS, et al. Characterization of smooth muscle, enteric nerve, interstitial cells of Cajal, and fibroblast-like cells in the gastric musculature of patients with diabetes mellitus. World J Gastroenterol. 2016;22:10131–9. https://doi.org/10.3748/wjg.v22.i46.10131.
• Zhang C, et al. Up-regulation of the Ang II/AT1 receptor may compensate for the loss of gastric antrum ICC via the PI3k/Akt signaling pathway in STZ-induced diabetic mice. Mol Cell Endocrinol. 2016;423:77–86. This study demonstrated that angiotensin II augmentation of tonic gastric smooth muscle contraction was increased in diabetic mice coincident with increased mSCF expression, cell proliferation, and Akt-Ser473 phosphorylation, and therefore implied that such pro-motility effects compensated for ICC loss.
Ejskjaer NT, et al. Novel surgical treatment, and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488–95. https://doi.org/10.1046/j.1464-5491.1999.00086.x.
Bhetwal BP, An C, Baker SA, Lyon KL, Perrino BA. Impaired contractile responses and altered expression and phosphorylation of Ca2+ sensitization proteins in gastric antrum smooth muscles from ob/ob mice. J Muscle Res Cell Motil. 2013;34:137–49.
Chen X, Fu XS, Li CP, Zhao HX. ER stress and ER stress-induced apoptosis are activated in gastric SMCs in diabetic rats. World J Gastroenterol. 2014;20:8260–7. https://doi.org/10.3748/wjg.v20.i25.8260.
• Herring BP, Hoggatt AM, Gupta A, Wo JM. Gastroparesis is associated with decreased FOXF1 and FOXF2 in humans, and loss of FOXF1 and FOXF2 results in gastroparesis in mice. Neurogastroenterol Motil. 2019;31:e13528. This study demonstrated that mRNA related to two smooth muscle transcription factors, FOXF1 and FOXF2, were decreased in patients with gastroparesis, and that the combined ablation of both factors in mice resulted in delayed gastric emptying for liquid and decreased expression of muscle contractile proteins in the muscularis.
Muller TD, et al. Ghrelin. Mol Metab. 2015;4:437–60. https://doi.org/10.1016/j.molmet.2015.03.005.
Sessenwein J, Lomax A. Ghrelin receptors as targets for novel motility drugs. Neurogastroenterol Motil. 2015;27:589–93.
Prinz P, Stengel A. Control of food intake by gastrointestinal peptides: Mechanisms of action and possible modulationn in the treatment of obesity. Neurogastroenterol Motil. 2017;23:180–96. https://doi.org/10.5056/jnm16194.
Alamri BN, Shin K, Chappe V, Anini Y. The role of ghrelin in the regulation of glucose homeostasis. Horm Mol Biol Clin Investig. 2016;26:3–11. https://doi.org/10.1515/hmbci-2016-0018.
Mosa RM, et al. Implications of ghrelin and hexarelin in diabetes and diabetes-associated heart diseases. Endocrine. 2015;49:307–23. https://doi.org/10.1007/s12020-015-0531-z.
Peeters T. Central and peripheral mechanisms by which ghrelin regulates gut motility. J Physiol Pharmacol. 2003;54:95–103.
Fujino K, et al. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227–40. https://doi.org/10.1113/jphysiol.2003.040600.
Zheng J, Ariga H, Taniguchi H, Ludwig K, Takahashi T. Ghrelin regulates gastric phase III-like contractions in freely moving conscious mice. Neurogastroenterol Motil. 2009;21:78–84. https://doi.org/10.1111/j.1365-2982.2008.01179.x.
Tack J, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–33. https://doi.org/10.1136/gut.2004.060426.
• Tack J, et al. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. 2005;22:847–853. https://doi.org/10.1111/j.1365-2036.2005.02658.x. This study observed, using a randomized, double-blind design in patients with idiopathic gastroparesis that ghrelin administration resulted in significantly enhanced gastric emptying and reduced meal related symptoms, thus supporting the use of ghrelin agonists in gastroparesis treatment.
McCallum R, Cynshi O, Team I. Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis–a randomized, multicenter, placebo-controlled study. Aliment Pharmacol Ther. 2007;26:1121–30.
Chapman MJ, et al. The effect of camicinal (GSK962040), a motilin agonist, on gastric emptying and glucose absorption in feed-intolerant critically ill patients: a randomized, blinded, placebo-controlled, clinical trial. Crit Care. 2016;20:232. https://doi.org/10.1186/s13054-016-1420-4.
Deloose E, et al. Manometric evaluation of the motilin receptor agonist camicinal (GSK 962040) in humans. Neurogastroenterol Motil. 2018;30: e13173.
Da Silva LM, et al. Vitamin C improves gastroparesis in diabetic rats: Effects on gastric contractile responses and and oxidative stress. Dig Dis Sci. 2017;62:2338–47. https://doi.org/10.1007/s10620-017-4632-9.
Gotfried J, Priest S, Schey R. Diabetes and the small intestine. Curr Treat Options Gastroenterol. 2017;15:490–507. https://doi.org/10.1007/s11938-017-0155-x.
•• Becker L, et al. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut. 2018;67:827–836. https://doi.org/10.1136/gutjnl-2016-312940. This study determined that aging in mice is associated with a conversion of intestinal macrophages from anti-inflammatory to pro-inflammatory state that is associated with a phenotypic neural response to inflammatory signals, loss of enteric neurons, and delayed intestinal transit.
Kenney MJ, Ganta CK. Autonomic nervous system and immune system interactions. Compr Physiol. 2014;4:1177–200. https://doi.org/10.1002/cphy.c130051.
• Zhou L, et al. Activation of alpha7nAChR protects against gastric inflammation and dysmotility in Parkinson's Disease rats. Front Pharmacol. 2021;12:793374. https://doi.org/10.3389/fphar.2021.793374. This study demonstrated that increased activation of α7 nicotinic receptors ((α7nAChR) on macrophages in the gastric muscularis of Parkinson's Disease rats by specific agonists caused decrease of nuclear factor κB (NF-κB) activation and monocyte chemotactic protein-1 mRNA expression ex vivo, whereas treatment in vivo with PNU-282987 caused decreased NF-κB activation, inflammatory mediator production, and improved gastric motility.
•• Borgmann D, et al. Gut-brain communication by distinct sensory neurons differently controls feeding and glucose metabolism. Cell Metab. 2021;33:1466–1482 e1467. https://doi.org/10.1016/j.cmet.2021.05.002. This study evaluated differential sensory input to the brain underlying feeding and glucose regulation and demonstrated that glucogon-like peptide 1 receptor (GLP1R) expressing vagal afferent activation promoted meal termination and improved glucose tolerance, whereas GPR65-expressing vagal afferent stimulation increased glucose production and control normoglycemia, but had no effect on feeding. These findings indicate the presence of complex afferent regulatory pathways affecting motility and metabolic control.
•• Gershon MD, Margolis KG. The gut, its microbiome, and the brain: Connections and communications. J Clin Invest. 2021;131:e143768. https://doi.org/10.1172/JCI143768. This paper reviews current knowledge of the bidrectional signaling process that relates the microbiota, the gut, and the brain, and proposes that this association be described in the form of "connectome" involving the roles of macrophages and other inflammatory components exhibiting functional plasticity, along with nerves and muscle manifesting complex signalling pathways to and from the central nervous system.
Ichijo T, Katafuchi T, Hori T. Central interleukin-1β enhances splenic sympathetic nerve activity in rats. Brain Res Bull. 1994;34:547–53.
Ek M, Kurosawa M, Lundeberg T, Ericsson A. Activation of vagal afferents after intravenous injection of interleukin-1beta: role of endogenous prostaglandins. J Neurosci. 1998;18:9471–9.
Siamwala JH, et al. Identification of human CD4+ sub-population of resident cardiac fibroblasts linked to inflammation-mediated cardiac fibrosis. bioRxiv 2021.
Nair DG, Miller KG, Lourenssen SR, Blennerhassett MG. Inflammatory cytokines promote growth of intestinal smooth muscle cells by induced expression of PDGF-Rβ. J Cell Mol Med. 2014;18:444–54. https://doi.org/10.1111/jcmm.12193.
• Baker SA, et al. Distribution and Ca2+ signalling of fibroblast‐like (PDGFRα+) cells in the murine gastric fundus. J Physiol. 2013;591:6193–6208. This study determined the biological responsivity of isolated and purified PDGFα positive fibroblast-like cells to purinergic activation principally by the demonstration of purinergic receptors and the specific elicitation of calcium transients, whereas purinergic inhibition abolished calcium transients.
Grover M, et al. Platelet-derived growth factor receptor α (PDGFRα)-expressing “fibroblast-like cells” in diabetic and idiopathic gastroparesis of humans. Neurogastroenterol Motil. 2012;24:844–52.
•• Ward SM, Sanders KM. Physiology and pathophysiology of the interstitial cell of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am J Physiol Gastrointest Liver Physiol. 2001;281:G602–611. https://doi.org/10.1152/ajpgi.2001.281.3.G602. This paper provides a comprehensive review of the development of ICC networks and associated phenotypic plasticity, and thereby suggests a basis for understanding the role of altered ICC function in the setting of pathological GI motility.
Chikkamenahalli LL, Pasricha PJ, Farrugia G, Grover M. Gastric biopsies in gastroparesis: Insights into gastric neuromuscular disorders to aid treatment. Gastroenterol Clin. 2020;49:557–70.
Harberson J, Thomas RM, Harbison SP, Parkman HP. Gastric neuromuscular pathology in gastroparesis: analysis of full-thickness antral biopsies. Dig Dis Sci. 2010;55:359–70. https://doi.org/10.1007/s10620-009-1071-2.
• Grover M, et al. Diabetic and idiopathic gastroparesis is associated with loss of CD 206‐positive macrophages in the gastric antrum. Neurogastroenterol Motil. 2017;29:e13018. This study assessed macrophage populations and ICC through immunolabeoing of full thickness antral biopsies from patients with diabetic or idiopathic gastroparesis, and determined that there was a loss of anti-inflammatory macrophages (CD206) in circular muscle and myenteric plexus correlated with the reduction of ICC.
Hemmer B, et al. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat Med. 1999;5:1375–82. https://doi.org/10.1038/70946.
Choi KM, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135:2055–2064, 2064 e2051–2052. https://doi.org/10.1053/j.gastro.2008.09.003.
Cipriani G, Gibbons SJ, Kashyap PC, Farrugia G. Intrinsic gastrointestinal macrophages: their phenotype and role in gastrointestinal motility. Cell Mol Gastroenterol Hepatol. 2016;2:120-130 e121.
• Grover M, et al. Transcriptomic signatures reveal immune dysregulation in human diabetic and idiopathic gastroparesis. BMC Med Genomics. 2018;11:62. https://doi.org/10.1186/s12920-018-0379-1. Transcriptomic analysis performed on full thickness antral tissue obtained from patients demonstrated increased expression of genes involved with macrophages, fibroblasts and the regulation of macriphage and T-cell cytokine production in diabetic gastroparesis and increased gene expression involved with granulocyte adhesion and macrophagea, fibroblast, and endothelial cell regulation in idiopathic gastroparesis. Specific immune profile analysis demonstrated increaseed gene expression associated with pro-ifnlammatory macrophages in tissues from idiopathic gastroparesis.
Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: Crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021;14:555–65.
• Bhuiyan P, Chen Y, Karim M, Dong H, Qian Y. Bidirectional communication between mast cells and the gut-brain axis in neurodegenerative diseases: Avenues for therapeutic intervention. Brain Res Bull. 2021;172:61–78. This review describes the role of the gut-microbiota on inflammatory signaling, and particularly dipicts multi-directional communication pathways involving inflammatory cytokines, neurocircuits within the brain-gut axis, and mast cells and microglia involved with neuroinflammation as it relates to neurodegenerative diseases.
Wrona D. Neural–immune interactions: an integrative view of the bidirectional relationship between the brain and immune systems. J Neuroimmunol. 2006;172:38–58.
Griffiths M, Neal JW, Gasque P. Innate immunity and protective neuroinflammation: new emphasis on the role of neuroimmune regulatory proteins. Int Rev Neurobiol. 2007;82:29–55. https://doi.org/10.1016/S0074-7742(07)82002-2.
De Schepper S, et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175:400-415 e413. https://doi.org/10.1016/j.cell.2018.07.048.
•• Muller P, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. This study demonstrated that muscularis macrophages, partially due to the effects of microbial factors in the gut lumen, promote smooth muscle contraction through the secretion of bone morphogenetic protein 2 (BMP2), which activates its receptor expressed in enteric neurorons, which in turn secretes a growth factor important for macrophage development. These findings provide evidence to support the presence of microbiota-dependent communication pathways involving muscularis macrophages, smooth muscle cells and enteric neurons for the regulation of gastrointestinal motility.
Stakenborg N, Viola MF, Boeckxstaens GE. Intestinal neuro-immune interactions: focus on macrophages, mast cells and innate lymphoid cells. Curr Opin Neurobiol. 2020;62:68–75. https://doi.org/10.1016/j.conb.2019.11.020.
Mogilevski T, Burgell R, Aziz Q, Gibson PR. Review article: the role of the autonomic nervous system in the pathogenesis and therapy of IBD. Aliment Pharmacol Ther. 2019;50:720–37. https://doi.org/10.1111/apt.15433.
•• Gabanyi I, et al. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. This study assessed how various regions of the gut wall exhibit differential gene expression of resident macrophages, specifically observing that lamina propria macrophages express a pro-inflammatory phenotype in relation to luminal pathogens and that muscularis macrophages express a protective phenotype related to the activation of specific neural pathways.
Van Diest SA, Stanisor OI, Boeckxstaens GE, de Jonge WJ, van den Wijngaard RM. Relevance of mast cell–nerve interactions in intestinal nociception. Biochim Biophys Acta BBA Mol Basis Dis. 2012;1822:74–84.
Forsythe P. Mast cells in neuroimmune interactions. Trends Neurosci. 2019;42:43–55.
Hu D, et al. Immunofluorescence characterization of innervation and nerve-immune cell interactions in mouse lymph nodes. Eur J Histochem: EJH. 2019;63.
Kaya D, et al. The effect of dopamine type-2 receptor blockade on autonomic modulation. Clin Auton Res. 2003;13:275–80. https://doi.org/10.1007/s10286-003-0097-3.
Hyser CL, Drake ME Jr. Myoclonus induced by metoclopramide therapy. Arch Intern Med. 1983;143:2201–2.
Ou LB, Moriello C, Douros A, Felon KB. Domperidone and the risks of sudden cardiac death and ventricular arrhythmia: A systematic review and meta-analysis of observational studies. Br J Clin Pharmacol. 2021;87:3649–58. https://doi.org/10.1111/bcp.14737.
Wickham RJ. Revisiting the physiology of nausea and vomiting-challenging the paradigm. Support Care Cancer. 2020;28:13–21. https://doi.org/10.1007/s00520-019-05012-8.
Razvi Y, et al. ASCO, NCCN, MASCC/ESMO: a comparison of antiemetic guidelines for the treatment of chemotherapy-induced nausea and vomiting in adult patients. Support Care Cancer. 2019;27:87–95. https://doi.org/10.1007/s00520-018-4464-y.
Mazzotta E, Villalobos-Hernandez EC, Fiorda-Diaz J, Harzman A, Christofi FL. Postoperative Ileus and postoperative gastrointestinal tract dysfunction: Pathogenic mechanisms and novel treatment strategies beyond colorectal enhanced recovery after surgery protocols. Front Pharmacol. 2020;11:583422. https://doi.org/10.3389/fphar.2020.583422.
Kuo B, et al. Randomized clinical trial: safety, pharmacokinetics and pharmacodynamics of trazpiroben (TAK-906), a dopamine D2/D3 receptor antagonist, in patients with gastroparesis. Aliment Pharmacol Ther. 2021;54:267–80.
Pasricha PJ, et al. Aprepitant has mixed effects on nausea and reduces other symptoms in patients with gastroparesis and related disorders. Gastroenterology. 2018;154:65-76. e11.
Weibel S, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anesthesia: a network meta‐analysis. Cochrane Database of Systematic Reviews; 2020.
Havelund T, Oster-Jorgensen E, Eshoj O, Larsen ML, Lauritsen K. Effects of cisapride on gastroparesis in patients with insulin-dependent diabetes mellitus. A double-blind controlled trial. Acta Med Scand. 1987;222:339–43. https://doi.org/10.1111/j.0954-6820.1987.tb10681.x.
Wang SH, et al. QT interval effects of cisapride in the clinical setting. Int J Cardiol. 2001;80:179–83. https://doi.org/10.1016/s0167-5273(01)00485-5.
Serra J, et al. European Society of Neurogastroenterology and Motility guidelines on functional constipation in adults. Neurogastroenterol Motil. 2020;32:e13762. https://doi.org/10.1111/nmo.13762.
Carbone F, et al. Prucalopride in gastroparesis: A randomized placebo-controlled crossover study. Am J Gastroenterol. 2019;114:1265–74. https://doi.org/10.14309/ajg.0000000000000304.
Kuo B, et al. Velusetrag accelerates gastric emptying in subjects with gastroparesis: a multicentre, double-blind, randomised, placebo-controlled, phase 2 study. Aliment Pharmacol Ther. 2021;53:1090–7. https://doi.org/10.1111/apt.16344.
Chedid V, et al. Randomized study: effects of the 5-HT4 receptor agonist felcisetrag vs placebo on gut transit in patients with gastroparesis. Aliment Pharmacol Ther. 2021;53:1010–20. https://doi.org/10.1111/apt.16304.
Kusunoki H, et al. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying, randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil. 2012;24(540–545):e250-541. https://doi.org/10.1111/j.1365-2982.2012.01897.x.
Tack J, et al. Influence of tegaserod on proximal gastric tone and on the perception of gastric distention in functional dyspepsia. Neurogastroenterol Motil. 2011;23:e32-39. https://doi.org/10.1111/j.1365-2982.2010.01613.x.
Vakil N, et al. Tegaserod treatment for dysmotility-like functional dyspepsia: results of two randomized, controlled trials. Am J Gastroenterol. 2008;103:1906–19. https://doi.org/10.1111/j.1572-0241.2008.01953.x.
Camilleri M, Acosta A. Emerging treatments in Neurogastroenterology: relamorelin: a novel gastrocolokinetic synthetic ghrelin agonist. Neurogastroenterol Motil. 2015;27:324–32. https://doi.org/10.1111/nmo.12490.
Camilleri M, et al. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, placebo-controlled study. Gastroenterology. 2017;153:1240-1250 e1242. https://doi.org/10.1053/j.gastro.2017.07.035.
Nelson AD, et al. Effects of ghrelin receptor agonist, relamorelin, on gastric motor functions and satiation in healthy volunteers. Neurogastroenterol Motil. 2016;28:1705–13. https://doi.org/10.1111/nmo.12870.
Kendall BJ, Chakravarti A, Kendall E, Soykan I, McCallum RW. The effect of intravenous erythromycin on solid meal gastric emptying in patients with chronic symptomatic post-vagotomy-antrectomy gastroparesis. Aliment Pharmacol Ther. 1997;11:381–5. https://doi.org/10.1046/j.1365-2036.1997.148324000.x.
Cipriani G, et al. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology. 2018;154:2122-2136 e2112. https://doi.org/10.1053/j.gastro.2018.02.027.
• Cipriani G, et al. Diabetic Csf1(op/op) mice lacking macrophages are protected against the development of delayed gastric emptying. Cell Mol Gastroenterol Hepatol. 2016;2:40–47. https://doi.org/10.1016/j.jcmgh.2015.09.001. This study assessed the role of macrophages in the generation of diabetic gastroparesis in mice for whom Csf1-dependent tissue macrophages were genetically ablated, and observed that the loss of macrophage expression was associated with the absence of ICC disruption and impaired gastric emptying.
Vieira-Frez FC, et al. Anti- and pro-oxidant effects of quercetin stabilized by microencapsulation on interstitial cells of Cajal, nitrergic neurons and M2-like macrophages in the jejunum of diabetic rats. Neurotoxicology. 2020;77:193–204. https://doi.org/10.1016/j.neuro.2020.01.011.
Choi KM, et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138(7):2399–409. https://doi.org/10.1053/j.gastro.2010.02.014.
Bharucha AE, et al. Effects of hemin on heme oxygenase-1, gastric emptying, and symptoms in diabetic gastroparesis. Neurogastroenterol Motil. 2016;28:1731–40. https://doi.org/10.1111/nmo.12874.
Federal Drug Administration, Humanitarian Device Exemption (HDE). Gastric electrical simulation (GES) system. HDE Number: H990014, Issued to Medtronic, Minneapolis, MN, 03/31/2000.
Lin ZY, McCallum RW, Schirmer BD, Chen JD. Effects of pacing parameters on entrainment of gastric slow waves in patients with gastroparesis. Am J Physiol. 1998;274:G186-191. https://doi.org/10.1152/ajpgi.1998.274.1.G186.
Abell T, et al. Gastric electrical stimulation for medically refractory gastroparesis. Gastroenterology. 2003;125:421–8. https://doi.org/10.1016/s0016-5085(03)00878-3.
van der Voort IR, et al. Gastric electrical stimulation results in improved metabolic control in diabetic patients suffering from gastroparesis. Exp Clin Endocrinol Diabetes. 2005;113:38–42. https://doi.org/10.1055/s-2004-830525.
McCallum R, Lin Z, Wetzel P, Sarosiek I, Forster J. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49–54. https://doi.org/10.1016/s1542-3565(04)00605-6.
Lin Z, McElhinney C, Sarosiek I, Forster J, McCallum R. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalizations. Dig Dis Sci. 2005;50:1328–34.
Oubre B, et al. Pilot study on gastric electrical stimulation on surgery-associated gastroparesis: long-term outcome. South Med J. 2005;98:693–7. https://doi.org/10.1097/01.smj.0000168660.77709.4d.
Cutts T, Luo J, Starkebaum W, Rashed H, Abell T. Is gastric electrical stimulation superior to standard pharmacologic therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35–43.
McCallum RW, et al. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947–954; quiz e116. https://doi.org/10.1016/j.cgh.2010.05.020.
McCallum RW, et al. Gastric electrical stimulation with Enterra therapy improves symptoms of idiopathic gastroparesis. Neurogastroenterol Motil. 2013;25:815-e636. https://doi.org/10.1111/nmo.12185.
• Ducrotte P, et al. Gastric electrical stimulation reduces refractory vomiting in a randomized crossover trial. Gastroenterology. 2020;158:506–514 e502. https://doi.org/10.1053/j.gastro.2019.10.018. This study employed a large, multicenter, randomized, double-blind trial with crossover to assess the efficacy of gastric electrical stimulation in patients with refractory vomiting, and found that gastric electrical stimulation reduced the frequency of vomiting but had no effect on gastric empyting or quality of life.
Karunaratne T, Yan Y, Eubanks A, Inman B, Rao S, Sharma A. Thoracic spinal nerve neuromodulation therapy for diabetic gastroparesis: A proof-of-concept study. Clin Gastroenterol Hepatol. 2022;S1542–3565(22):00910–7. https://doi.org/10.1016/j.cgh.2022.09.012.
Arts J, et al. Influence of intrapyloric botulinum toxin injection on gastric emptying and meal-related symptoms in gastroparesis patients. Aliment Pharmacol Ther. 2006;24:661–7. https://doi.org/10.1111/j.1365-2036.2006.03019.x.
Shada AL, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc. 2016;30:1326–32.
Arts J, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251–8. https://doi.org/10.1111/j.1365-2036.2007.03467.x.
Rodriguez J, et al. Per-oral pyloromyotomy (POP) for medically refractory gastroparesis: short term results from the first 100 patients at a high volume center. Annals Surgery. 2018;268:421–30.
Gonzalez JM, et al. Gastric per-oral endoscopic myotomy with antropyloromyotomy in the treatment of refractory gastroparesis: clinical experience with follow-up and scintigraphic evaluation. Gastrointest Endosc. 2017;85:132–9. https://doi.org/10.1016/j.gie.2016.07.050.
Geyl S, et al. Peroral endoscopic pyloromyotomy accelerates gastric emptying in healthy pigs: proof of concept. Endosc Int Open. 2016;4:E796-799. https://doi.org/10.1055/s-0042-108192.
Malik Z, et al. Gastric per-oral endoscopic myotomy (G-POEM) for the treatment of refractory gastroparesis: Early experience. Dig Dis Sci. 2018;63:2405–12. https://doi.org/10.1007/s10620-018-4976-9.
Jacques J, et al. Peroral endoscopic pyloromyotomy is efficacious and safe for refractory gastroparesis: prospective trial with assessment of pyloric function. Endoscopy. 2019;51:40–9.
Abdelfatah MM, et al. Long-term outcome of gastric per-oral endoscopic pyloromyotomy in treatment of gastroparesis. Clin Gastroenterol Hepatol. 2021;19:816–24. https://doi.org/10.1016/j.cgh.2020.05.039.
• Oscar Víctor HM, et al. Gastroparesis peroral endoscopic myotomy outcomes after 4 years of follow-up in a large cohort of patients with refractory gastroparesis. Gastro Endosc. 2022. https://doi.org/10.1016/j.gie.2022.03.025. This study assessed the efficacy of G-POEM in 374 patients over 4 years in terms of symptoms and quality of life and established that G-POEM was effective in 77.5%, thereby proposing its use as first-line therapy.
Ward MA, Hasan SS, Whitfield EP, Ogola GO, Leeds SG. Endoscopic per-oral pyloromyotomy for gastroparesis: Initial experience and postoperative comparison to predicted complications following laparoscopic pyloromyotomy as calculated by the ACS risk calculator. Surg Laparosc Endosc Percutan Tech. 2020;31:142–5. https://doi.org/10.1097/SLE.0000000000000862.
Xu J, et al. Gastric peroral endoscopic myotomy (G-POEM) as a treatment for refractory gastroparesis: Long-term outcomes. Can J Gastroenterol Hepatol. 2018;2018:6409698. https://doi.org/10.1155/2018/6409698.
Mohan BP, et al. Clinical efficacy of gastric per-oral endoscopic myotomy (G-POEM) in the treatment of refractory gastroparesis and predictors of outcomes: a systematic review and meta-analysis using surgical pyloroplasty as a comparator group. Surg Endosc. 2020;34:3352–67.
Meybodi MA, et al. Efficacy and feasibility of G-POEM in management of patients with refractory gastroparesis: a systematic review and meta-analysis. Endoscopy International Open. 2019;7:E322–9.
Li P, Ma B, Gong S, Zhang X, Li W. Gastric per-oral endoscopic myotomy for refractory gastroparesis: A meta-analysis. J Gastrointest Surg. 2021;25:1108–16. https://doi.org/10.1007/s11605-020-04520-x.
Vosoughi K, et al. Gastric per-oral endoscopic myotomy (G-POEM) for refractory gastroparesis: results from an international prospective trial. Gut. 2022;71:25–33. https://doi.org/10.1136/gutjnl-2020-322756.
Martinek J, Hustak R, Mares J, Vackova Z, Spicak J, Kieslichova E, Buncova M, Pohl D, Amin S, Tack J. Endoscopic pyloromyotomy for the treatment of severe and refractory gastroparesis: a pilot, randomized, sham-controlled trial. Gut. 2022;71(11):2170–8. https://doi.org/10.1136/gutjnl-2022-326904.
Vosoughi K, et al. Role of endoscopic functional luminal imaging probe in predicting the outcome of gastric peroral endoscopic pyloromyotomy (with video). Gastrointest Endosc. 2020;91:1289–99. https://doi.org/10.1016/j.gie.2020.01.044.
Kamal F, et al. Systematic review with meta-analysis: one-year outcomes of gastric peroral endoscopic myotomy for refractory gastroparesis. Aliment Pharmacol Ther. 2022;55:168–77. https://doi.org/10.1111/apt.16725.
Papasavas PK, et al. Gastric bypass surgery as treatment of recalcitrant gastroparesis. Surg Obes Relat Dis. 2014;10:795–9.
Sun Z, et al. Surgical treatment of medically refractory gastroparesis in the morbidly obese. Surg Endosc. 2015;29:2683–9. https://doi.org/10.1007/s00464-014-3990-z.
Author information
Authors and Affiliations
Contributions
RJG was the principal author, having performed all analyses of the literature, generated the initial draft and concepts underlying the pathogenesis model and contributed substantially to the editing of all versions.
JHS contributed substantially to editing and revisions to all versions of the manuscript and was responsible for the generation of the immunopathogenesis section.
VK contributed substantially to the editing and revisions made to all versions of the manuscript and made significant contributions to the analyses of pathogenesis shown in text, tables, and figures.
CCT contributed substantially to the editing and revisions made to all versions of the manuscrip and made significant contributions to the analysis of the literature related to endoscopic therapy.
SAS participated in the conceptualization of the manuscript, contributed significantly to the editing and revisions, and made significant contributions to the analysis related to anatomic treatment.
Corresponding author
Ethics declarations
Competing Interests
RJG, JHS, VK, and SAS have no conflicts or appearances of conflict to report relevant to the content of this manuscript. CCT discloses the following potentially competing interests related to endoscopic treatments for GI disorders, including research support/consulting from Apollo Endo-surgery, Aspire Bariatrics, Boston Scientific, Erbe, GI Dynamics, Lumendi, Olympus, USGI Medical, and Covidien/Medtronic, as well as equity interest in Enterasense and Envision.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gilbert, R.J., Siamwala, J.H., Kumar, V. et al. Reconsideration of the Gastroparetic Syndrome. Curr Gastroenterol Rep 25, 75–90 (2023). https://doi.org/10.1007/s11894-023-00865-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11894-023-00865-w