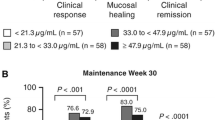
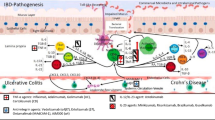
Abstract
Knowledge about the clinical pharmacology of medical therapy of inflammatory bowel disease has incrementally advanced. Small studies with mesalamine have suggested that intestinal mucosal concentrations of mesalamine may predict clinical response to mesalamine therapy. Increased expression of glucocorticoid receptor β and increased expression of the multidrug resistance drug pump P-glycoprotein 170 have been proposed as markers of drug resistance to glucocorticoids. A baseline determination of thiopurine methyltransferase phenotype or genotype may predict early leukopenia in patients treated with azathioprine or 6-mercaptopurine. Serial measurement of erythrocyte 6-thioguanine nucleotides may be useful in tailoring the dose of these medications. A loading dose of intravenous azathioprine does not accelerate the time to response in patients with steroid-treated Crohn's disease; however, standard azathioprine may work more quickly than previously reported. Methotrexate, 15 to 25 mg/wk, is effective for the treatment of Crohn's disease (active or in remission), and there is no significant difference in the erythrocyte concentrations of methotrexate polyglutamate in patients with inflammatory bowel disease receiving 15 mg, compared with 25 mg, subcutaneously on a weekly basis.
Similar content being viewed by others
References and Recommended Reading
De Vos M, Verdievel H, Schoonjans R, et al.: High-performance liquid chromatographic assay for the determination of 5-aminosalicylic acid and acetyl-5-aminosalicylic acid concentrations in endoscopic intestinal biopsy in humans. J Chromatogr 1991, 564:296–302.
Palumbo G, Carlucci G, Mazzeo P, et al.: Simultaneous determination of 5-aminosalicylic acid, acetyl-5-aminosalicylic acid and 2,5-dihydroxybenzoic acid in endoscopic intestinal biopsy samples in humans by high-performance liquid chromatography with electrochemical detection. J Pharmacol Biomed Anal 1995, 14:175–180.
De Vos M, Verdievel H, Schoonjans R, et al.: Concentrations of 5-ASA and Ac-5-ASA in human ileocolonic biopsy homogenates after oral 5-ASA preparations [see comments]. Gut 1992, 33:1338–1342.
Schoonjans R, De Vos M, Schelfhout AM, et al.: Distribution and concentrations of 5-aminosalicylic acid in rectosigmoid biopsy specimens after rectal administration. Dis Colon Rectum 1996, 39:788–793.
Frieri G, Giacomelli R, Pimpo M, et al.: Mucosal 5-aminosalicylic acid concentration inversely correlates with severity of colonic inflammation in patients with ulcerative colitis. Gut 2000, 47:410–414.
Frieri G, Pimpo MT, Palumbo GC, et al.: Rectal and colonic mesalazine concentration in ulcerative colitis: oral vs. oral plus topical treatment. Aliment Pharmacol Ther 1999, 13:1413–1417.
Frieri G, Pimpo MT, Andreoli A, et al.: Prevention of postoperative recurrence of Crohn's disease requires adequate mucosal concentration of mesalazine. Gruppo Italiano per lo Studio del Colon e del Retto. Aliment Pharmacol Ther 1999, 13:577–582.
Frieri G, Pimpo MT, Palumbo G, et al.: Anastomotic configuration and mucosal 5-aminosalicyclic acid (5-ASA) concentrations in patients with Crohn's disease: a GISC study. Gruppo Italiano per lo Studio del Colon e del Retto. Am J Gastroenterol 2000, 95:1486–1490.
D'albasio G, Pacini F, Camarri E, et al.: Combined therapy with 5-aminosalicylic acid tablets and enemas for maintaining remission in ulcerative colitis: a randomized doubleblind study. Am J Gastroenterol 1997, 92:1143–1147.
Safdi M, DeMicco M, Sninsky C, et al.: A double-blind comparison of oral versus rectal mesalamine versus combination therapy in the treatment of distal ulcerative colitis. Am J Gastroenterol 1997, 92:1867–1871.
Camma C, Giunta M, Rosselli M, Cottone M: Mesalamine in the maintenance treatment of Crohn's disease: a meta- analysis adjusted for confounding variables. Gastroenterology 1997, 113:1465–1473.
Lochs H, Mayer M, Fleig WE, et al.: Prophylaxis of postoperative relapse in Crohn's disease with mesalamine: European Cooperative Crohn's Disease Study VI. Gastroenterology 2000, 118:264–273.
Gronemeyer H: Control of transcription activation by steroid hormone receptors. FASEB J 1992, 6:2524–2529.
Hollenberg SM, Weinberger C, Ong ES, et al.: Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature 1985, 318:635–641.
Giguere V, Hollenberg SM, Rosenfeld MG, Evans RM: Functional domains of the human glucocorticoid receptor. Cell 1986, 46:645–652.
Bamberger CM, Bamberger AM, de Castro M, Chrousos GP: Glucocorticoid receptor beta: a potential endogenous inhibitor of glucocorticoid action in humans. J Clin Invest 1995, 95:2435–2441.
Webster JC, Cidlowski JA: Mechanisms of glucocorticoidreceptor- mediated repression of gene expression. Trends Endocrinol Metab 1999, 10:396–402.
Ueda K, Okamura N, Hirai M, et al.: Human P-glycoprotein transports cortisol, aldosterone, and dexamethasone, but not progesterone. J Biol Chem 1992, 267:24248–24252.
Bourgeois S, Gruol DJ, Newby RF, Rajah FM: Expression of an mdr gene is associated with a new form of resistance to dexamethasone-induced apoptosis. Mol Endocrinol 1993, 7:840–851. Initial report indicating that increased glucocorticoid receptor b concentrations are associated with steroid resistance in patients with ulcerative colitis.
Honda M, Orii F, Ayabe T, et al.: Expression of glucocorticoid receptor beta in lymphocytes of patients with glucocorticoidresistant ulcerative colitis. Gastroenterology 2000, 118:859–866.
Schottelius A, Wedel S, Weltrich R, et al.: Higher expression of glucocorticoid receptor in peripheral mononuclear cells in inflammatory bowel disease. Am J Gastroenterol 2000, 95:1994–1999. Initial report indicating that increased multidrug resistence-1 gene product, P-glycoprotein 170, is associated with steroid resistance in patients with ulcerative colitis.
Farrell RJ, Murphy A, Long A, et al.: High multidrug resistance (P-glycoprotein 170) expression in inflammatory bowel disease patients who fail medical therapy. Gastroenterology 2000, 118:279–288.
Chan GL, Erdmann GR, Gruber SA, et al.: Azathioprine metabolism: pharmacokinetics of 6-mercaptopurine, 6- thiouric acid and 6-thioguanine nucleotides in renal transplant patients. J Clin Pharmacol 1990, 30:358–363.
Weinshilboum RM, Sladek SL: Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet 1980, 32:651–662.
Otterness D, Szumlanski C, Lennard L, et al.: Human thiopurine methyltransferase pharmacogenetics: gene sequence polymorphisms. Clin Pharmacol Ther 1997, 62:60–73.
Yates CR, Krynetski EY, Loennechen T, et al.: Molecular diagnosis of thiopurine S-methyltransferase deficiency: genetic basis for azathioprine and mercaptopurine intolerance [see comments]. Ann Intern Med 1997, 126:608–614. Initial report that early leukopenia is associated with low or intermediate thiopurine methyltransferase genotype in patients with Crohn's disease treated with azathioprine.
Colombel JF, Ferrari N, Debuysere H, et al.: Genotypic analysis of thiopurine S-methyltransferase in patients with Crohn's disease and severe myelosuppression during azathioprine therapy. Gastroenterology 2000, 118:1025–1030.
Lennard L, Rees CA, Lilleyman JS, Maddocks JL: Childhood leukaemia: a relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br J Clin Pharmacol 1983, 16:359–363.
Lennard L, Van Loon JA, Weinshilboum RM: Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther 1989, 46:149–154.
Black AJ, McLeod HL, Capell HA, et al.: Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine. Ann Intern Med 1998, 129:716–718.
Snow JL, Gibson LE: A pharmacogenetic basis for the safe and effective use of azathioprine and other thiopurine drugs in dermatologic patients. J Am Acad Dermatol 1995, 32:114–116.
Snow JL, Gibson LE: The role of genetic variation in thiopurine methyltransferase activity and the efficacy and/or side effects of azathioprine therapy in dermatologic patients [see comments]. Arch Dermatol 1995, 131:193–197.
Anstey A, Lennard L, Mayou SC, Kirby JD: Pancytopenia related to azathioprine: an enzyme deficiency caused by a common genetic polymorphism: a review. J R Soc Med 1992, 85:752–756.
Cuffari C, Theoret Y, Latour S, Seidman G: 6-Mercaptopurine metabolism in Crohn's disease: correlation with efficacy and toxicity. Gut 1996, 39:401–406. First larger report indicating that measurement of 6-thioguanine in the erythrocytes of patients with inflammatory bowel disease treated with 6-mercaptopurine or azathioprine may help to tailor therapy.
Dubinsky MC, Lamothe S, Yang HY, et al.: Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology 2000, 118:705–713. Report from a randomized, controlled trial demonstrating no benefit for an intravenous loading dose of azathioprine for accelerating remission in active, steroid-treated Crohn's disease.
Sandborn WJ, Tremaine WJ, Wolf DC, et al.: Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn's disease: North American Azathioprine Study Group [see comments]. Gastroenterology 1999, 117:527–535.
Cross-sectional study of IBD patients taking azathioprine (AZA) or 6-mercaptopurine (6MP): lack of correlation between disease activity and 6-thioguanine nucleotide (6TGN) concentration [abstract]. Gastroenterology 2000, 118:A788.
Sandborn WJ, Van OE, Zins BJ, et al.: An intravenous loading dose of azathioprine decreases the time to response in patients with Crohn's disease. Gastroenterology 1995, 109:1808–1817.
Feagan BG, Rochon J, Fedorak RN, et al.: Methotrexate for the treatment of Crohn's disease: the North American Crohn's Study Group Investigators [see comments]. N Engl J Med 1995, 332:292–297.
Feagan BG, Fedorak RN, Irvine EJ, et al.: A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease: North American Crohn's Study Group Investigators [see comments]. N Engl J Med 2000, 342:1627–1632.
Arora S, Katkov W, Cooley J, et al.: Methotrexate in Crohn's disease: results of a randomized, double-blind, placebocontrolled trial. Hepatogastroenterology 1999, 46:1724–1729.
Oren R, Moshkowitz M, Odes S, et al.: Methotrexate in chronic active Crohn's disease: a double-blind, randomized, Israeli multicenter trial. Am J Gastroenterol 1997, 92:2203–2209.
Oren R, Arber N, Odes S, et al.: Methotrexate in chronic active ulcerative colitis: a double-blind, randomized, Israeli multicenter trial [see comments]. Gastroenterology 1996, 110:1416–1421.
Egan LJ, Sandborn WJ, Tremaine WJ, et al.: A randomized dose-response and pharmacokinetic study of methotrexate for refractory inflammatory Crohn's disease and ulcerative colitis. Aliment Pharmacol Ther 1999, 13:1597–1604.
Moshkowitz M, Oren R, Tishler M, et al.: The absorption of low-dose methotrexate in patients with inflammatory bowel disease. Aliment Pharmacol Ther 1997, 11:569–573. Report from a pharmacokinetic study demonstrating that subcutaneously administered methotrexate, 15 to 25 mg/wk, achieves pharmacologically active concentrations in the gut lumen and mucosa at the 1-week trough time point.
Egan LJ, Sandborn WJ, Mays DC, et al.: Systemic and intestinal pharmacokinetics of methotrexate in patients with inflammatory bowel disease. Clin Pharmacol Ther 1999, 65:29–39.
Egan LJ, Sandborn WJ, Mays DC, et al.: Dialysis of the rectum for sampling drug concentrations in the luminal extracellular fluid of the gut: technique and precision. Aliment Pharmacol Ther 1998, 12:679–684.
Egan LJ, Sandborn WJ, Mays DC, et al.: Plasma and rectal adenosine in inflammatory bowel disease: effect of methotrexate. Inflamm Bowel Dis 1999, 5:167–173.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sandborn, W.J., Faubion, W.A. Clinical pharmacology of inflammatory bowel disease therapies. Curr Gastroenterol Rep 2, 440–445 (2000). https://doi.org/10.1007/s11894-000-0005-0
Issue Date:
DOI: https://doi.org/10.1007/s11894-000-0005-0