Abstract
Purpose of Review
The field of inpatient diabetes has advanced significantly over the last 20 years, leading to the development of personalized treatment approaches. However, outdated guidelines still recommend the use of basal-bolus insulin therapy as the preferred treatment approach, and against the use of non-insulin anti-hyperglycemic agents.
Recent Findings
Several observational and prospective randomized controlled studies have demonstrated that oral anti-hyperglycemic agents are widely used in the hospital, including studies of DPP-4 agents and GLP-1 agonists.
Summary
With advances in the field of inpatient diabetes management, a paradigm shift has occurred, from an approach of recommending “basal-bolus regimens” for all patients to a more precision medicine option for hospitalized non-critically ill patients with type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inpatient hyperglycemia, defined as blood glucose ≥ 140 mg/dl, is a common scenario in hospitals across the world, affecting patients with prior diagnosis of diabetes or those with stress hyperglycemia (i.e., without a prior diagnosis of diabetes) [1–3]. It is reported that about 20–40% of hospitalized patients in the USA [4–13], up to 18–31% in the UK [14] and 18.4% in Spain [15], have inpatient hyperglycemia. In addition, data from the UK also suggest that over 20% of people without a diagnosis of diabetes presenting to an emergency department may have newly recognized hyperglycemia [16]. Extensive evidence suggests a strong association between inpatient hyperglycemia, in persons with and without diabetes, and increased morbidity, mortality, and healthcare utilization [1–4, 17, 18]. In this article, we review the scientific evidence for the management of inpatient hyperglycemia in non-critically ill patients with type 2 diabetes, with emphasis on recent studies assessing non-insulin therapies (Table 1).
Historical Overview of the Management of Hyperglycemia in Non-critical Hospital Settings
It may appear unreasonable that 20 years ago, there was not a standard of care for the management of hospitalized patients with hyperglycemia. In some hospitals, many clinicians did not pay attention to hyperglycemia, with the management relying on continuing home medications, including metformin, and sulfonylureas, with the addition of sliding scale insulin (SSI) therapy [9]. In addition, there has been a heavy reliance on the reactive approach of using SSI [23, 24]. In a prospective observational study in the late 1990s, Queale et al. demonstrated that over 75% of patients hospitalized were treated with SSI regimens, which was associated with increased risk of hyperglycemia [23]. In recent retrospective studies at large academic medical centers in the USA, 31–40% of non-critical patients with hyperglycemia were treated with SSI alone [9, 22].
During the past two decades, an increased focus has been placed on the inpatient management of hyperglycemia, particularly in the intensive care unit (ICU). The initial study from Van den Berghe et al., as a prospective, randomized trial in 2001, showed an impressive 42% relative reduction in mortality in intensively treated patients in the surgical ICU [13]. The Leuven protocol used in this trial included the administration of an insulin infusion with an intensive glycemic target of a blood glucose between 80 and 110 mg/dl. Based on these single-center results in a surgical ICU, the use of intensive insulin (subcutaneous and intravenous) regimens was adopted in all ICU and non-ICU settings around the world. However, the same investigators and several large multi-center randomized control trials failed to replicate the benefits of intensive insulin therapy in the ICU, reporting no mortality benefits [25]. Definitive data against intensive insulin therapy in the ICU was available with the publication of the multinational Normoglycemia in Intensive Care Evaluation–Survival Using Glucose Algorithm Regulation (NICE-SUGAR) trial [26]. The results of the NICE-SUGAR trial showed an absolute increase in the rate of death at 90 days with intensive glucose control (27.5%, vs. 24.9% with conventional control; odds ratio, 1.14; P = 0.02). In addition, severe hypoglycemia occurred more frequently in the intensive treatment group than in the conventional-control group (6.8% vs. 0.5%, P<0.001). From this point on, a more relaxed glycemic target of 140–180 mg/dl has been recommended for most patients in the ICU.
In non-ICU settings, the lack of evidence triggered randomized controlled trials to assess benefits of improved glycemic outcomes in general medicine and surgical patients. The RABBIT2 trial showed that the basal-bolus insulin regimen was superior to SSI alone [27]. This was further confirmed by other studies, with observational and prospective randomized trials also supporting the benefits of a proactive approach to the management of inpatient hyperglycemia in non-critically ill hospitalized patients [28, 29•, 30]. Several scientific papers from the medical community, patient advocacy, and regulatory bodies raised the need for the creation of clinical practice guidelines and a change in the standard of care [31]. Consequently, several organizations in the US and in Europe developed their guidelines, with pioneers such as the American Association of Clinical Endocrinology, The Endocrine Society, the Joint British Diabetes Societies for Inpatient Care, and the Spanish Diabetes Society leading the movement [1–3, 32, 33]. While these clinical practice guidelines represent a step forward in the field, many of the recommendations are based on consensus expertise, due to the lack of randomized controlled trials, particularly in the use of non-insulin therapy and optimal glycemic targets.
Since the late 2000s, the American Diabetes Association and American Association of Clinical Endocrinology’s guidelines for the management of diabetes in the hospital recommended basal bolus insulin therapy as a proactive and physiological approach, opposing to the reactive approach of sliding insulin scale [1, 3]. The basal bolus regimen includes three components: (1) basal insulin, (2) bolus (nutritional/prandial) insulin given before meals, and (3) supplemental or correctional insulin. The latter term refers to the addition of supplemental rapid acting insulin to scheduled bolus doses, to correct residual hyperglycemia—not to be confused with sliding scale insulin (SSI) or variable dose subcutaneous insulin regimen as also known in the UK. The guidelines emphasized that the prolonged use of sliding scale insulin as the only regimen to mitigate inpatient hyperglycemia was not effective for most patients. Similarly, the Endocrine Society guideline for the management of diabetes recommended against using SSI alone for most patients with type 2 diabetes, with basal-bolus insulin regimen being the preferred approach for most of these patients [2]. Several other scientific societies, including the American Diabetes Association [1], the American College of Physicians [34], the Society of Critical Care Medicine [35], the Joint British Diabetes Societies for Inpatient Care [33], and the Spanish Diabetes Society [32], produced comprehensive guidelines to guide clinicians on the management of ICU and non-ICU patients with hyperglycemia.
Across the USA, several hospitals have implemented system-wide initiatives to expand the use of basal bolus insulin therapy, and decrease the use of insulin sliding scale (use of short or rapid-acting insulin without basal insulin) and non-insulin agents in non-ICU settings. In addition, several healthcare systems have implemented “Specialized Diabetes Consult Service,” including endocrinologists, advanced practitioners such as nurse practitioners and physician assistants, pharmacists, and diabetes care and education specialists to manage persons with diabetes in the hospital. The benefits of these dedicated inpatient teams have been validated in different hospital environments including academic, community, and specialized hospital services36. In the UK, the use of the diabetes inpatient nursing teams has been shown to significantly reduce length of stay and improve patient satisfaction while in hospital [37, 38].
Similarly, there is very limited evidence on the management of hospitalized patients specifically with type 1 diabetes, as opposed to insulin treated type 2 diabetes [39]. People with type 1 diabetes are a high-risk population with a higher risk of large glycemic excursions, and frequently requiring specialist care by endocrinologists or diabetologists. Recent developments on the use of continuous glucose monitoring (CGM) and automated insulin delivery systems (AIDs) have improved glycemic care for ambulatory patients with type 1 diabetes, with consensus meeting guidelines recently published—a topic beyond the scope of this article [40, 41]. Work on the safety and efficacy of closed loop devices in the hospital are ongoing [42, 43].
Evolution of Personalized Management of Diabetes in the Hospital—Role of Non-insulin Therapies
Recent evidence from randomized and observational studies, as well as the experience from inpatient diabetes services, have shown that not all patients require basal-bolus regimens as the single or universal approach to managing diabetes in the hospital setting. A need for a more personalized approach has become evident in clinical practice. The development of newer anti-hyperglycemic agents for ambulatory diabetes care, with different mechanisms of action and lower risk of hypoglycemia, has triggered the need to assess innovative approaches to the traditional basal bolus approach. Indeed, non-insulin agents are used widely in Europe and outside the USA [44–47], despite guideline recommendations against it. There are many potential reasons for using non-insulin agents which include, but not limited to, the fact that the basal bolus regimen is complex, labor-intensive, and associated with risk of hypoglycemia [47]. The lack of confidence or knowledge of medical staff in managing diabetes in general is also likely to be a factor [48, 49].
Predictors of response to basal-bolus therapy have been identified, including high HbA1c levels [50]. Several prospective randomized trials and observational studies have shown that patients with mild inpatient hyperglycemia may be managed with simpler regimens, such as non-insulin therapy [51••, 9], or combination of basal or correctional insulin therapy with incretin therapy [52••, 53••, 54–56], instead of basal-bolus insulin therapy.
Basal-Plus Regimen
A basal-plus approach may be preferred for patients with mild hyperglycemia, decreased oral intake, or for patients undergoing surgery. This regimen consists of a single dose of basal insulin (~0.1–0.25 units/kg/day) along with correction doses of insulin for elevated glucose levels before meals or every 6 h (if “nil by mouth”). A randomized clinical trial in 370 general medicine and surgical patients with type 2 diabetes reported that the basal-plus approach resulted in similar improvements in mean daily blood glucose levels compared to basal-bolus regimens, with a trend in reducing hypoglycemic events < 70 mg/dl (17% vs 13%)—albeit the study was not powered for this outcome. The ADA Standard of Care recommends starting with the basal-plus correction approach in patients with type 2 diabetes with poor or uncertain nutritional intake [1].
Use of DPP-4 Inhibitors
Given the safety profile of incretin agents, several investigators have assessed the efficacy of DPP-4 inhibitors alone or in combination with basal insulin in hospitalized non-ICU patients with type 2 diabetes. Several prospective randomized and observational studies have shown that therapy with DPP-4 inhibitors is well tolerated and associated with lower risk of hypoglycemia, and in non-inferior glycemic outcomes compared to basal-bolus insulin regimens in patients with mild hyperglycemia. In a multi-center, prospective, non-inferiority, randomized trial, Pasquel et al. compared the safety and efficacy of sitagliptin with basal insulin to the traditional basal-bolus regimen among hospitalized, non-critically ill patients with type 2 diabetes [51••]. The authors reported that mean blood glucose in the basal plus sitagliptin group was not inferior to that in the basal-bolus group (mean difference 1.8 mg/dl), with no differences in hypoglycemia or hospital complications. Hence, they concluded that basal plus DPP-4 regimen may be an alternative to the labor-intensive basal-bolus regimen in select patients. In an accompanying editorial article, Nauck and Meier suggested that this study was a step forward in the era of personalized and individualized care of inpatient hyperglycemia [57]. Taking into consideration that patients with high insulin needs were excluded, up to 26% have recommended glycemic outcomes (HbA1c < 7%), and 38% had admission glucose < 200 mg/dl, this study suggested that patients with mild-to-moderate inpatient hyperglycemia, frail or elderly patients, and those with renal failure or high risk of hypoglycemia may be safely managed with this simplified approach of DPP-4 inhibitors with basal insulin combination. Other studies using saxagliptin [58] in general medicine and linagliptin in general surgery patients [53••] have confirmed these findings.
Overall, therapy with DPP-4 inhibitors, particularly when combined with low-dose basal insulin, has shown to provide similar glycemic outcomes compared to basal-bolus insulin, and is associated with less hypoglycemia and lower treatment burden [59]. Hence, it is reasonable to recommend that for hospitalized patients with admission glucose below 180–200 mg/dl, the use of basal insulin plus correctional insulin and/or DPP-4 inhibitors may be considered. Since previous HbA1c can be a predictor for achieving recommended inpatient glycemic outcomes, patients having previous target or near-target glycemic outcomes—as evident by HbA1c < 8%, or with low insulin requirements (< 0.5 units/kg/day), or with poor oral intake—particularly those undergoing non-cardiac surgery, can be candidates for therapy with DPP-4 inhibitors plus correction by rapid insulin or combined therapy with basal insulin.
Use of GLP-1 Agents
Following the validation studies of GLP-1 agonists on the initial positive outcomes in the prevention of cardiovascular events, there was an increased interest in assessing the safety and efficacy of these agents in hospitalized patients—particularly patients with cardiac disease or undergoing cardiac surgery. Few pilot studies using intravenous formulations of GLP-1 agonists or native GLP-1 in patients undergoing cardiac surgery showed no safety signals, but there was a need for larger studies to confirm their preliminary results [60–63].
A few randomized controlled studies using liraglutide have reported improvement in glycemic outcomes. Polderman et al. administered low-dose liraglutide (0.6 mg) the evening before surgery and found that mean glucose 1-h post-surgery was lower, compared to insulin infusion or bolus insulin, but with more nausea reported [64]. In another study in non-critically ill patients, Fayfman et al. found that treatment with short-acting exenatide twice daily plus basal insulin resulted in better glycemic outcomes compared to either basal-bolus or exenatide alone, but also demonstrated higher rates of gastro-intestinal side effects [52••]. While these pilot studies showed promising results, there is a need for larger studies, and using newer longer-acting agents, to determine if there is a benefit of using GLP-1 agonists in the hospital to counterbalance the gastro-intestinal side effects. With newer, weekly, and more potent GLP-1 agonists already approved for diabetes, weight management and cardiovascular prevention—benefits beyond glycemic outcomes—and with ongoing developments of twin-incretins, one unanswered question remains on the impact of these agents administered before admission and continued effect during hospitalization, but no data is available to our knowledge. The use of GLP-1 agonists in hospitalized patients has been reviewed in detail elsewhere [65].
Use of SGLT2 Inhibitors
SGLT2 inhibitors, along with GLP-1 inhibitors, have changed the treatment paradigm in ambulatory patients with type 2 diabetes, shifting from a glucose-centric approach to include prevention of cardiovascular events and kidney disease progression, weight loss, and prevention of hypoglycemia1. Previous work looked at the effect of dapagliflozin on corticosteroid-induced hyperglycemia in people hospitalized with an acute exacerbation of chronic obstructive pulmonary disease (COPD), where it failed to demonstrate an improvement in hyperglycemia [66]. Another recent pilot randomized controlled trial evaluated the safety and efficacy of empagliflozin compared to placebo in patients hospitalized with acute decompensate heart failure with (83%) and without diabetes (17%). The authors reported no differences in dyspnea score, diuretic response, N-terminal pro-brain natriuretic peptide (NT-ProBNP), and length of stay (LOS). As expected, and confirming ambulatory studies, there was a decrease in deaths, and hospital readmission for heart failure during the outpatient follow-up to 60 days [67]. Another pilot study of persons with type 2 diabetes assessed the efficacy and safety of empagliflozin as add-on therapy when admitted for acute heart failure [68]. During the initial seven days of hospitalization, there was no difference in left ventricular function, NT-ProBNP, creatinine, and hematocrit. While these two studies are just preliminary data, both confirmed that use of SGLT2 inhibitors in the inpatient setting may be safe, but with limited efficacy benefits. These results are expected since efficacy outcomes are likely be noticed after longer follow-up, and not during a short period of inpatient stay (~< 7 days).
More recently, a study looking to see if people hospitalized with COVID-19 and at least one cardiovascular risk factor (i.e., not limited to those with diabetes) would benefit from dapagliflozin showed that there were no differences in outcomes compared to placebo [7]. Other preliminary work has suggested that the use of SGLT2 inhibitors is relatively safe, with low levels of hypoglycemia, acidosis (as measured by plasma bicarbonate), or ketonemia [69]. However, these data are complicated by the dropout rate of almost 55% of those taking these drugs. Concerns for diabetes ketoacidosis (DKA) have also limited the use of these agents in the hospital, with the DARE study reporting only 2 “mild” cases [7]. A further review of the literature identified 42 reports of peri-operative euglycemic DKA up to 2019, with presentation ranging from few hours up to 6 weeks after surgery [70].
At this stage, the use of SGLT2 inhibitors in the hospital is still experimental in patients with diabetes, but adding this therapy during the discharge process may improve clinical inertia and allow more patients to benefits from these agents. These drugs are potentially beneficial if their safety can be assured [71]. International consensus guidelines for management and prevention of euglycemic DKA in patients with type 1 diabetes have been published and can provide excellent guidance [72], but there are not specific recommendations for patients with type 2 diabetes.
Use of Metformin
The use of metformin in the hospital have not been recommended by clinical guidelines due to concerns with lactic acidosis risk and other side effects [1–3]. Several single-center retrospective studies, however, have reported no increased risk of adverse events. Metformin is used in up to 20–50% of patients admitted to hospitals in developing countries [44, 73, 74]. While there is controversy, it is customary to stop metformin in patients undergoing radiological studies with utilization of intravenous contrast for 72 h from the start of the procedure, such as percutaneous coronary intervention (PCI) [75, 76]. However, several observational studies have shown a low risk of metformin-associated lactic acidosis after radiological procedures or PCI [77, 78], particularly in patients with GFR > 30–60 mL/min/1.73 m2. However, metformin should not be used in patients at risk of lactic acidosis, such as those with renal failure, sepsis, hypoxia, liver failure, and alcoholism. A recent review of metformin use in the hospital confirmed the lack of sound quality evidence to support its continued use [79]. The authors concluded that if perons are relatively stable, with no renal impairment and no increased risk of lactic acidosis, then it may be possible to safely continue it.
Use of Sulfonylureas
Sulfonylureas has been used extensively for many years. Their benefits beyond glucose-lowering effects are limited, with concerns for hypoglycemia [80, 81]. Several observational studies have previously shown increased risk of cardiovascular events in patients treated with sulfonylureas [82–84]. However, the CAROLINA trial showed non-inferiority of glimepiride to linagliptin in regard to first occurrence of cardiovascular events in persons with elevated cardiovascular risk and type 2 diabetes [46]. Despite these concerns, sulfonylureas are used in up to 20% of hospitalized patients in the USA and UK, but data on prevalence use is limited [45, 74, 85]. As suggested by two recent risk prediction models, the use of sulfonylureas in the hospital is associated with hypoglycemia [86, 87]. The rates of hypoglycemia ranged from ~20 to 30% [45, 85, 88]. In a single-center study in the USA, Deusenberry et al. found that up to 19% of patients that continued or initiated therapy with sulfonylureas in the hospital experience a hypoglycemic episode, similar to therapy with insulin [85]. The highest risk for hypoglycemia was noted among elderly patients, with renal failure or using concurrent insulin therapy [85].
In a retrospective study of 11 acute hospitals in the UK, hypoglycemia related to sulfonylureas affected up to 30% of the cohort, with many experienced recurrent hypoglycemic episodes [12], and most hypoglycemic episodes occurring during the night or early-morning shifts. This is of great concern because the use of point-of-care capillary glucose testing performed before fasting and each meal is inadequate to detect nocturnal or prolonged hypoglycemia, compared to continuous glucose monitoring [89]. Additionally, the hypoglycemic events caused by sulfonylureas (particularly the old and long-acting agents) are frequently more severe, difficult to solve, and longer, something particularly concerning in the hospital setting [90].
There are differences in recommendations between scientific societies, such as US guidelines recommended against the use of sulfonylureas in the hospital [2], while UK guidelines allow the use of sulfonylureas to manage glucose excursions in patients taking once-daily oral steroids in the morning, if necessary, along with morning administration of basal human insulin [91]. Other European countries, like Spain, consider that secretagogues (sulfonylureas, glinides) have a relative contraindication during hospitalization, especially the long-acting sulfonylureas [32].
Use of Thiazolidinediones
Use of thiazolidinediones in ambulatory settings has decreased in recent years, trending from 28.5 to 5.6% from 2006 to 2013 [92]. Thiazolidinedione use in the hospital is less common, with estimated prevalence reports of < 10% [74]. Concerns about the time they take to achieve effective glycemic outcomes (i.e., several weeks to months) and their predisposition for fluid retention, and heart failure decompensation—particularly when combined with insulin—make these agents less attractive for inpatient use [2].
Future of Inpatient Diabetes Management
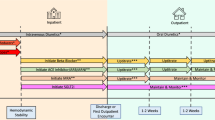
An individualized approach has been recommended to select an effective and safe treatment approach for the management of inpatient hyperglycemia in patients with type 2 diabetes. The best treatment approach should incorporate patient characteristics (e.g., duration of diabetes, BMI), severity of hyperglycemia (admission glucose, HbA1c), hypoglycemia risk (elderly, renal, or liver failure), and diabetes treatment complexity prior to admission (Table 2).
Personalized treatment in non-ICU hospitalized patients with T2D **Regimen complexity refers to the number and type of agents (oral agents, GLP-1RA, and insulin) used in the outpatient setting, with more complex regimens referring to those including multiple agents and/or insulin therapy. SSI refers to use of correctional sliding scale insulin. Patients on multiple agents are likely to have worsening hyperglycemia if all preadmission agents are stopped and may respond better to basal + OAD or a basal-bolus approach [95]
Among frail patients (i.e., elderly, advanced kidney disease) or those with target glycemic outcomes, less aggressive regimens reduce the risk of iatrogenic hypoglycemia. The use of correction insulin alone usually works in insulin naïve and recently diagnosed patients with BG levels < 180 mg/dL [9]. If glycemic targets are not achieved in 24–48 h, adding a basal insulin (“basal-plus” regimen) at starting dose of 0.1 to 0.25 units per kg of actual body weight, plus correction and prandial insulin before meals for BG levels > 180 mg/dL, is likely to manage most patients with T2D, particularly patients who are insulin naïve. [93]
For patients with moderate (BG >200 mg/dL) or severe hyperglycemia (BG >300 mg/dl), more intensive insulin regimens are indicated, Figure 1. For patients receiving insulin therapy prior to admission, the home regimen is used to calculate the total daily dose (TDD). Insulin-naïve patients with significant hyperglycemia and adequate oral intake can be started at a TDD between 0.3 and 0.5 units/kg divided on a ratio of basal/bolus insulin of 50:50%. [94] Lower doses are recommended for those at high hypoglycemia risk.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Diabetes Association. Chapter 16. Diabetes care in the hospital: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl_1):S244-S253
Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38.
Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–31.
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–82.
Bersoux S, Cook CB, Kongable GL, Shu J. Trends in glycemic control over a 2-year period in 126 US hospitals. J Hosp Med. 2013;8(3):121–5.
Cook CB, Kongable GL, Potter DJ, Abad VJ, Leija DE, Anderson M. Inpatient glucose control a glycemic survey of 126 US hospitals. J Hosp Med. 2009;4(9):7–14.
Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9(9):586–94.
McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810–5.
Migdal AL, Fortin-Leung C, Pasquel F, Wang H, Peng L, Umpierrez GE. Inpatient glycemic control with sliding scale insulin in noncritical patients with type 2 diabetes: who can slide? J Hosp Med. 2021;16(8):462–8.
Mulla I, Schmidt K, Cashy J, et al. Comparison of glycemic and surgical outcomes after change in glycemic targets in cardiac surgery patients. Diabetes Care. 2014;37(11):2960–5.
Schmeltz LR, DeSantis AJ, Thiyagarajan V, et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care. 2007;30(4):823–8.
Swanson CM, Potter DJ, Kongable GL, Cook CB. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011;17(6):853–61.
van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–67.
NHS Digital. National Diabetes Inpatient Audit (NaDIA). 2021. https://digital.nhs.uk/supplementary-information/2021/nadia-insulin-use-oral-hypoglycaemic-agent-use-insulin-infusion-use
Ena J, Gomez-Huelgas R, Romero-Sanchez M, et al. Hyperglycemia management in patients admitted to internal medicine in Spain: A point-prevalence survey examining adequacy of glycemic control and guideline adherence. Eur J Intern Med. 2015;26(6):392–8.
Ghosh S, Manley SE, Nightingale PG, et al. Prevalence of admission plasma glucose in “diabetes” or “at risk” ranges in hospital emergencies with no prior diagnosis of diabetes by gender, age and ethnicity. Endocrinol Diabetes Metab. 2020;3(3):e00140.
Evans NR, Dhatariya KK. Assessing the relationship between admission glucose levels, subsequent length of hospital stay, readmission and mortality. Clin Med (Lond). 2012;12(2):137–9.
Galindo RJ, Fayfman M, Umpierrez GE. Perioperative management of hyperglycemia and diabetes in cardiac surgery patients. Endocrinol Metab Clin North Am. 2018;47(1):203–22.
Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. US Dept of Health and Human Services. 2020.
Soriguer F, Goday A, Bosch-Comas A, et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@bet.es Study. Diabetologia. 2012;55(1):88–93.
Kosiborod M, Inzucchi SE, Spertus JA, et al. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation. 2009;119(14):1899–907.
Sadhu AR, Patham B, Vadhariya A, Chikermane SG, Johnson ML. Outcomes of “real-world” insulin strategies in the management of hospital hyperglycemia. J Endocr Soc. 2021;5(8):bvab101.
Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545–52.
Gearhart JG, Duncan JL 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313–22.
Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–61.
Sugar Investigators NICE, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97.
Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181–6.
Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256–61.
• Christensen MB, Gotfredsen A, Norgaard K. Efficacy of basal-bolus insulin regimens in the inpatient management of non-critically ill patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2017;33(5). This meta-analysis concluded that basal-bolus insulin regimen results in lower mean glucose compared to sliding scale, but it's associated with increased risk of hypoglycemia.
Colunga-Lozano LE, Gonzalez Torres FJ, Delgado-Figueroa N, et al. Sliding scale insulin for non-critically ill hospitalised adults with diabetes mellitus. Cochrane Database Syst Rev. 2018;11:011296.
Umpierrez GE, Palacio A, Smiley D. Sliding scale insulin use: myth or insanity? Am J Med. 2007;120(7):563–7.
Perez Perez A, Conthe Gutierrez P, Aguilar Diosdado M, et al. Hospital management of hyperglycemia. Med Clin (Barc). 2009;132(12):465–75.
Sampson M, Jones C, Joint British Diabetes Societies for Inpatient C. Joint British Diabetes Societies for Inpatient Care clinical guidelines and improving inpatient diabetes care. Diabet Med. 2018;35(8):988–91.
Qaseem A, Humphrey LL, Chou R, Snow V, Shekelle P. Clinical Guidelines Committee of the American College of P Use of intensive insulin therapy for the management of glycemic control in hospitalized patients a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;154(4):260–7.
Jacobi J, Bircher N, Krinsley J, et al. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40(12):3251–76.
Haque WZ, Demidowich AP, Sidhaye A, Golden SH, Zilbermint M. The Financial impact of an inpatient diabetes management service. Curr Diab Rep. 2021;21(2):5.
Sampson MJ, Crowle T, Dhatariya K, et al. Trends in bed occupancy for inpatients with diabetes before and after the introduction of a diabetes inpatient specialist nurse service. Diabet Med. 2006;23(9):1008–15.
Rutter CL, Jones C, Dhatariya KK, et al. Determining in-patient diabetes treatment satisfaction in the UK–the DIPSat study. Diabet Med. 2013;30(6):731–8.
Mendez CE, Umpierrez GE. Management of type 1 diabetes in the hospital setting. Curr Diab Rep. 2017;17(10):98.
Galindo RJ, Umpierrez GE, Rushakoff RJ, et al. Continuous glucose monitors and automated insulin dosing systems in the hospital consensus guideline. J Diabetes Sci Technol. 2020;14(6):1035–64.
Grunberger G, Sherr J, Allende M, et al. American Association of Clinical Endocrinology Clinical Practice Guideline: the use of advanced technology in the management of persons with diabetes mellitus. Endocr Pract. 2021;27(6):505–37.
Boughton CK, Bally L, Martignoni F, et al. Fully closed-loop insulin delivery in inpatients receiving nutritional support: a two-centre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7(5):368–77.
Boughton CK, Daly A, Thabit H, et al. Day-to-day variability of insulin requirements in the inpatient setting: observations during fully closed-loop insulin delivery. Diabetes Obes Metab. 2021;23(8):1978–82.
Sultana G, Kapur P, Aqil M, Alam MS, Pillai KK. Drug utilization of oral hypoglycemic agents in a university teaching hospital in India. J Clin Pharm Ther. 2010;35(3):267–77.
Rajendran R, Kerry C, Rayman G, Ma GICsg. Temporal patterns of hypoglycaemia and burden of sulfonylurea-related hypoglycaemia in UK hospitals: a retrospective multicentre audit of hospitalised patients with diabetes. BMJ Open. 2014;4(7):e005165.
Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322(12):1155–66.
Pasquel FJ, Lansang MC, Dhatariya K, Umpierrez GE. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021;9(3):174–88.
George JT, Warriner D, McGrane DJ, et al. Lack of confidence among trainee doctors in the management of diabetes: the Trainees Own Perception of Delivery of Care (TOPDOC) Diabetes Study. QJM. 2011;104(9):761–6.
Horton WB, Law S, Darji M, et al. A multicenter study evaluating perceptions and knowledge of inpatient glycemic control among resident physicians: analyzing themes to inform and improve care. Endocr Pract. 2019;25(12):1295–303.
Pasquel FJ, Gomez-Huelgas R, Anzola I, et al. Predictive value of admission hemoglobin A1c on inpatient glycemic control and response to insulin therapy in medicine and surgery patients with type 2 diabetes. Diabetes Care. 2015;38(12):e202-203.
•• Pasquel FJ, Gianchandani R, Rubin DJ, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol. 2017;5(2):125–33. This multicentric randomized controlled trial validated the safety and efficacy of DPPIV inhibitors in non-critically ill hospitalized patients, admitted to medical and surgical wards, with mild-moderate hyperglycemia.
•• Fayfman M, Galindo RJ, Rubin DJ, et al. A randomized controlled trial on the safety and efficacy of exenatide therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes. Diabetes Care. 2019;42(3):450–6. This multicentric randomized controlled trial demostrated the safety and efficacy of short-acting GLP1 agonists in combination with basal insulin therapy in non-critically ill hospitalized patients with mild-moderate hyperglycemia.
•• Vellanki P, Rasouli N, Baldwin D, et al. Glycaemic efficacy and safety of linagliptin compared to a basal-bolus insulin regimen in patients with type 2 diabetes undergoing non-cardiac surgery: a multicentre randomized clinical trial. Diabetes Obes Metab. 2019;21(4):837–43. This multicentric randomized controlled trial validated the safety and efficacy of DPPIV inhibitors in non-critically ill hospitalized patients, admitted to surgical wards for non-cardiac surgery, with mild-moderate hyperglycemia.
Perez-Belmonte LM, Osuna-Sanchez J, Millan-Gomez M, et al. Glycaemic efficacy and safety of linagliptin for the management of non-cardiac surgery patients with type 2 diabetes in a real-world setting: Lina-Surg study. Ann Med. 2019;51(3–4):252–61.
Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care. 2013;36(11):3430–5.
Lorenzo-Gonzalez C, Atienza-Sanchez E, Reyes-Umpierrez D, et al. Safety and efficacy of Ddp4-inhibitors for management of hospitalized general medicine and surgery patients with type 2 diabetes. Endocr Pract. 2020.
Nauck MA, Meier JJ. Sitagliptin plus basal insulin: simplifying in-hospital diabetes treatment? Lancet Diabetes Endocrinol. 2017;5(2):83–5.
Garg R, Schuman B, Hurwitz S, Metzger C, Bhandari S. Safety and efficacy of saxagliptin for glycemic control in non-critically ill hospitalized patients. BMJ Open Diabetes Res Care. 2017;5(1):e000394.
Hulst AH, Plummer MP, Hollmann MW, et al. Systematic review of incretin therapy during peri-operative and intensive care. Crit Care. 2018;22(1):299.
Besch G, Perrotti A, Mauny F, et al. Clinical effectiveness of intravenous exenatide infusion in perioperative glycemic control after coronary artery bypass graft surgery: a phase II/III randomized trial. Anesthesiology. 2017;127(5):775–87.
Lips M, Mraz M, Klouckova J, et al. Effect of continuous exenatide infusion on cardiac function and peri-operative glucose control in patients undergoing cardiac surgery: a single-blind, randomized controlled trial. Diabetes Obes Metab. 2017;19(12):1818–22.
Kohl BA, Hammond MS, Cucchiara AJ, Ochroch EA. Intravenous GLP-1 (7–36) amide for prevention of hyperglycemia during cardiac surgery: a randomized, double-blind, placebo-controlled study. J Cardiothorac Vasc Anesth. 2014;28(3):618–25.
Abuannadi M, Kosiborod M, Riggs L, et al. Management of hyperglycemia with the administration of intravenous exenatide to patients in the cardiac intensive care unit. Endocr Pract. 2013;19(1):81–90.
Polderman JAW, van Steen SCJ, Thiel B, et al. Peri-operative management of patients with type-2 diabetes mellitus undergoing non-cardiac surgery using liraglutide, glucose-insulin-potassium infusion or intravenous insulin bolus regimens: a randomised controlled trial. Anaesthesia. 2018;73(3):332–9.
Mustafa OG, Whyte MB. The use of GLP-1 receptor agonists in hospitalised patients: an untapped potential. Diabetes Metab Res Rev. 2019;35(8):e3191.
Gerards MC, Venema GE, Patberg KW, et al. Dapagliflozin for prednisone-induced hyperglycaemia in acute exacerbation of chronic obstructive pulmonary disease. Diabetes Obes Metab. 2018;20(5):1306–10.
Damman K, Beusekamp JC, Boorsma EM, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020;22(4):713–22.
Correction to: Effect of empagliflozin as an add-on therapy on decongestion and renal function in patients with diabetes hospitalized for acute decompensated heart failure: a prospective randomized controlled study. Circ Heart Fail. 2021;14(4):e000067.
Huang W, Whitelaw J, Kishore K, et al. The comparative epidemiology and outcomes of hospitalized patients treated with SGLT2 or DPP4 inhibitors. J Diabetes Complications. 2021:108052.
Thiruvenkatarajan V, Meyer EJ, Nanjappa N, Van Wijk RM, Jesudason D. Perioperative diabetic ketoacidosis associated with sodium-glucose co-transporter-2 inhibitors: a systematic review. Br J Anaesth. 2019;123(1):27–36.
Koufakis T, Mustafa OG, Ajjan RA, et al. The use of sodium-glucose co-transporter 2 inhibitors in the inpatient setting: Is the risk worth taking? J Clin Pharm Ther. 2020;45(5):883–91.
Danne T, Garg S, Peters AL, et al. International Consensus on Risk Management of Diabetic Ketoacidosis in Patients With Type 1 Diabetes Treated With Sodium-Glucose Cotransporter (SGLT) Inhibitors. Diabetes Care. 2019;42(6):1147–54.
Satpathy SV, Datta S, Upreti B. Utilization study of antidiabetic agents in a teaching hospital of Sikkim and adherence to current standard treatment guidelines. J Pharm Bioallied Sci. 2016;8(3):223–8.
Khalam A, Dilip C, Shinu C. Drug use evaluation of diabetes mellitus in hospitalized patients of a tertiary care referral hospital. J Basic Clin Physiol Pharmacol. 2012;23(4):173–7.
van der Molen AJ, Reimer P, Dekkers IA, et al. Post-contrast acute kidney injury Part 2 risk stratification role of hydration and other prophylactic measures patients taking metformin and chronic dialysis patients Recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2018;28(7):2856–69.
Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315.
Yu Q, Zhu JJ, Liu WX. Effect of continuous use of metformin on kidney function in diabetes patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. BMC Cardiovasc Disord. 2020;20(1):187.
Zeller M, Labalette-Bart M, Juliard JM, et al. Metformin and contrast-induced acute kidney injury in diabetic patients treated with primary percutaneous coronary intervention for ST segment elevation myocardial infarction: Amulticenter study. Int J Cardiol. 2016;220:137–42.
Koufakis T, Mustafa OG, Zebekakis P, Kotsa K. Oral antidiabetes agents for the management of inpatient hyperglycaemia: so far, yet so close. Diabet Med. 2020;37(9):1418–26.
Schopman JE, Simon AC, Hoefnagel SJ, Hoekstra JB, Scholten RJ, Holleman F. The incidence of mild and severe hypoglycaemia in patients with type 2 diabetes mellitus treated with sulfonylureas: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2014;30(1):11–22.
Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–99.
Azoulay L, Suissa S. Sulfonylureas and the risks of cardiovascular events and death: a methodological meta-regression analysis of the observational studies. Diabetes Care. 2017;40(5):706–14.
Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15(10):938–53.
Rao AD, Kuhadiya N, Reynolds K, Fonseca VA. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality?: a meta-analysis of observational studies. Diabetes Care. 2008;31(8):1672–8.
Deusenberry CM, Coley KC, Korytkowski MT, Donihi AC. Hypoglycemia in hospitalized patients treated with sulfonylureas. Pharmacotherapy. 2012;32(7):613–7.
Mathioudakis NN, Abusamaan MS, Shakarchi AF, et al. Development and validation of a machine learning model to predict near-term risk of iatrogenic hypoglycemia in hospitalized patients. JAMA Netw Open. 2021;4(1):e2030913.
Kyi M, Gorelik A, Reid J, et al. Clinical prediction tool to identify adults with type 2 diabetes at risk for persistent adverse glycemia in hospital. Can J Diabetes. 2021;45(2):114-121e113.
Stuart K, Adderley NJ, Marshall T, et al. Predicting inpatient hypoglycaemia in hospitalized patients with diabetes: a retrospective analysis of 9584 admissions with diabetes. Diabet Med. 2017;34(10):1385–91.
Galindo RJ, Migdal AL, Davis GM, et al. Comparison of the freestyle Libre Pro Flash Continuous Glucose Monitoring (CGM) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care. 2020;43(11):2730–5.
Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–12.
Roberts A, James J, Dhatariya K. Joint British Diabetes Societies for Inpatient Care. Management of hyperglycaemia and steroid (glucocorticoid) therapy: a guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care group. Diabet Med. 2018;35(8):1011–7.
Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care. 2017;40(4):468–75.
Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a Basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169–74.
Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38.
Pasquel FJ, Umpierrez GE. Annals for hospitalists inpatient notes - how we treat hyperglycemia in the hospital. Ann Intern Med. 2021;174(8):HO2–4.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under Award Numbers P30DK111024 and K23DK123384 to R.J.G. G. E. U. is partly supported by NIH/NATS UL1 TR002378 from the Clinical and Translational Science Award program, and by 1P30DK111024-01 from the NIH/NIDDK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
R. J. G. received research support to Emory University for investigator-initiated studies from Novo Nordisk, Dexcom, and Eli Lilly and consulting fees from Abbott Diabetes Care, Sanofi, Valeritas, Eli Lilly, Novo Nordisk, and Weight Watchers, outside of this work. GEU has also received unrestricted research support for research studies (to Emory University) from Merck, Novo Nordisk, Dexcom Inc., and Sanofi. K. D. Has received speaker fees, travel or taken part in advisory boards for AstraZeneca, Sanofi Diabetes, Boehringer Ingelheim, Lilly or Novo Nordisk. F. G. P. has taken part in advisory panels for Sanofi and Novo Nordisk; has received research support from Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, and Lilly; and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, and Lilly.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US government.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hospital Management of Diabetes
Rights and permissions
About this article
Cite this article
Galindo, R.J., Dhatariya, K., Gomez-Peralta, F. et al. Safety and Efficacy of Inpatient Diabetes Management with Non-insulin Agents: an Overview of International Practices. Curr Diab Rep 22, 237–246 (2022). https://doi.org/10.1007/s11892-022-01464-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-022-01464-1