Abstract
Purpose of Review
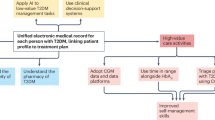
This article reviews recent clinical efficacy research and economic analysis of the use of personal continuous glucose monitoring (CGM) in type 2 diabetes (T2D).
Recent Findings
Studies from the past 5 years include a variety of randomized controlled trials, meta-analyses, and other studies which generally favor CGM over self-monitoring of blood glucose (SMBG) in T2D, especially among people with T2D treated with insulin. Concurrently, some studies show no significant difference, but there is no evidence of worse outcomes with CGM. CGM is frequently associated with greater reduction in HbA1c than is SMBG. HbA1c reductions tend to be greater when baseline HbA1c is higher. Reductions in hypoglycemia and hyperglycemia have also been demonstrated with CGM in people with T2D, as have comfort with, preference for, and psychosocial benefits of CGM compared to SMBG. There is a small but growing evidence base on the economics and cost-effectiveness of CGM in T2D.
Summary
CGM has been clearly demonstrated to have clinical benefits in people with T2D, especially among those treated with insulin. Economic and cost-effectiveness data are more scant but are generally favorable. CGM should be an important consideration in the management of T2D, and its use is likely to increase as efficacy data accumulate further and as costs associated with CGM gradually decrease.
Similar content being viewed by others
Introduction
The American Diabetes Association (ADA) significantly updated its thoroughly evidence-based Standards of Medical Care in Diabetes in 2021 around use of continuous glucose monitoring (CGM) [1••]. ADA had already noted that CGM could be useful with multiple daily injections (MDI) or continuous subcutaneous insulin infusion [2], but they added “other forms of insulin therapy,” which was absent previously. No longer excluding any particular insulin regimen, ADA noted that CGM can be helpful in reducing and/or maintaining HbA1c, reducing hypoglycemia, and reducing or even replacing self-monitoring of blood glucose (SMBG). Previous recommendations were restricted to those not achieving glycemic targets, with hypoglycemia unawareness, and/or experiencing hypoglycemia. With the updated standards, there is broader support even for people who are achieving glycemic targets to help them maintain this achievement. Recommendations from the American Association of Clinical Endocrinology and American College of Endocrinology are similar and even further-reaching, clearly stating their preferred recommendation of CGM over SMBG, and with one of their 11 principles of comprehensive management of T2D being that “CGM is highly recommended, as available, to assist patients in reaching goals safely” [3••]. They cite the clarity with which CGM can reveal glycemic patterns, more easily and more quickly than SMBG, noting also that this enables more rapid titration of therapy to achieve glycemic targets. They further note CGM’s utility in reducing the risk and frequency of hypoglycemia.
Despite growing evidence of CGM’s clinically efficacy among many people with T2D, CGM has seen limited expansion into clinical care. The goal of this article is to review research published within the past 5 years specific to personal CGM (where the patient can view their glucose data) among adults with T2D and to provide an overview of current knowledge of the economic impacts of CGM use in T2D.
Overview of Evidence in Type 2 Diabetes
This brief summary discusses evolution of the evidence, study types reviewed, outcomes measured, and findings. Detailed review of individual studies follows in the next section.
As CGM systems have evolved, data have accumulated about CGM use and associations with outcomes. Most earlier work focused on type 1 diabetes (T1D), and T2D-focused CGM research has emerged over the past few years. While some early work remains relevant, the technology has changed dramatically, with improvements in accuracy such that some may now be used for therapeutic and dosing decisions, rather than their prior status as purely adjunctive to SMBG. Because of this rapid evolution, we restrict this review to new evidence within the past 5 years, focusing on personal (where the patient can view their glucose data on their own device, any time they want) rather than professional CGM (where the glucose data are blinded to the patient and reviewed retrospectively by a diabetes care professional).
In most randomized controlled trials (RCTs) and meta-analyses, CGM use was evaluated on HbA1c change from baseline to after some period of CGM use. Most RCTs compared CGM to SMBG, usually employing blinded CGM readings among participants using SMBG to allow direct comparisons of glycemia. Most studies found greater HbA1c reductions with CGM than SMBG, though the magnitude varied among studies and populations. Some differences are likely due to varying average baseline HbA1c between studies; in general, greater HbA1c reductions were seen in studies with higher baseline HbA1c, with smaller reductions seen with lower baseline HbA1c. Some differences are also likely due to treatment regimen among participants. Most recent studies involved intensive insulin therapy (IIT) among participants with T2D, but the variety of diabetes treatment regimens has broadened, with more studies now including people using basal-only or even non-insulin regimens. Not surprisingly, higher baseline HbA1c correlated with greater HbA1c reduction with CGM use. Some studies found no significant difference, but none found a greater HbA1c reduction with SMBG than with CGM. Various quality of life and treatment satisfaction measures also generally favored CGM over SMBG. When evaluated, time in range (TIR), time above range (TAR), and average sensor glucose correlated well with HbA1c and change in HbA1c, again generally favoring CGM over SMBG. Hypoglycemia results were mixed, with some studies finding less hypoglycemia with CGM use and some finding no difference. One meta-analysis looked specifically at the correlations of HbA1c with various CGM metrics, and therefore how CGM can be used to reflect not only HbA1c-like measures of glycemia, but also how CGM can be used to evaluate aspects of glycemia not reflected by HbA1c (notably, hypoglycemia). One study found more frequent hypoglycemia with CGM, though the method of measuring hypoglycemia with CGM was quite different than with SMBG. Another study found a difference in its primary outcome of change in treatment satisfaction, while others included such satisfaction and quality of life measures as secondary outcomes and were therefore not powered to find such differences. Nearly all studies that evaluated such domains, including treatment or monitoring satisfaction, ease of management, likelihood to recommend, and various quality of life measures, found CGM to be superior. On most clinical and behavioral/psychosocial outcomes, and with only rare exceptions, CGM has consistently been demonstrated to be as beneficial as or superior to SMBG. Likewise, CGM was usually found to be beneficial for TIR, TAR, and time below range (TBR). The studies reviewed include several RCTs, several meta-analyses, and several observational studies, which are reviewed in turn below and summarized in Table 1.
Recent Studies
Randomized Controlled Trials
Most studies found a greater decrease in HbA1c among participants using CGM compared to SMBG, with some exceptions where no difference was found. For example, the 24-week adjusted difference in HbA1c was − 0.4% (− 0.9% for CGM, − 0.5% for SMBG; p < 0.001) in a 2017 RCT of 116 adults aged 60 or older and using MDI (82 with T2D and 34 with T1D) randomized to real-time CGM (rtCGM) or SMBG [6]. There were also significant benefits in the CGM group at 24 weeks in mean glucose, TIR, and TAR. There were too few episodes of hypoglycemia to allow for meaningful comparisons, with both groups already experiencing fewer episodes of hypoglycemia than recommended as acceptable limits even at baseline.
Similar findings regarding HbA1c change were seen among participants with T2D treated with MDI, where adjusted mean HbA1c was changed 0.3% greater at 12 and 24 weeks with rtCGM than with SMBG (p = 0.005 and p = 0.02, respectively) [4•]. In this study, baseline HbA1c decreased from 8.5 to 7.5% with rtCGM and to 7.9% with SMBG at 12 weeks and to 7.7% with rtCGM and 8.0% with SMBG at 24 weeks among 158 adults with T2D treated with MDI randomized to rtCGM or four daily SMBG checks. Participants’ CGM usage was quite high, with 6.9 days of use per week in month 1 and sustained at 6.7 days/week in months 3 and 6. No meaningful differences were seen in secondary outcomes of measured hypoglycemia or quality of life.
A 2017 RCT found no difference in HbA1c change at 6 months for the whole study population of 224 adults with T2D treated with IIT, but among participants < 65 years old, participants randomized to intermittently scanned CGM (isCGM) experienced more substantial change in HbA1c (− 0.53%) than those randomized to SMBG (− 0.20%), p = 0.03 [5•]. There was also a significant decrease in hypoglycemia, for both time below 70 mg/dL and time below 55 mg/dL, which decreased by 43% and 53% more with CGM, respectively, compared to SMBG. This corresponds to 0.47 h per day less (or − 1.9% time below 70 mg/dL), which is noteworthy especially in light of the consensus target of < 4% time below 70 mg/dL. Likewise, time below 55 mg/dL decreased by 0.9%, again noteworthy with a consensus target of < 1% time below 55 mg/dL.
A 2021 RCT conducted among primary care centers demonstrated greater HbA1c reduction at 8 months (adjusted difference − 0.4%, p = 0.02) among 116 adults with T2D treated with basal insulin and randomized to CGM compared to 59 randomized to SMBG [8]. TIR was greater in the CGM group (59% vs. 43%, adjusted difference 15%, p < 0.001), time above 250 mg/dL was less (11% vs. 27%, adjusted difference − 16%, p < 0.001), and mean glucose was lower (179 mg/dL vs. 206 mg/dL, adjusted difference − 26 mg/dL, p < 0.001).
A shorter-term RCT also found greater HbA1c change at 10 weeks (− 0.53%, p < 0.0001) with isCGM than with SMBG among 101 adults with T2D treated with MDI [9]. This study also assessed treatment satisfaction, and participants expressed greater preference for CGM than SMBG regarding flexibility and likelihood to recommend. While no difference in measured hypoglycemia was found (perhaps because participants randomized to SMBG were not followed by blinded CGM, while CGM users had much greater opportunity for detection of hypoglycemia by the CGM), CGM users reported a greater decrease in perceived hypoglycemia.
In 2020, RCTs of CGM in T2D moved further beyond CGM alone as the intervention. For example, one study showed greater HbA1c reduction for a medication management intervention combining a lifestyle education intervention to minimize glycemic excursion with rtCGM, compared to conventional medication management [11]. There was 1.2% greater HbA1c reduction in the intervention group than the control group (− 1.3% vs. − 0.1%, p = 0.03) among 30 adults with T2D not treated with insulin and with HbA1c > 7.0%. Intervention participants also experienced significant benefits compared to controls in secondary measures including quality of life, diabetes empowerment, diabetes distress, and glucose monitoring satisfaction.
Another 2020 RCT specifically focused on hypoglycemia using isCGM or SMBG, with severe hypoglycemia as the primary outcome and any hypoglycemia as a secondary outcome [10]. It found no difference in severe hypoglycemia between the isCGM and SMBG groups but also had relatively low power to detect such a difference, with 30 participants—predominantly people with T1D, with less than 1/3 of participants with T2D—in each group and low baseline estimates of severe hypoglycemia events. However, for the secondary outcome of any reported hypoglycemia, the CGM group demonstrated twice as many events (205 per person-year) than the SMBG group (96 per person-year), p < 0.001. The authors offer potential explanations for this, including the lower accuracy of the isCGM system in lower blood glucose ranges, in which it has a tendency to measure falsely lower interstitial glucose concentration than simultaneous venous measurement. It is also notable that hypoglycemia was measured by self-reported symptoms, SMBG value, and/or CGM value. Therefore, another plausible explanation is that isCGM provides many more data points (96 per day with the system in the study) than SMBG (4 per day in this study) and therefore roughly 24-fold as many opportunities to detect hypoglycemia. Much asymptomatic hypoglycemia, which accounts for a significant proportion of all hypoglycemia [25,26,27], could therefore be captured by isCGM but missed by SMBG. This is supported by the lack of blinded CGM for any period of the follow-up in the SMBG group, whereas most studies comparing hypoglycemia between CGM and control groups use blinded CGM in the controls for some period to enable direct comparison during that time. Further, the analysis was conducted on the entire study population without sub-analysis by diabetes type, making it difficult to draw significant conclusions about this use of CGM for T2D specifically.
Finally, another 2020 RCT found that when stopping glucose monitoring by either CGM or SMBG after a 12-week period of monitoring, participants who had been using CGM sustained their 12-week HbA1c decrease at 24 weeks, while those who had been using SMBG did not [7•]. In this study, 100 adults with T2D not treated with insulin and not previously monitoring glucose via either CGM or SMBG were randomized to receive either isCGM or SMBG for 12 weeks, after which the devices were made unavailable to participants. Benefits extended beyond sustained HbA1c decreases; both groups experienced increases in TIR, but participants using CGM experienced 9.8% TIR more than those using SMBG, or 2.35 h per day, p < 0.001. CGM users’ mean TIR increased from 59.8%, below the consensus target for TIR of > 70%, to 78.0%. SMBG users’ TIR increased from 65.1 to 69.4%, a more modest rise not quite reaching target. There was no significant change in hypoglycemia, but baseline rates were very low, quite possibly because all participants were non-users of insulin and therefore at lower risk of hypoglycemia than other populations.
This group of RCTs illustrates the recent demonstration and replication of HbA1c benefits of using CGM in the treatment of T2D, as well as the movement toward investigating outcomes beyond HbA1c and interventions incorporating CGM without consisting solely of CGM.
Meta-analyses
Rather than relying only on RCTs, it is also instructive to review meta-analyses of other studies. Several recent meta-analyses have been reported, often involving overlapping groups of studies, including data pooled from some of the RCTs mentioned above. One meta-analysis pooled data from 7 RCTs and 3 cohort studies [12]. Among the studies’ 1384 patients using rtCGM or professional CGM (they did not separate these CGM types for analysis), they found a greater decrease in HbA1c with CGM compared to controls (mean difference − 0.20%, 95% confidence interval − 0.09 to − 0.31). Among 4902 patients using isCGM, they found no difference compared to controls. With newer meta-analyses conducted since then, and when restricted to RCTs only, the evidence becomes clearer. A meta-analysis of RCTs of isCGM involving 1023 adults with T1D and T2D found a mean HbA1c change of − 0.56% in the pooled CGM groups, with the HbA1c decrease occurring within the first 2 months and sustained at 12 months [15]. Sub-analysis showed no difference between T1D and T2D, concluding that there was significant and sustained HbA1c decrease among adults with T2D using isCGM. A meta-analysis of RCTs restricted to T2D concluded that CGM reduced HbA1c by 0.25% more than SMBG did (p = 0.01), with 4/5 included studies favoring CGM [14]. Another meta-analysis of RCTs of CGM restricted to T2D demonstrated a significantly greater change in HbA1c with any personal CGM (rtCGM or isCGM) than with SMBG (mean difference − 0.42%, p = 0.004) [13]. Sub-analysis by CGM type showed similar benefits for rtCGM compared to SMBG (mean difference − 0.45%, p < 0.001), with a non-significant trend of similar magnitude also favoring isCGM (− 0.43%, p = 0.13). The CGM groups also spent less time in hypoglycemia than the SMBG groups (− 0.35 h/day, p = 0.006), corresponding to a decrease in time below range (TBR) of 1.46%.
Other Studies
Beyond RCTs and meta-analyses, some recent pilot and observational studies bear review. A 2016 pilot studied 4 subjects with T2D using CGM in addition to a lifestyle education intervention designed to minimize postprandial glycemic excursion [17]. Participants’ baseline HbA1c was higher than a group in a prior pilot of the intervention without CGM, yet their HbA1c decrease was slightly greater, demonstrating that CGM might augment the effect of the lifestyle education program. HbA1c decreases of 1.33% by 2 months and 1.21% at 6 months (p < 0.001, p = 0.009, respectively) were seen in a 2016 cross-sectional study of isCGM use among 31 patients (25 with T2D, 6 with T1D) using MDI with deliberately higher mean baseline HbA1c (8.9%) [16]. All participants reported high satisfaction with and desire to continue using isCGM, and they reported that it was painless and easy to use. Physicians in the study found the data reporting (using the standardized ambulatory glucose profile report generated by the isCGM system) to be excellent and to enable better and easier glucose management. In Australia, isCGM for at least 2 weeks demonstrated reduction in HbA1c from a mean of 11.9 to 8.5% (a decrease of 3.4%) in a cross-sectional study involving chart review of 22 patients with T2D [18]. While this change is more pronounced than seen in most other studies, baseline HbA1c was also higher, and other studies have found that HbA1c decreases with CGM tend to be greater with higher baseline HbA1c. A 2019 cross-sectional European study demonstrated HbA1c decrease overall (− 0.9%, p < 0.0001, from 8.9 to 8.0%) and in each participating country (Austria − 0.9%, France − 0.8%, Germany − 0.9%; all p < 0.0001) by chart review of 363 adult patients (92 in Austria, 88 in France, 183 in Germany) with T2D treated with IIT for at least 1 year, on CGM for at least 3 months, and with baseline HbA1c of 8.0–12.0% [20]. A recent 2021 cross-sectional study involved 248 patients on IIT (182 with T1D and 66 with T2D) and new to rtCGM, assessing change in HbA1c and quality of life [23]. Those with T2D experienced a decrease in HbA1c of 1.4% (from 8.5 to 7.1%), p < 0.001. Likewise, 21% had HbA1c < 7.0% before CGM use, while 50.0% achieved HbA1c < 7.0% after CGM use (p < 0.001). Among the T2D cohort, quality of life, as measured by diabetes distress and hypoglycemia concerns, improved significantly. A large 2021 retrospective cohort study found a greater HbA1c decrease (adjusted difference − 0.40%, p < 0.001) among 3,806 insulin-treated patients who initiated rtCGM compared to 37,947 insulin-treated patients who did not initiate CGM [24]. The cohorts included 41,753 patients, of whom 36,080 (86.4%) had T2D and 5,673 (13.6%) had T1D. CGM initiators also experienced a decrease in hypoglycemia-related ED visits or hospital admissions from 5.1 to 3.0%, compared to an increase among noninitiators from 1.9 to 2.3% (net difference estimate − 2.7%, p = 0.001). 9.6% more of the CGM initiators than the noninitiators achieved HbA1c < 7% (p < 0.001), 13.1% more achieved HbA1c < 8% (p < 0.001), and 7.1% fewer achieved HbA1c > 9% (p < 0.001).
Beyond the more traditional face-to-face care delivery of most study environments and healthcare delivery environments, at least before the upturn in telehealth seen with COVID-19, there are emerging data about diabetes care delivery, including CGM training and support, via telehealth. Mean HbA1c reduction of 1.6% (p < 0.001) was seen in a 2020 report of a pilot study of 55 adults with T2D treated through a virtual diabetes specialty clinic, including with rtCGM [21]. Greater reductions were seen in participants with baseline HbA1c > 9.0% (− 2.4%, p < 0.001) than for those with baseline HbA1c of 8.0–9.0% (− 1.2%, p < 0.001). TIR increased significantly, from 65.4 to 75.5%. Time > 180 mg/dL and time > 250 mg/dL also decreased significantly. In a recent survey of 594 adults with T2D who were remotely initiated on rtCGM via telehealth, mean HbA1c change was − 0.6% (from 7.7% at baseline to 7.1% after mean follow-up of 10.2 months), p < 0.001 [22]. Respondents reported very high satisfaction (4.5/5), with 94.7% agreeing or strongly agreeing that they were comfortable with rtCGM insertion, 97.0% that rtCGM improved their understanding of the impact of foods, 95.7% that rtCGM use increased their knowledge, and 79.4% that rtCGM use helped improve their treatment even when not wearing a sensor.
Finally, beyond examining efficacy or quality of life around CGM, a 2019 study evaluated associations of CGM metrics with HbA1c [19]. The investigators found that HbA1c correlated strongly with mean glucose (r = 0.80), with TIR (r = 0.75), and with TAR (r = 0.72), but only moderately with time below 70 mg/dL (r = 0.39) and weakly with time below 55 mg/dL (r = 0.21). These findings are consistent with the sensitivity of HbA1c to reflect euglycemia and hyperglycemia and its insensitivity to reflect hypoglycemia.
Having reviewed clinical and psychosocial studies of the past several years, we turn now to consider the economics and value of CGM in T2D.
Health Economics and the Value of CGM in T2D
A health economics perspective on value of CGM in T2D considers both clinical benefits of CGM, as discussed above, and cost of CGM. Here we explore a socioecological view of CGM costs and value to patients, provider and healthcare systems, payers, and society overall. CGM-specific cost data for individuals with T2D in the USA is limited to descriptive data on CGM materials and equipment costs and classic cost effectiveness analysis.
Patient Perspective
Patient out-of-pocket costs for CGM include co-pays for materials and equipment (sensors, and in some cases, transmitters and receivers) and range from $2500 to $6000 annually [28]. As CGM is often not covered by health insurance for T2D, this cost can be largely borne by patients. However, Medicare recently expanded CGM coverage to include anyone using insulin pumps or multiple daily insulin injections [28]. Additionally, Medicaid covers CGM in a growing number of states [29]. CGM may be covered under durable medical equipment and/or pharmacy benefits, depending on the insurance. The value of CGM to patients may lie in less time and pain associated with checking blood glucose, managing medication (including insulin dosing), and lower rates of complications and disability, with improved quality of life and ability to work [30]. Further research on the patient perspective of the cost and value of CGM is needed. However, patient out-of-pocket cost may limit continual CGM use, in which case intermittent use may still be valuable, which has been demonstrated in two studies below.
Payer Perspective
Two studies have explored the cost-effectiveness of CGM from the payer perspective and have favored their cost-effectiveness. One study examined third-party payer data (N = 100, not on insulin) to project lifetime clinical and economic outcomes for CGM (Dexcom SEVEN, which required SMBG calibrations) versus SMBG, by comparing cohort experiences, outcomes, and costs between the rtCGM and SMBG cohorts in a study of an RCT [31]. Two scenarios were presented: scenario 1, 8 weeks of usage (4 periods of 2 weeks on/1 week off, over 12 weeks) in year 1, and scenario 2, a repeat of similar usage in years 1 and 2. In scenario 1, life expectancy (LE) and quality-adjusted life expectancy (QALE) from CGM were 0.10 and 0.07, indicating a 1.25 month LE gain with CGM compared to SMBG. Incremental cost of CGM was $653/patient over a lifetime ($66,094 vs. $65,441). Incremental cost-effectiveness ratios were $6293 per life year (LY) gained and $8898 per quality-adjusted life year (QALY) gained. Scenario 2 resulted in incremental LE and QALE of 0.14 and 0.10, translating to 1.69 months and 1.20 quality-adjusted life months. Incremental costs were estimated to be approximately double at $1312 per patient over a lifetime ($66,763 vs. $65,423). The incremental cost-effectiveness ratios were $9,319 per LY gained and $13,030 per QALY gained. These estimates favor CGM as being cost-effective for those with T2D not using prandial insulin. CGM costs related to more recent CGMs that do not require calibration or standalone receivers may elicit additional cost savings.
While this article focuses on personal rather than professional CGM, we include here a study of professional CGM because there are so few economic studies of CGM. Sierra et al. sought to understand how professional CGM affects clinical and financial outcomes, examining laboratory and third-party payer data among a large US claims and lab dataset for patients with T2D who received a professional CGM compared to SMBG only [32]. There was no difference demonstrated in growth of total annual costs for people who used professional CGM compared to those who did not. However, patients using professional CGM more than once per year had a − $3,376 difference in the growth of total healthcare costs (p = 0.05). Patients who used professional CGM while changing their diabetes treatment regimen also had a difference of − $3,327 in growth of total costs (p = 0.002). Those patients who used professional CGM were described as slightly younger with more comorbid conditions compared to controls. Though limited, these studies support the cost-effectiveness of personal and professional CGM from the payer perspective.
Provider and Health System Perspective
The value of CGM care to health systems and providers may lie in its impact on population health and quality metrics. With slow movement towards value-based payment models in the USA, there are enhanced provider and health system incentives to improve population-level quality metrics while reducing costs, resource utilization, and burden. Several quality metrics, e.g., Healthcare Effectiveness Data and Information Set (HEDIS) measures [33], which are used to establish reimbursement and bundled payment rates (i.e., per-member-per-month (PMPM) payments), relate to diabetes care processes and outcomes, such as achieving target HbA1c levels. Technologies like CGM may allow for more efficient use of resources while simultaneously improving diabetes quality metrics. With CGM demonstrated to lower HbA1c, one would expect CGM to improve population-level quality performance, but this hypothesis has not been explicitly tested. In their report on a virtual diabetes clinic providing CGM and diabetes to people with T2D, Bergenstal et al. presented their data not only by HbA1c change, but also by proportion before and after participation whose HbA1c was < 8%—directly in the context of this core HEDIS quality measure [22]. Insulin users satisfying this HEDIS measure increased from 46.0 to 65.3%, while noninsulin users increased from 78.6 to 93.1% at 6 months.
Healthcare utilization and total cost of care may also improve with use of CGM compared to SMBG, as suggested by Isaacson et al. [34]. In a parallel randomized multi-site prospective trial, primary care patients with diabetes (N = 99; 93 with T2D, 6 with T1D) using rtCGM significantly decreased total visits (CGM 5.6, SMBG 7.0; p = 0.009), emergency department encounters (CGM 0.2, SMBG 0.5; p = 0.018), and labs ordered (CGM 7.7, SMBG 11.0; p = 0.001). In a sub-analysis of 36 people (18 CGM, 18 SMBG), CGM users had all-cost average savings of $417 PMPM for non-Medicare and $426 PMPM for Medicare Advantage members over 6 months. Thus, there is some evidence that CGM use may simultaneously improve quality and reduce utilization and costs in T2D. From a provider and health system perspective, these studies demonstrate that CGM use, with and without insulin use in T2D, was cost-effective and clinically effective.
Societal Perspective
The cost to society of diagnosed diabetes is $327 billion, including $237 billion in direct healthcare expenditures and $90 billion in reduced and lost productivity [35]. Fonda et al. additionally examined CGM in relation to societal costs, though exact dollar amounts were not reported [31]. CGM use translated into reduced cumulative rates of diabetes complications and deaths in cardiovascular disease, ulcers and amputations, and renal disease. The one exception was stroke (death and event), which, paradoxically, is predicted to be higher for patients who use CGM due to longer-term survival, or the “survival paradox.” Long-term studies are needed to better understand how CGM use relates to diabetes complications.
Gaps and Future Research
Generally, there is limited cost-analysis research on CGM in T2D, likely due to lower use in this population due to limited access and insurance coverage and only recently emerging clinical evidence of benefit in T2D. We identified three main gaps in the literature, related to (1) the potential influence of CGM on healthcare workforce efficiency, especially in primary care; (2) study or analysis of CGM’s potential costs and benefits in achieving quality metrics, especially related to value-based payment models; and (3) lack of data on initial and downstream costs of healthcare utilization related to CGM use and its consequences. These are described in Table 2.
Another significant gap revolves around barriers to CGM use, some of which contribute to disparities in care and access. For example, most patients with T2D in the USA are managed by primary care, yet CGM remains more accessible to endocrinology practices. This also contributes to geographic disparities, given the concentration of specialists in less rural areas, balanced by the nearly universal availability of primary care clinicians across the USA [36]. There are significant efforts under way to increase awareness and use of CGM in primary care, which will help improve access to those not treated by endocrinology. Likewise, there are device language barriers, with only limited support and resources available in languages other than English. Device use can also be challenging for those with vision or hearing impairment. Insurance coverage remains a substantial barrier, with CGM among the services excluded from coverage by many states’ Medicaid programs, which introduces further disparities in access between commercially insured patients and patients with fewer resources. We hope to see these barriers and disparities reduced over the coming months and years.
Conclusions
With the well-established and still growing evidence base supporting personal CGM in T1D, more recently in T2D with IIT, and most recently in T2D with any insulin therapy, CGM recommendations in professional guidelines and standards have likewise expanded. It seems increasingly clear that CGM is clinically efficacious for those on insulin. It is not yet clear what the role of CGM will be for those not on insulin, but evidence is beginning to accumulate. As CGM products evolve and as clinical and economic evidence continues to amass, we will likely see more data, more indications, more recommendations, and more standards around the rapidly changing role of personal CGM in the management of diabetes.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• American Diabetes Association. Diabetes technology: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl 1):S85–99. https://doi.org/10.2337/dc21-S007. The most current (2021) ADA Standards regarding technology use in diabetes, including CGM, and thorough consideration of evidence.
American Diabetes Association. Diabetes technology: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S77–88. https://doi.org/10.2337/dc20-S007.
•• Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abramson MJ, Barzillay JI, Blonde L, Bush MA, DeFronzo RA, Garber JR, Garvey WT, Hirsch IB, Jellinger JS, McGill JB, Mechanick JI, Perreault L, Rosenblit PD, Samson S, Umpierrez GE. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26(1):107–39. https://doi.org/10.4158/CS-2019-0472. The most current (2020) AACE/ACE statement on type 2 diabetes management, including preferred use of CGM over SMBG and reasons for this preference.
• Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D, McGill JB, Polonsky W, Price D, Aronoff S, Aronson R. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–74. https://doi.org/10.7326/M16-2855. RCT demonstrating greater HbA1c decrease in rtCGM than SMBG, with additional benefits in mean glucose, TIR, and TAR.
• Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55–73. https://doi.org/10.1007/s13300-016-0223-6. RCT demonstrating substantial decreases in hypoglycemia in adults with T2D treated with IIT.
Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol. 2017;11(6):1138–46. https://doi.org/10.1177/1932296817704445.
• Wada E, Onoue T, Kobayashi T, Handa T, Hayase A, Ito M, Furukawa M, Okuji T, Okada N, Iwama S, Sugiyama M. Flash glucose monitoring helps achieve better glycemic control than conventional self-monitoring of blood glucose in non-insulin-treated type 2 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2020;8(1):e001115. https://doi.org/10.1136/bmjdrc-2019-001115. Among the first RCTs to evaluate CGM in adults with T2D who do not use insulin therapy, this demonstrated greater increase in TIR for CGM than for SMBG, with improvements later sustained for CGM but not for SMBG.
Martens T, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, Pop-Busui R, Philis-Tsimikas A, Bao S, Umpierrez G, Davis G, Kruger D, Bhargava A, Young L, McGill JB, Aleppo G, Nguyen QT, Orozco I, Biggs W, Lucas KJ, Polonsky WH, Buse JB, Price D, Begenstal RM. Effects of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized controlled trial. JAMA. 2021;325(22):2262–72. https://doi.org/10.1001/jama.2021.7444.
Yaron M, Roitman E, Aharon-Hananel G, Landau Z, Ganz T, Yanuv I, Rozenberg A, Karp M, Ish-Shalom M, Singer J, Wainstein J, Raz I. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019;42(7):1178–84. https://doi.org/10.2337/dc18-0166.
Davis TM, Dwyer P, England M, Fegan PG, Davis WA. Efficacy of intermittently scanned continuous glucose monitoring in the prevention of recurrent severe hypoglycemia. Diabetes Technol Ther. 2020;22(5):367–73. https://doi.org/10.1089/dia.2019.0331.
Cox DJ, Banton T, Moncrief M, Conaway M, Diamond A, McCall AL. Minimizing glucose excursions (GEM) with continuous glucose monitoring in type 2 diabetes: a randomized clinical trial. J Endocr Soc. 2020;4(11):bvaa118. https://doi.org/10.1210/jendso/bvaa118.
Park C, Le QA. The effectiveness of continuous glucose monitoring in patients with type 2 diabetes: a systematic review of literature and meta-analysis. Diabetes Technol Ther. 2018;20(9):613–21. https://doi.org/10.1089/dia.2018.0177.
Ida S, Kaneko R, Murata K. Utility of real-time and retrospective continuous glucose monitoring in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. J Diabetes Res. 2019;2019:4684815. https://doi.org/10.1155/2019/4684815.
Janapala RN, Jayaraj JS, Fathima N, Kashif T, Usman N, Dasari A, Jahan N, Sachmechi I. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11(9):e5634. https://doi.org/10.7759/cureus.5634.
Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta-analysis of clinical trials and real-world observational studies. Diabetes Ther. 2020;11(1):83–95. https://doi.org/10.1007/s13300-019-00720-0.
Ish-Shalom M, Wainstein J, Raz I, Mosenzon O. Improvement in glucose control in difficult-to-control patients with diabetes using a novel flash glucose monitoring device. J Diabetes Sci Technol. 2016;10(6):1412–3. https://doi.org/10.1177/1932296816653412.
Cox DJ, Taylor AG, Moncrief M, Diamond A, Yancy WS, Hegde S, McCall AL. Continuous glucose monitoring in the self-management of type 2 diabetes: a paradigm shift. Diabetes Care. 2016;39(5):e71–3. https://doi.org/10.2337/dc15-2836.
Weiss J, Cohen N, Zajac JD, Ekinci EI. Flash glucose monitoring—using technology to improve outcomes for patients with diabetes. Aust J Rural Health. 2018;26(6):453–4. https://doi.org/10.1111/ajr.12440.
Hirsch IB, Welsh JB, Calhoun P, Puhr S, Walker TC, Price DA. Associations between HbA1c and continuous glucose monitoring-derived glycaemic variables. Diabet Med. 2019;36(12):1637–42. https://doi.org/10.1111/dme.14065.
Kröger J, Fasching P, Hanaire H. Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther. 2020;11(1):279–91. https://doi.org/10.1007/s13300-019-00741-9.
Majithia AR, Kusiak CM, Lee AA, Colangelo FR, Romanelli RJ, Robertson S, Miller DP, Erani DM, Layne JE, Dixon RF, Zisser H. Glycemic outcomes in adults with type 2 diabetes participating in a continuous glucose monitor–driven virtual diabetes clinic: prospective trial. J Med Internet Res. 2020;22(8):e21778. https://doi.org/10.2196/21778.
Bergenstal RM, Layne JE, Zisser H, Gabbay RA, Barleen NA, Lee AA, Majithia AR, Parkin CG, Dixon RF. Remote application and use of real-time continuous glucose monitoring by adults with type 2 diabetes in a virtual diabetes clinic. Diabetes Technol Ther. 2021;23(2):128–32. https://doi.org/10.1089/dia.2020.0396.
Gilbert TR, Noar A, Blalock O, Polonsky WH. Change in hemoglobin A1c and quality of life with real-time continuous glucose monitoring use by people with insulin-treated diabetes in the landmark study. Diabetes Technol Ther. 2021;23(S1):S35–9. https://doi.org/10.1089/dia.2020.0666.
Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real-time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin-treated diabetes. JAMA. 2021;325(22):2273–84. https://doi.org/10.1001/jama.2021.6530.
Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, Shubrook JH. Hypoglycemia in type 2 diabetes – more common than you think: a continuous glucose monitoring study. J Diabetes Sci Technol. 2015;9(5):999–1005. https://doi.org/10.1177/1932296815581052.
Reddy M, Oliver N. Self-monitoring of blood glucose requirements with the use of intermittently scanned continuous glucose monitoring: a follow-up analysis using real-life data. Diabetes Technol Ther. 2020.https://doi.org/10.1089/dia.2020.0477.
Abdelhamid YA, Bernjak A, Phillips LK, Summers MJ, Weinel LM, Lange K, Chow E, Kar P, Horowitz M, Heller S, Deane AM. Nocturnal hypoglycemia in patients with diabetes discharged from ICUs: a prospective two-center cohort study. Crit Care Med. 2021;49(4):636–49. https://doi.org/10.1097/CCM.0000000000004810.
Robertson SL, Shaughnessy AF, Slawson DC. Continuous glucose monitoring in type 2 diabetes is not ready for widespread adoption. Am Fam Physician. 2020;101(11):646.
Anderson JE, Gavin JR, Kruger DF. Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Ther. 2020;22(3):169–73. https://doi.org/10.1089/dia.2019.0303.
Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, Beck R. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–40. https://doi.org/10.2337/dc17-1600.
Fonda SJ, Graham C, Munakata J, Powers JM, Price D, Vigersky RA. The cost-effectiveness of real-time continuous glucose monitoring (RT-CGM) in type 2 diabetes. J Diabetes Sci Technol. 2016;10(4):898–904. https://doi.org/10.1177/1932296816628547.
Sierra JA, Shah M, Gill MS, Flores Z, Chawla H, Kaufman FR, Vigersky R. Clinical and economic benefits of professional CGM among people with type 2 diabetes in the United States: analysis of claims and lab data. J Med Econ. 2018;21(3):225–30. https://doi.org/10.1080/13696998.2017.1390474.
National Committee on Quality Assurance. In: Health Insurance Exchange 2021 Quality Rating System Measure Technical Specifications. 2020. https://www.cms.gov/files/document/2021-qrs-measure-technical-specifications.pdf. Accessed 20 Mar 2021.
Isaacson B, Kaufusi S, Sorensen J, Joy E, Jones C, Ingram V, Mark N, Phillips M, Briesacher M. Demonstrating the clinical impact of continuous glucose monitoring within an integrated healthcare delivery system. J Diabetes Sci Technol. 2020:1932296820955228. https://doi.org/10.1177/1932296820955228.
American Diabetes Association. Economic costs of diabetes in the US in 2017. Diabetes Care. 2018;41(5):917–28. https://doi.org/10.2337/dci18-0007.
Oser SM, Oser TK. Diabetes technologies: we are all in this together. Clin Diabetes. 2020;38(2):188–9. https://doi.org/10.2337/cd19-0046.
Acknowledgements
The authors thank Jessica Parascando and Marilyn Stasinopoulos for their assistance with literature search.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review conception and design. Literature review and compilation for the clinical review were performed by Sean Oser and Tamara Oser. Literature review and compilation for the economic review were performed by Michelle Litchman, Bethany Kwan, and Nancy Allen. The first draft of the manuscript was written by Tamara Oser, Sean Oser, and Michelle Litchman, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors did not receive support from any organization for the submitted work. Financial interests: SO and TO have served on advisory boards for Cecelia Health, DiabetesWise.org, and Dexcom. LF has served as an advisor to Ascensia, Dexcom, and Eli Lilly. WP has received research funding from and served as an advisor to Abbott and Dexcom. NA and ML have received equipment grants from Dexcom to conduct unrelated studies. ML has received an investigator-initiated grant from Abbott to conduct an unrelated study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Economics and Policy in Diabetes
Rights and permissions
About this article
Cite this article
Oser, T.K., Litchman, M.L., Allen, N.A. et al. Personal Continuous Glucose Monitoring Use Among Adults with Type 2 Diabetes: Clinical Efficacy and Economic Impacts. Curr Diab Rep 21, 49 (2021). https://doi.org/10.1007/s11892-021-01408-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11892-021-01408-1