Abstract
Women with type 1 diabetes (T1DM) have unique needs during the preconception, pregnancy, and postpartum periods. Preconception counseling is essential for women with T1DM to minimize pregnancy risks. The goals of preconception care should be tight glycemic control with a hemoglobin A1c (A1C) < 7 % and as close to 6 % as possible, without significant hypoglycemia. This will lower risks of congenital malformations, preeclampsia, and perinatal mortality. The safety of medications should be assessed prior to conception. Optimal control of retinopathy, hypertension, and nephropathy should be achieved. During pregnancy, the goal A1C is near-normal at <6 %, without excessive hypoglycemia. There is no clear evidence that continuous subcutaneous insulin infusion (CSII) versus multiple daily injections (MDI) is superior in achieving the desired tight glycemic control of T1DM during pregnancy. Data regarding continuous glucose monitoring (CGM) in pregnant women with T1DM is conflicting regarding improved glycemic control. However, a recent CGM study does provide some distinct patterns of glucose levels associated with large for gestational age infants. Frequent eye exams during pregnancy are essential due to risk of progression of retinopathy during pregnancy. Chronic hypertension treatment goals are systolic blood pressure 110–129 mmHg and diastolic blood pressure 65–79 mmHg. Labor and delivery target plasma glucose levels are 80–110 mg/dl, and an insulin drip is recommended to achieve these targets during active labor. Postpartum, insulin doses must be reduced and glucoses closely monitored in women with T1DM because of the enhanced insulin sensitivity after delivery. Breastfeeding is recommended and should be highly encouraged due to maternal benefits including increased insulin sensitivity and weight loss and infant and childhood benefits including reduced prevalence of overweight. In this article, we discuss the care of pregnant patients with T1DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes (T1DM) affects about 0.1–0.2 % of all pregnancies. Education, effective contraception, preconception planning, tight glycemic control, and comprehensive medical care can decrease maternal, fetal, and pregnancy risks associated with T1DM. Therefore, all women of childbearing age should be counseled about the increased pregnancy risks associated with T1DM to ensure that pregnancies are planned. This review article will discuss the current standards of care and latest research for T1DM and pregnancy in the preconception, pregnancy, and postpartum periods.
Preconception
Counseling
The goals of preconception care should be tight glycemic control with an A1C <7 % and as close to 6 % as possible without significant hypoglycemia. Since the hemoglobin A1C (A1C) at conception significantly affects pregnancy outcomes, pregnancy planning and preconception counseling regarding tight glycemic control are extremely important for women with T1DM [1]. Elevated blood glucose levels at conception and during the early first trimester are associated with increased rates of congenital malformations, most commonly cardiac and neural tube defects. Compared with the general population rate of 2 %, the prevalence of congenital malformations increases with increasing first trimester A1C (Fig. 1) [2]. Higher A1Cs early in pregnancy are also associated with higher prevalence of spontaneous abortions [3, 4], intrauterine fetal demise [5], preeclampsia [6•], preterm deliveries [7, 8], and perinatal mortality [1].
Preconception A1C vs absolute risk of congenital anomaly (with permission from American Diabetes Association: Guerin A, Nisenbaum R, Ray JG. Use of maternal GHb concentration to estimate the risk of congenital anomalies in the offspring of women with prepregnancy diabetes. Diabetes Care 2007;30:1920-5. Copyright and all rights reserved. Material from this publication has been used with the permission of American Diabetes Association) [2]
To improve pregnancy outcomes, preconception care should be comprehensive and include contraception planning, counseling about risks, and optimization of glycemic control, body mass index (BMI), and nutrition. Patients with T1DM who have planned pregnancies enjoy better outcomes, including reduced prevalence of congenital malformations [9], greater gestational age at delivery [10], lower A1C before and during pregnancy [9], lower cesarean delivery rates [11], and decreased perinatal mortality [9]. Tight glycemic control is the cornerstone of preconception care to improve outcomes in this patient population, and stopping contraception only after the goal A1C has been achieved is recommended for best outcomes [12].
Approximately 40–60 % of patients with pre-existing diabetes report that their pregnancies were not planned [13, 14]. Factors associated with planned pregnancies include higher income, higher education levels, private health insurance, endocrinology care prior to pregnancy, married, Caucasian, and encouragement from their physician [14]. Further, physicians often overlook preconception counseling [15]. We recommend ongoing education about contraception and preconception planning starting in the teen years. Contraceptive methods must fit the woman’s lifestyle and be used reliably. Methods include abstinence, barriers, progestin-only and estrogen-progestin pills, injections, implants, patches, vaginal rings, and intrauterine devices. We do not review the advantages and disadvantages of each method in this chapter but do emphasize that for the majority of women, the risks of an unplanned pregnancy greatly outweigh the risks of most forms of contraception. The exception to this is the preference for non-hormonal contraception for women with known increased clotting risk or prior history of venous thrombosis or patent foramen ovale. In general, the intrauterine device is an excellent choice for most women because it is highly effective and is associated with few side effects and does not increase clotting risk. Physicians should outline goals for medical clearance prior to conception and encourage patients to contact them immediately, if they suspect they are pregnant.
Nutrition
Medical nutrition therapy is needed to optimize glucose management with a focus on consistent timing and quality of healthy meals and snacks and accurate carbohydrate counting. Prenatal vitamins with folic acid reduce the risk of congenital malformations in infants of diabetic mothers [12, 16].
Diabetes control
Women with T1DM should have monthly visits until control is achieved. After that, medical appointments can be spaced out further. Basal-bolus regimens are beneficial to patients with T1DM [17]. Women with T1DM should begin a basal-bolus regimen preconception, if they are not already on one with either multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII) therapy with a goal of achieving target fasting and pre-meal blood glucose and reducing peak postprandial glucose [18]. MDI methods may use either a sliding scale protocol or alternatively match insulin to carbohydrate using a ratio (I:CHO). In pregnancy, superiority of CSII over MDI has not been demonstrated [19, 20]. However, most studies are observational and not randomized controlled trials (RCTs). Optimal A1C levels and timing and targets for self-monitoring of blood glucose (SMBG) are debated. An A1C < 7 % and as close to 6 %, as possible without causing significant hypoglycemia, is recommended, with fasting targets of 80–110 mg/dl and 1-h postmeal targets of 100–155 mg/dl suggested [18, 21].
Insulin generally does not cross the placenta and is thought to be safe in pregnancy. However, the insulin analog glargine has increased affinity for the IGF-1 receptor [22], which plays an important role in fetal development. A recent meta-analysis of studies comparing safety of insulin glargine to NPH insulin concluded that there was no evidence of adverse outcomes related to glargine use in pregnancy [23]. However, long-term risks of glargine in pregnancy are not known, and the FDA has designated glargine as class C. Detemir, another long acting insulin analog, was found to be non-inferior to NPH in an RCT [24, 25•] and is FDA class B in pregnancy. NPH and detemir provide safe and effective basal insulin coverage in pregnancy. For short acting insulin, both aspart and lispro are considered safe in pregnancy [26]. Newer insulin analogs are not well studied.
Blood Pressure
Preconception blood pressure (BP) goals are now systolic BP < 140 mmHg and diastolic BP < 90 mmHg [27•]. There is strong evidence that ACE-Is and ARBs cause oligohydramnios and renal insufficiency in infants exposed during the second and third trimesters [28]. However, there is some debate about their safety in the first trimester. A study in 2006 suggested that ACE-Is may increase the risk of cardiac and central nervous system malformations in infants exposed in the 1st trimester [29], although recent additional studies on ACE-I and/or ARB exposure during the first trimester of pregnancy have suggested that they are not teratogenic [30–32]. As a result, ACE inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) may be continued while tight glycemic control is being achieved. The decision to stop or continue them once contraception has been stopped and prior to a positive pregnancy test depends on individual benefits. As such, women with proteinuria, in whom there may be a delay in becoming pregnant, might derive greater benefit from the renal protective effects by continuing them until there is a positive pregnancy test. In most cases, ACE-Is and ARBs should be stopped when clearance to conceive is given. During preconception, BP control can be achieved with methyldopa, calcium channel blockers [33], or the beta blocker labetalol [34•]. Atenolol has been associated with intrauterine growth retardation (IUGR) and is not recommended [35].
Nephropathy
Kidney disease increases the risk of hypertension, preterm delivery, preeclampsia, IUGR, neonatal jaundice, and mechanical ventilation of the infant. The severity of renal disease is predictive of adverse outcomes, with increasing proteinuria and creatinine inversely associated with gestational age and birth weight [36]. Chronic kidney disease is related to a high risk of both maternal renal failure and perinatal mortality. In women with elevated serum creatinine 1.4–2.7 mg/dl, 43 % had persistent loss of renal function at 6 months postpartum, 59 % of these pregnancies were complicated by preterm delivery, and 37 % had IUGR [37]. Pregnancy does not seem to worsen maternal renal outcomes in women without chronic kidney disease [38]. Preconception assessment should include blood pressure (BP) measurements, serum creatinine, and urine microalbumin.
Retinopathy
Pregnancy may worsen retinopathy and macular edema [39]. The DCCT showed that diabetic retinopathy was more likely to progress during pregnancy [40]. Risk factors for retinopathy progression during pregnancy include poor glycemic control prior to pregnancy [39–42], rapid improvement of glycemic control [40, 41], previous diabetic retinopathy severity [41], diabetes duration [42], and hypertension [39, 42]. Preconception care should include a dilated retinal exam performed by a retina specialist. Proliferative diabetic retinopathy (PDR) should be treated and quiescent prior to conception.
Obesity
Obesity is becoming more common in the T1DM patient and is associated with increased risk of intrauterine fetal demise [43], preeclampsia [43, 44], perinatal mortality, and preterm delivery [43]. Therefore, it is imperative to address obesity with lifestyle interventions. Bariatric surgery has not been studied in patients with T1DM who become pregnant. In obese women without diabetes, it has been associated with an increased prevalence of small for gestational age and shorter gestation [45•]. In non-pregnant women with T1DM, gastric bypass reduces weight and BMI at 1 and 5 years, but there is no improvement in A1C levels [46•]. We do not recommend gastric bypass in women with T1DM at this time.
Thyroid
Autoimmune thyroid disease is common in women with T1DM [47]. Even those patients without known thyroid disease should have a TSH level checked preconception.
Summary
See preconception checklist (Table 1).
Pregnancy
Pregnant patients with T1DM are complex and require a team approach to their care [48, 49], which should include a diabetologist, obstetrician (perinatal specialist), neonatologist, certified diabetes educator, dietitian, and the patient’s partner. Patients with T1DM have insufficient insulin, causing higher maternal glucose levels. The excess glucose from the maternal circulation results in elevated glucose levels in the fetal circulation, which in turn causes excess fetal adipose growth and increases the risk for large for gestational age (LGA) or macrosomia in infants, especially when the mother is obese [50]. In addition, excessive fuel to the fetus results in increased risk of obesity [51] and impaired glucose tolerance [52, 53] in children of diabetic mothers.
CSII, MDI, and CGM
Tight glycemic control should be continued throughout the entire pregnancy with an A1C goal <6 % to reduce the occurrence of maternal, fetal, and neonatal complications [18, 54•]. The goal of insulin therapy with either MDI or CSII is to achieve fasting, preprandial and postprandial, and overnight glucose levels in the target range without hypoglycemia. CSII necessitates commitment to frequent glucose monitoring, carbohydrate counting, and rapid implementation of sick day rules in the setting of pump malfunction or illness. Pregnant women with T1DM may benefit from real-time continuous glucose monitoring (CGM), but data on this subject is limited. An RCT did not show that intermittent use of CGM in pregnant women with pre-gestational diabetes improved glycemic control or pregnancy outcomes compared to self-monitoring of plasma glucose multiple times daily [55•]. However, a recent CGM study does provide some distinct patterns of glucose levels associated with large for gestational age infants [56•].
Insulin Requirements
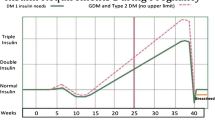
Insulin requirements vary throughout pregnancy, increasing in the first 9 weeks, decreasing weeks 9–16, and increasing until the 37th week [57]. In the final month of pregnancy, insulin requirements usually decrease [58]. Over the entire pregnancy, insulin requirements may double, and there is a shift toward more prandial insulin relative to basal insulin. [59]. We have observed that insulin delivered right at mealtimes may result in peak postprandial hyperglycemia due to mismatch of glucose absorption into the blood stream and timing of insulin delivery. We recommend giving the mealtime bolus 10–15 min prior to eating if the premeal glucose level is >70 mg/dl and the patient does not have symptoms of hypoglycemia.
Based on the ADA guidelines [18, 60], we recommend fasting glucose targets of 60–99 mg/dl, peak postprandial glucose targets of 100–129 mg/dl, and an A1C of <6 % during pregnancy, without significant hypoglycemia. During labor and delivery, we recommend glucose levels in the 80–110-ng/dl range [61] and use of the insulin drip with D10 [60] to achieve this goal.
Hypoglycemia
Severe hypoglycemia occurs in up to 50 % of pregnancies in women with T1DM [62], with most occurring in the first trimester [63] and at night [64]. It is most common in women with hypoglycemia unawareness [63]. Hypoglycemia may occur in patients who reactively bolus with correction insulin for an elevated 1 h postprandial blood glucose (bolus stacking) instead of adjusting mealtime bolus insulin (personal obs). Hypoglycemia does not increase the risk of birth defects or fetal death [64], and it does not affect fetal heart fate, breathing, body movement, umbilical artery Doppler wave forms [65], or neonatal intelligence [66]. However, a severe hypoglycemic event could result in a catastrophic event, such as a motor vehicle accident, which could have detrimental effects on the mother [13] and fetus [67]. Pregnant women with T1DM should test glucose levels premeals and postmeals, before and after physical activity, and occasionally in the middle of the night and before driving to monitor for hypoglycemia. CGMs have low glucose alarms which may alert patients of hypoglycemia before the onset of symptoms, especially in those individuals with hypoglycemia unawareness.
Hyperglycemia
DKA occurs at lower glucose levels because pregnancy is a ketosis-prone state [68, 69]. Factors that may predispose to DKA include infection, insulin omission, pump infusion problems, and use of medications such as terbutaline (to prevent preterm labor) or glucocorticoids (to induce fetal lung maturity) [70–72]. Most are avoidable if close attention is paid to glucose monitoring and sick day rules. Hyperglycemia should be treated promptly with insulin injection by syringe and changing the infusion set and if necessary, presentation to triage for hydration and intravenous insulin and correction of electrolyte abnormalities. For anticipated hyperglycemia when betamethasone is given for fetal lung maturation, Mathiesen et al. describe a useful algorithm for insulin adjustment—also see below “preterm labor” [73]. Neonatal macrosomia, hypoglycemia, and respiratory distress can be decreased when average antepartum glucose levels are <110 mg/dl [61]. Intrapartum glucose levels affect the risk of neonatal hypoglycemia more than antepartum glucose levels; neonatal hypoglycemia risks are lowest when the intrapartum glucose levels are <100 mg/dl [74]. Increased stillbirth rates occur in poorly controlled T1DM [75].
HTN
For patients who have chronic hypertension on antihypertensives, target blood pressure is systolic blood pressure 110–129 mmHg and diastolic blood pressure 65–79 mmHg [18, 54•, 60]. Goals of therapy for pregnant women with T1DM are derived from data on non-pregnant women with diabetes and pregnant women without diabetes. Hypertension management should ideally start prior to conception, but for women that present for care already pregnant, antihypertensive medications that are safe in pregnancy (described in preconception care) should replace those that are associated with adverse side effects. An RCT of pregnant hypertensive women (not stated as having diabetes) comparing tight (target BP < 140/90 mmHg) vs very tight (target BP < 130/80 mmHg) blood pressure control showed less severe hypertension, fewer hospitalizations, more advanced gestational age, without a difference in preterm delivery or difference in birth weight in the very tight control group [76]. It is important to avoid targeting blood pressure significantly below the mean because of possible increased risk of fetal growth restriction and mortality [77]. Antihypertensives should be initiated to treat chronic hypertension, but not preeclampsia or gestational hypertension [78]. A Cochrane study reviewing RCTs of antihypertensive therapy in pregnancy and a recent RCT demonstrated a halving of the risk of developing severe hypertension with antihypertensive treatment but no improvement in other outcomes [79•, 80•]. Methyldopa appears to be non-inferior to labetalol in preventing adverse outcomes [34•]. Diuretics [81], the beta blocker atenolol and clonidine [35, 82], are generally not used in pregnancy. Fetopathy associated with ACE-Is and ARBs in the second and third trimesters is well established. Severe hypertension may be treated with nifedipine [83•].
Nephropathy
In uncomplicated T1DM, urinary albumin excretion (UAE) rates are mildly elevated but return to normal by 6 weeks postpartum [84]. Glomelular filtration rate (GFR) increases by 50 % during pregnancy [85]. Patients with diabetic kidney disease may have a marked increase in UAE with nephrotic range proteinuria in some, but in most patients, UAE rates return to baseline levels after delivery [86, 87]. Conversely, patients with diabetic nephropathy with renal insufficiency are at significant risk of GFR decline [88] and renal failure during and after pregnancy (60, see Table 11.19). A retrospective cohort study found increased risk of preterm delivery at 32 weeks in patients with diabetic kidney disease and suboptimal blood pressure control using BP > 130/80 vs <130/80 mmHg (38.1 vs 4.6 %) [84]. Preeclampsia may be difficult to distinguish from worsening diabetic kidney disease and hypertension [78].
Retinopathy
Diabetic retinopathy (DR) is classified in the same manner in pregnancy as in the non-pregnant population. Pregnancy may cause a temporary worsening of retinopathy [39, 40]. The exact mechanism is uncertain [42, 89]. Known risk factors for progression of retinopathy during pregnancy [60, 90] include diabetes duration, elevated A1C at conception, rapid normalization of glycemic control [41], retinal status at conception (60, see Table 11.22), hypertension, nephropathy, and preeclampsia. Screening eye exams are recommended every trimester during pregnancy, and more frequently if there is significant baseline retinopathy or macular edema. Laser therapy is the treatment of choice based on Diabetic Retinopathy Study [91] and the Early Treatment Diabetic Retinopathy Study [92]. Data on anti-vascular endothelial growth factors (VEGF) exposure in pregnancy is limited to case reports. Theoretically, anti-VEGF mechanisms may be involved in preeclampsia [93] and therefore could negatively impact pregnancy.
Neuropathy
Diabetes is associated with hyperemesis [94]. Preexisting gastroparesis may worsen during pregnancy, causing severe hyperemesis gravidarum [95]. In a recent RCT of pregnant non-diabetic subjects, ondansetron and metoclopramide both had similar anti-nausea and anti-emetic effects, but ondansetron had fewer side effects and reduced ketonuria but higher cost [96•]. However, a recent review cautions regarding possible increased risk of cardiac congenital malformations with using ondansetron in the first trimester of pregnancy [97•]. Carpal tunnel syndrome is more common in pregnant patients with diabetes [98•]; wrist splints are a non-invasive and safe treatment. There is little data on painful peripheral neuropathy during pregnancy in diabetes, and more data is needed on this subject.
CV Disease
Only case reports of cardiovascular disease (CVD) in pregnant women with T1DM exist. Age and renal function determine the incidence of CVD in non-pregnant women with T1DM [99•]. Pregnancy may exacerbate pre-existing cardiac disease because it increases cardiac output, heart rate, and hypercoagulability. If a woman has atypical symptoms, ECG, transthoracic echocardiography, or exercise echocardiography should be considered [100]. It may be difficult to discern normal pregnancy symptoms such as dyspnea and fatigue from anginal symptoms. To optimize risk factors, good glycemic control [101], smoking cessation, blood pressure control (targets as above), and lipid management are essential. Dietary modifications to reduce trans and saturated fats may decrease LDL cholesterol. Aspirin therapy is not routinely used for primary prevention in patients with diabetes [102]. The management of myocardial infarction in pregnancy is similar to the non-pregnant state except that ACE-I and ARBs and statins are not used [103, 104].
Hyperlipidemia
Patients with uncomplicated T1DM who are not pregnant have similar lipid profiles to patients without diabetes [105, 106]. Conversely, patients with T1DM complicated by albuminuria or retinopathy have elevated LDL cholesterol and triglyceride levels compared to patients with uncomplicated T1DM [107]. In pregnant patients, total cholesterol and HDL increase by 50 %, while triglycerides may double; HDL cholesterol increases mid-pregnancy and returns to baseline at the end of pregnancy [108]. Pregnant women with T1DM have similar lipid changes during pregnancy, except that there is less of an increase in HDL mid-gestation [109]. A baseline lipid panel should be obtained at the initial visit if a preconception one is not available [18]. This may help to recognize patients with higher risk of hypertriglyceridemia (those with poorly controlled DM and albuminuria) [107]. Nutrition counseling should be provided on reducing intake of saturated fats to <7 % of caloric intake, elimination of trans fats from the diet, and initiation of exercise walking program if there is no contraindication. In patients with severe hypertriglyceridemia (>1000 mg/dl), omega 3 fatty acids may prevent pancreatitis, although this is rare in the patient with T1DM.
Thyroid Hypothyroid
Women with Hashimoto’s should have a TSH level every 4–6 weeks during pregnancy. Goal TSH concentrations in pregnancy are 0.1–2.5 μU/ml in the first trimester, 0.2–3 μU/ml in the second trimester, and 0.3–3 μU/ml in the third trimester [110, 111]. The target reference range for total T4 in pregnancy is 1.5 times the normal total T4 due to estrogen-mediated rise in thyroxine-binding globulin in pregnancy [112]. Thyroid hormone replacement requirements rapidly go up between 6 and 16 weeks of gestation, and then plateau after 20 weeks. Usually by 10 weeks, the thyroid hormone dose increases by 30 % compared to baseline; by 20 weeks, the dose is usually increased by 50 % compared to baseline and usually remains stable after [113, 114]. A recommended up titration strategy is to have the patient with hypothyroidism take an extra two pills per week (i.e., nine tabs instead of seven) once they find out they are pregnant until the first prenatal visit [113]. After delivery, thyroid hormone doses usually return to preconception doses.
Hyperthyroidism
Hyperthyroidism is much less common than hypothyroidism, but it is more common in patients with T1DM than in the general population. Thyroid receptor antibodies (such as TSI or TBII) may help with establishing the diagnosis of Graves’ disease as well as monitoring the disease activity of Graves’ disease who have been previously treated with radioactive iodine or surgery. After delivery, the infant must be monitored for hyperthyroidism with thyroid function tests and heart rate monitoring for tachycardia when the mother has a history of Graves’ disease, even if her disease has been treated. This is because a high thyroid receptor antibody titer in the mother during pregnancy can cause neonatal Graves’ disease. The American Thyroid Association and the American Association of Clinical Endocrinologists recommends using propylthiouracil in the first trimester and methimazole in the second and third trimesters of pregnancy [115]. Radioactive iodine is contraindicated in pregnancy, and thyroidectomy is recommended only in cases where antithyroidal drugs cannot be used.
LGA
In infants, LGA is defined as weight > 90%ile, while macrosomia is defined as weight > 4000 g at birth. An LGA incidence of 47 % has been noted [116]. The rates of reported macrosomia vary between 29 and 56 % [5, 117, 118]. There is conflicting literature regarding measures of glycemia that predispose to LGA and macrosomia in T1DM pregnancies. Risks associated with LGA/macrosomia include poor glycemic control (A1C > 7 %) in the periconception to 12-week gestation period [119], A1C > 6.5 % in the third trimester [63], elevated fasting [61], and/or preprandial and postprandial blood glucose [120•, 121, 122], elevated average daily blood glucose [123], and episodic elevated postprandial blood glucose despite normal A1C level [124]. Furthermore, increasing A1C > 6 % at 26- and 34-week gestation was associated with increasing prevalence of LGA [120•]. A recent study using CGM data found that women whose infants had LGA had glucose levels that were significantly lower midmorning and early evening in the first trimester, significantly higher in the early morning and throughout the afternoon in the second trimester, and significantly higher during the evening in the third trimester [56•]. We have found that even women who have an A1C < 6 % in the third trimester of pregnancy have LGA prevalence of 25 % (unpublished data, FM. Brown). Thus, it appears that even patients with outstanding diabetes control during pregnancy (A1C < 6 %) have a high risk of having an LGA baby. Other factors that could contribute to LGA may need to be considered to improve this outcome.
Preterm Delivery
Preterm delivery is birth before 37-week gestation; prevalence varies between 21 and 37 % in T1DM [125] compared to 5.1 % in controls. Risk factors for indicated preterm delivery include A1C > 7 %, worsening nephropathy, preeclampsia, and nulliparity [126]. Increasing levels of third trimester A1C > 6.5 % are associated with increasing prevalence of preterm delivery [120•]. Treatment with antenatal steroids is associated with a decrease in neonatal morbidity and mortality [127]. A recommended algorithm for insulin dosing to control glucose levels after betamethasone 12 mg IM and repeated at 24 h is as follows: increase from baseline total insulin dose of 27, 45, 40, 31, and 11 %, respectively, on days 1–5 from start of steroid therapy [73]. Baby aspirin 81 mg daily is recommended from 12–36 weeks to help reduce risk of preeclampsia in patients with T1DM [128•].
Summary
See pregnancy checklist (Table 2).
Postpartum
Insulin Dosing
After delivery, there is a significant increase in insulin sensitivity; so, a reduction of the dose of insulin to approximately 50 % of the preconception dose is advised. Women who breastfeed will likely have lower basal insulin needs than women who do not breastfeed [129].
Breastfeeding
Breastfeeding is beneficial for mothers and infants, but women with T1DM may find breastfeeding particularly beneficial due to increased insulin sensitivity [129], weight loss [130], potentially improved sleep if exclusively breastfeeding [131], and improved maternal-fetal bonding. It is unclear if exposure to complex cow’s milk proteins increases the risk of T1DM in susceptible individuals. An intervention trial evaluating a hydrolyzed infant formula to investigate the risk of beta-cell autoimmunity compared with standard formula in women who have discontinued breastfeeding is underway. Early results have not demonstrated a benefit. However, the endpoint will not be reached until 2017 [132•]. Other infant and childhood benefits include reduced prevalence of overweight [133]. Unfortunately, breastfeeding rates in women with T1DM may be lower than in the general population, likely due to both maternal and infant complications [134]. These may include cesarean delivery or a stay in the neonatal intensive care unit resulting in separation of the mother and infant [5, 135], biomechanical issues such as difficulty latching (more common in infants of mothers with T1DM) [136] or delayed lactogenesis [137] which is more common in women with T1DM, or increased episodes of hypoglycemia [129] in the mother. As a result, clinicians treating patients with T1DM in the postpartum period should assess the patient for potential barriers to breastfeeding and provide support to increase rates of initiation and duration of breastfeeding. Ensuring that mothers and infants are not separated when not medically necessary may improve breastfeeding initiation and duration, including early skin to skin contact [138] and night-time feedings [139].
Medications During Lactation
It is important to take into consideration if a medication has impact on milk production and infant and maternal risks.
Thyroid
Levothyroxine is FDA-approved for hypothyroid patients during breastfeeding. Antithyroidal medications are safe in breastfeeding in moderate doses (methimazole up to 20–30 mg per day and PTU < 300 mg per day). Methimazole is preferred during breastfeeding over PTU [140]. Infants whose mothers are taking antithyroid medications should have thyroid function testing. Mothers should take their antithyroid drugs immediately after each feeding to reduce infant exposure [111]. Women with T1DM have an increased prevalence of postpartum thyroiditis. Therefore, a TSH level at 3 and 6 months postpartum is recommended in euthyroid women with T1DM [110].
Lipids
The risk of lipid medications may outweigh the benefits during the short period of lactation. Therefore, these therapies are usually not recommended during breastfeeding. Because atherosclerosis is a chronic condition, stopping these medications for a brief period during lactation should not have a significant effect on long-term cardiovascular disease. The decision to use a lipid lowering agent should be made on a case-by-case basis.
Contraception
Postpartum contraception with no impact on lactation is preferred [141]. The lactation amenorrhea method or natural family planning with barrier protection is ideal since they do not affect lactation or glucose levels. Intrauterine devices are extremely effective and do not change metabolic status. Progestin-only hormonal contraceptives are a second choice for postpartum contraception and may be administered in pill, implant, or injection form. However, they may lower milk production, particularly if given in the first 6 weeks postpartum. Hormonal contraceptives with both estrogen and progestin usually lower milk production in a dose-dependent manner and are not recommended during lactation.
Postpartum Weight Retention
Postpartum weight retention is defined as the weight difference between the first year postpartum and the preconception weight; excessive postpartum weight retention is defined as weighing more than 5 kg at 1 year postpartum compared to preconception. Women with T1DM should be encouraged to return to their preconception weight to for optimal glucose control [142•].
Conclusions
Patients with T1DM who are of reproductive age should be informed about the increased risks associated with pregnancy. Early counseling, pregnancy planning, good glycemic control, and a multi-specialist approach to care before and during pregnancy can all improve pregnancy outcomes for mothers with T1DM and their infants. More research is needed in this patient population, especially in the areas of improving rates of preconception counseling and education, ways to predict and decrease preeclampsia risk, fertility data, treatment of diabetic nephropathy in pregnancy, prevention of infants with LGA and macrosomia, insulin dosing with relation to meal times during pregnancy, medication use during lactation in women with T1DM, and how to improve breastfeeding rates.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Jensen DM, Korsholm L, Ovesen P, Beck-Nielsen H, Moelsted-Pedersen L, Westergaard JG, et al. Peri-conceptional A1C and risk of serious adverse pregnancy outcome in 933 women with type 1 diabetes. Diabetes Care. 2009;32(6):1046–8.
Guerin A, Nisenbaum R, Ray JG. Use of maternal GHb concentration to estimate the risk of congenital anomalies in the offspring of women with prepregnancy diabetes. Diabetes Care. 2007;30:1920–5.
Galindo A, Burguillo AG, Azriel S, Fuente PL. Outcome of fetuses in women with pregestational diabetes mellitus. J Perinat Med. 2006;34:323–31.
Greene MF, HareJW CJP, Benacerraf BR, Soeldner JS. First-trimester hemoglobin A1 and risk for major malformation and spontaneous abortion in diabetic pregnancy. Teratology. 1989;39:225–31.
Evers IM, de Valk HW, Visser GH. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ. 2004;328:915.
Cohen AL, Wenger JB, James-Todd T, et al. The association of circulating angiogenic factors and HbA1c with the risk of preeclampsia in women with preexisting diabetes. Hypertens Pregnancy. 2014;33(1):81–92. Demonstrates strong association between 1st trimester A1C and risk of preeclampsia. No association was found with 3rd trimester A1C.
Jensen DM, Damm P, Moelsted-Pedersen L, et al. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care. 2004;27:2819–23.
Casson IF. Pregnancy in women with diabetes--after the CEMACH report, what now? Diabet Med. 2006;23:481–4.
Wahabi H, Alzeidan R, Bawazeer G, Alansari L, Esmaeil S. Preconception care for diabetic women for improving maternal and fetal outcomes: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2010;10:63.
Temple RC, Aldridge VJ, Murphy HR. Prepregnancy care and pregnancy outcomes in women with type 1 diabetes. Diabetes Care. 2006;29:1744–9.
Gunton JE, Morris J, Boyce S, Kelso I, McElduff A. Outcome of pregnancy complicated by pre-gestational diabetes – improvement in outcomes. Aust N Z J Obstet Gynaecol. 2002;42:478–81.
Pearson DW. The relationship between pre-pregnancy care and early pregnancy loss, major congenital anomaly or perinatal death in type I diabetes mellitus. BJOG: Int J Obstet Gynaecol. 2007;114:104–7.
Pregnancy outcomes in the diabetes control and complications trial. Am J Obstet Gynecol. 1996;174:1343-53.
Holing EV, Beyer CS, Brown ZA, Connell FA. Why don’t women with diabetes plan their pregnancies? Diabetes Care. 1998;21:889–95.
Varughese GI, Chowdhury SR, Warner DP, Barton DM. Preconception care of women attending adult general diabetes clinics--Are we doing enough? Diabetes Res Clin Pract. 2007;76:142–5.
Reader D, Thomas AM. Medical nutrition therapy: goals for pregnancy complicated by pre-existing diabetes mellitus. In: Kitzmiller JL, Jovanovic LB, Brown FM, Coustan DR, Reader D, editors. Managing preexisting diabetes and pregnancy: technical reviews and consensus recommendations. Alexandria: ADA; 2008.
Garg S, Ampudia-Blasco FJ, Pfohl M. Rapid-acting insulin analogues in Basal-bolus regimens in type 1 diabetes mellitus. Endocr Pract : Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2010;16:486–505.
Kitzmiller JL, Block JM, Brown FM, et al. Managing preexisting diabetes for pregnancy: summary of evidence and consensus recommendations for care. Diabetes Care. 2008;31:1060–79.
Mukhopadhyay A, Farrell T, Fraser RB, Ola B. Continuous subcutaneous insulin infusion vs intensive conventional insulin therapy in pregnant diabetic women: a systematic review and metaanalysis of randomized, controlled trials. Am J Obstet Gynecol. 2007;197:447–56.
Ranasinghe PD, Maruthur NM, Nicholson WK, et al. Comparative effectiveness of continuous subcutaneous insulin infusion using insulin analogs and multiple daily injections in pregnant women with diabetes mellitus: a systematic review and meta-analysis. J Womens Health (Larchmt). 2015;24(3):237–49.
Kitzmiller JL, Buchanan TA, Kjos S, Combs CA, Ratner RE. Pre-conception care of diabetes, congenital malformations, and spontaneous abortions. Diabetes Care. 1996;19:514–41.
Tennagels N, Werner U. The metabolic and mitogenic properties of basal insulin analogues. Arch Physiol Biochem. 2013;119(1):1–14.
Pollex E, Moretti ME, Koren G, Feig DS. Safety of insulin glargine use in pregnancy: a systematic review and meta-analysis. Ann Pharmacother. 2011;45:9–16.
Mathiesen ER, Hod M, Ivanisevic M, Duran Garcia S, Brøndsted L, Jovanovic L, et al. Maternal efficacy and safety outcomes in a randomized, controlled trial comparing insulin detemir with NPH insulin in 310 pregnant women with type 1 diabetes. Diabetes Care. 2012;35(10):2012–7.
Hod M, Mathiesen ER, Jovanovič L, McCance DR, Ivanisevic M, Durán-Garcia S, et al. A randomized trial comparing perinatal outcomes using insulin detemir or neutral protamine Hagedorn in type 1 diabetes. J Matern Fetal Neonatal Med. 2014;27(1):7–13. Detemir is non inferior to NPH in perinatal outcomes in pregnant women with type 1 diabetes.
de Valk HW, Visser GH. Insulin during pregnancy, labour and delivery. Best Pract Res Clin Obstet Gynaecol. 2011;25:65–76.
James PA, Oparil S, Carter BL, et al. Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8). JAMA. 2014;311(5):507–20. Evidence based JNC8 consensus statement on blood pressure management for patients with preexisting diabetes. Used rigorous evidence based evidence to develop blood pressure treatment recommendations.
Bos-Thompson M-A, Hillaire-Buys D, Muller F, et al. Fetal toxic effects of angiotensin II receptor antagonists: case report and follow-Up after birth. Ann Pharmacother. 2005;39:157–61.
Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354:2443–51.
Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: a retrospective cohort study. BMJ. 2011;343:d5931.
Diav-Citrin O, Shechtman S, Halberstadt Y, et al. Pregnancy outcome after in utero exposure to angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Reprod Toxicol. 2011;31:540–5.
Porta M, Hainer JW, Jansson SO, et al. Exposure to candesartan during the first trimester of pregnancy in type 1 diabetes: experience from the placebo-controlled DIabetic REtinopathy Candesartan Trials. Diabetologia. 2011;54:1298–303.
Weber-Schoendorfer C, Hannemann D, Meister R, et al. The safety of calcium channel blockers during pregnancy: a prospective, multicenter, observational study. Reprod Toxicol. 2008;26:24–30.
Magee LA, von Dadelszen P, et al, CHIPS Study Group. Do labetalol and methyldopa have different effects on pregnancy outcome? Analysis of data from the Control of Hypertension In pregnancy study (CHIPS) trial. BJOG. 2015. Demonstrates that methyldopa is noninferior to labetalol for the treatment of hypertension in pregnancy.
Butters L, Kennedy S, Rubin PC. Atenolol in essential hypertension during pregnancy. BMJ. 1990;301:587–9.
Gordon M, Landon MB, Samuels P, Hissrich S, Gabbe SG. Perinatal outcome and long-term follow-up associated with modern management of diabetic nephropathy. Obstet Gynecol. 1996;87:401–9.
Jones DC, Hayslett JP. Outcome of pregnancy in women with moderate or severe renal insufficiency. N Engl J Med. 1996;335:226–32.
Rossing K, Jacobsen P, Hommel E, et al. Pregnancy and progression of diabetic nephropathy. Diabetologia. 2002;45:36–41.
Klein BEMS, Klein R. Effect of pregnancy on progression of diabetic retinopathy. Diabetes Care. 1990;13:34–40.
The Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. Diabetes Care. 2000;23:1084–91.
Chew EYMJ, Metzger BE, Remaley NA, Jovanovic-Peterson L, Knopp RH, Conley M, et al. Metabolic control and progression of retinopathy. The diabetes in early pregnancy study. National institute of child health and human development diabetes in early pregnancy study. Diabetes Care. 1995;18:631–7.
Rahman WRF, Yassin S, Al-Suleiman SA, Rahman J. Progression of retinopathy during pregnancy in type 1 diabetes mellitus. Clin Exp Ophthalmol. 2007;35:231–6.
Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–52.
Chung JH, Melsop KA, Gilbert WM, Caughey AB, Walker CK, Main EK. Increasing pre-pregnancy body mass index is predictive of a progressive escalation in adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2012;0:1–23.
Johansson K, Cnattingius S, Näslund I, Roos N, Trolle Lagerros Y, Granath F, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med. 2015;372(9):814–24. Demonstrates increased risk of small for gestational age risk of infants in non diabetic women who underwent prior bariatric surgery.
Middelbeek RJ, James-Todd T, Cavallerano JD, Schlossman DK, Patti ME, Brown FM. Gastric bypass surgery in severely obese women with type 1 diabetes: anthropometric and cardiometabolic effects at 1 and 5 years postsurgery. Diabetes Care. 2015;38(7). Patients with T1DM who have undergone bariatric surgery have reductions in BMI but no improvement in glycemic control.
Duntas LH, Orgiazzi J, Brabant G. The Interface between thyroid and diabetes mellitus. Clin Endocrinol. 2011.
Bailey BK, Cardwell MS. A team approach to managing preexisting diabetes complicated by pregnancy. Diabetes Educ. 1996;22:111–2. 5.
Quevedo SF, Coustan DR. Diabetes and pregnancy. Use of an integrated “team” approach provides the necessary comprehensive care. R I Med J. 1989;72:129–32.
Catalano PM, Hauguel-De MS. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011;204:479–87.
Lindsay RS, Nelson SM, Walker JD, et al. Programming of adiposity in offspring of mothers with type 1 diabetes at age 7 years. Diabetes Care. 2010;33:1080–5.
Silverman BL, Metzger BE, Cho NH, Loeb CA. Impaired glucose tolerance in adolescent offspring of diabetic mothers. Relationship to fetal hyperinsulinism. Diabetes Care. 1995;18:611–7.
Buinauskiene J, Baliutaviciene D, Zalinkevicius R. Glucose tolerance of 2- to 5-yr-old offspring of diabetic mothers. Pediatr Diabetes. 2004;5:143–6.
American Diabetes Association. Management of diabetes in pregnancy. Sec. 12. In standards of medical care in diabetes 2016. Diabetes Care. 2016;39(1):S94–8. An up to date summary of ADA recommendations for the management of preexisting diabetes in pregnancy.
Secher AL, Ringholm L, Andersen HU, et al. The effect of real-time continuous glucose monitoring in pregnant women with diabetes. Diabetes Care. 2013;36(7):1877–83. A RCT of CGM as the intervention vs no CGM, which demonstrated no improvement in glycemic control.
Law GR, Ellison GT, Secher A, et al. Analysis of continuous glucose monitoring in pregnant women with diabetes: disting temportal patterns of glucose associated with large-for-gestational-age infants. Diabetes Care. 2015;38:1319–24. Different patterns of glucose excursion on CGM that were associated with LGA.
Garcia-Patterson A, Gich I, Amini SB, Catalano PM, de Leiva A, Corcoy R. Insulin requirements throughout pregnancy in women with type 1 diabetes mellitus: three changes of direction. Diabetologia. 2010;53:446–51.
McManus RM, Ryan EA. Insulin requirements in insulin-dependent and insulin-requiring GDM women during final month of pregnancy. Diabetes Care. 1992;15:1323–7.
Callesen NF, Ringholm L, Stage E, Damm P, Mathiesen ER. Insulin requirements in type 1 diabetic pregnancy: Do twin pregnant women require twice as much insulin as singleton pregnant women? Diabetes Care. 2012;35:1246–8.
Kitzmiller JL, Jovanovic L, Brown F, Coustan D, Reader DM, American Diabetes Association, editors. Managing preexisting diabetes and pregnancy : technical reviews and consensus recommendations for care. Alexandria: American Diabetes Association; 2008.
Landon MB, Gabbe SG, Piana R, Mennuti MT, Main EK. Neonatal morbidity in pregnancy complicated by diabetes mellitus: predictive value of maternal glycemic profiles. Am J Obstet Gynecol. 1987;156:1089–95.
Nielsen LR, Pedersen-Bjergaard U, Thorsteinsson B, Johansen M, Damm P, Mathiesen ER. Hypoglycemia in pregnant women with type 1 diabetes: predictors and role of metabolic control. Diabetes Care. 2008;31:9–14.
Evers IM, ter Braak EW, de Valk HW, van Der Schoot B, Janssen N, Visser GH. Risk indicators predictive for severe hypoglycemia during the first trimester of type 1 diabetic pregnancy. Diabetes Care. 2002;25:554–9.
Kimmerle R, Heinemann L, Delecki A, Berger M. Severe hypoglycemia incidence and predisposing factors in 85 pregnancies of type I diabetic women. Diabetes Care. 1992;15:1034–7.
Bjorklund AO, Adamson UK, Almstrom NH, et al. Effects of hypoglycaemia on fetal heart activity and umbilical artery Doppler velocity waveforms in pregnant women with insulin-dependent diabetes mellitus. Br J Obstet Gynaecol. 1996;103:413–20.
Rizzo T, Metzger BE, Burns WJ, Burns K. Correlations between antepartum maternal metabolism and child intelligence. N Engl J Med. 1991;325:911–6.
Zisser HC, Biersmith MA, Jovanovic LB, Yogev Y, Hod M, Kovatchev BP. Fetal risk assessment in pregnancies complicated by diabetes mellitus. J Diabetes Sci Technol. 2010;4:1368–73.
Cullen MT, Reece EA, Homko CJ, Sivan E. The changing presentations of diabetic ketoacidosis during pregnancy. Am J Perinatol. 1996;13:449–51.
Guo RX, Yang LZ, Li LX, Zhao XP. Diabetic ketoacidosis in pregnancy tends to occur at lower blood glucose levels: case-control study and a case report of euglycemic diabetic ketoacidosis in pregnancy. J Obstet Gynaecol Res. 2008;34:324–30.
Schneider MB, Umpierrez GE, Ramsey RD, Mabie WC, Bennett KA. Pregnancy complicated by diabetic ketoacidosis: maternal and fetal outcomes. Diabetes Care. 2003;26:958–9.
Bernstein IM, Catalano PM. Ketoacidosis in pregnancy associated with the parenteral administration of terbutaline and betamethasone. A case report. J Reprod Med. 1990;35:818–20.
Lindenbaum C, Menzin A, Ludmir J. Diabetic ketoacidosis in pregnancy resulting from insulin pump failure. A case report. J Reprod Med. 1993;38:306–8.
Mathiesen ER, Christensen AB, Hellmuth E, Hornnes P, Stage E, Damm P. Insulin dose during glucocorticoid treatment for fetal lung maturation in diabetic pregnancy: test of an algorithm [correction of analgoritm]. Acta Obstet Gynecol Scand. 2002;81:835–9.
Curet LB, Izquierdo LA, Gilson GJ, Schneider JM, Perelman R, Converse J. Relative effects of antepartum and intrapartum maternal blood glucose levels on incidence of neonatal hypoglycemia. J Perinatol. 1997;17:113–5.
Dudley DJ. Diabetic-associated stillbirth: incidence, pathophysiology, and prevention. Obstet Gynecol Clin N Am. 2007;34:293–307. ix.
El Guindy AA, Nabhan AF. A randomized trial of tight vs. less tight control of mild essential and gestational hypertension in pregnancy. J Perinat Med. 2008;36:413–8.
von Dadelszen P, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: an updated metaregression analysis. Journal of obstetrics and gynaecology. Canada : JOGC = Journal d’obstetrique et gynecologie du Canada : JOGC. 2002;24:941–5.
ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet : Off Organ Int Fed Gynaecol Obstet. 2002;77:67–75.
Abalos E, Duley L, Steyn DW. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2014;6:2. A systematic review of antihypertensive drugs that were used to lower blood pressure and prevent progression to more severe disease and improved outcomes in pregnancy.
Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372(5):407–17. A randomized controlled trial demonstrating that tight vs not tight glucose control is associated with a reduced risk of developing severe hypertension.
Sibai BM, Grossman RA, Grossman HG. Effects of diuretics on plasma volume in pregnancies with long-term hypertension. Am J Obstet Gynecol. 1984;150:831–5.
Rothberger S, Carr D, Brateng D, Hebert M, Easterling TR. Pharmacodynamics of clonidine therapy in pregnancy: a heterogeneous maternal response impacts fetal growth. Am J Hypertens. 2010;23:1234–40.
Firoz T, Magee LA, MacDonell K, Payne BA, Gordon R, Vidler M, et al. Oral antihypertensive therapy for severe hypertension in pregnancy and postpartum: a systematic review. BJOG. 2014;121(10):1210–8. A systematic review of oral antihypertensive therapy in severe hypertension in pregnancy and postpartum identifying nifedipine and possibly labetalol and methyldopa as suitable options for treatment.
Carr DB, Koontz GL, Gardella C, et al. Diabetic nephropathy in pregnancy: suboptimal hypertensive control associated with preterm delivery. Am J Hypertens. 2006;19:513–9.
Davison JM, Noble MC. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol. 1981;88:10–7.
Reece EA, Coustan DR, Hayslett JP, et al. Diabetic nephropathy: pregnancy performance and fetomaternal outcome. Am J Obstet Gynecol. 1988;159:56–66.
Kitzmiller JL, Brown ER, Phillippe M, et al. Diabetic nephropathy and perinatal outcome. Am J Obstet Gynecol. 1981;141:741–51.
Biesenbach GS, Zazgornik HJ. Influence of pregnancy on progression of diabetic nephropathy and subsequent requirement of renal replacement therapy in female type 1 diabetic patients with impaired renal function. Nephrol Dial Transplant. 1992;7:105–9.
Chang LK, Sarraf D. Current and future approaches in the prevention and treatment of diabetic retinopathy. Clin Ophthalmol. 2008;2:425–33.
Kaaja R, Loukovaara S. Progression of retinopathy in type 1 diabetic women during pregnancy. Curr Diabetes Rev. 2007;3:85–93.
The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88:583–600.
Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98:766--85.
Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005.
Roseboom TJ, Ravelli AC, van der Post JA, Painter RC. Maternal characteristics largely explain poor pregnancy outcome after hyperemesis gravidarum. Eur J Obstet Gynecol Reprod Biol. 2011;156:56–9.
Macleod AF, Smith SA, Sonksen PH, Lowy C. The problem of autonomic neuropathy in diabetic pregnancy. Diabet Med : J Br Diabet Assoc. 1990;7:80–2.
Abas MN, Tan PC, Azmi N, Omar SZ. Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2014;123(6):1272–9. A randomized controlled trial of of ondansetron vs metoclopramide with demonstrating similar antinausea and antiemetic effects, but ondansetron had fewer side effects and reduced ketonuria.
Koren G. Treating morning sickness in the United States--changes in prescribing are needed. Am J Obstet Gynecol. 2014;211(6):602–6. A review of ondansetron recommending caution in its use in early pregnancy for the treatment of hyperemesis gravedarum because of possible increased risk of cardiac congenital malformations.
Stevens JC, Beard CM, O’Fallon WM, Kurland LT. Conditions associated with carpal tunnel syndrome. Mayo Clinic Proc Mayo Clinic. 1992;67:541–8.
Krolewski AS, Kosinski EJ, Warram JH, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–5.
Raggi P, Bellasi A, Ratti C. Ischemia imaging and plaque imaging in diabetes: complementary tools to improve cardiovascular risk management. Diabetes Care. 2005;28:2787–94.
Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53.
Pignone M, Alberts MJ, Colwell JA, et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the american diabetes association, a scientific statement of the american heart association, and an expert consensus document of the american college of cardiology foundation. Diabetes Care. 2010;33:1395–402.
Taguchi N, Rubin ET, Hosokawa A, et al. Prenatal exposure to HMG-CoA reductase inhibitors: effects on fetal and neonatal outcomes. Reprod Toxicol. 2008;26:175–7.
Pacheco LD, Saade GR, Hankins G. Acute myocardial infarction during pregnancy. Clin Obstet Gynecol. 2014;57(4):835–43.
Effect of intensive diabetes management on macrovascular events and risk factors in the diabetes control and complications trial. Am J Cardiol. 1995;75:894-903.
Perez A, Wagner AM, Carreras G, et al. Prevalence and phenotypic distribution of dyslipidemia in type 1 diabetes mellitus: effect of glycemic control. Arch Intern Med. 2000;160:2756–62.
Chaturvedi N, Fuller JH, Taskinen MR. Differing associations of lipid and lipoprotein disturbances with the macrovascular and microvascular complications of type 1 diabetes. Diabetes Care. 2001;24:2071–7.
Knopp RH, Warth MR, Carrol CJ. Lipid metabolism in pregnancy. I. Changes in lipoprotein triglyceride and cholesterol in normal pregnancy and the effects of diabetes mellitus. J Reprod Med. 1973;10:95–101.
Knopp RH, Van Allen MI, McNeely M, et al. Effect of insulin-dependent diabetes on plasma lipoproteins in diabetic pregnancy. J Reprod Med. 1993;38:703–10.
De Groot LD, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and the postpartum: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(8):2543–65.
Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid : Off J Am Thyroid Assoc. 2011;21:1081–125.
Wojcicki JM, Heyman MB. Maternal omega-3 fatty acid supplementation and risk for perinatal maternal depression. J Matern Fetal Neonatal Med : Off J Eur Assoc Perinat Med Fed Asia Oceania Perinatal Societies, Int Soc Perinatal Obstet. 2011;24:680–6.
Alexander EK, Marqusee E, Lawrence J, Jarolim P, Fischer GA, Larsen PR. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med. 2004;351:241–9.
Mandel SJ, Larsen PR, Seely EW, Brent GA. Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med. 1990;323:91–6.
Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the american thyroid association and american association of clinical endocrinologists. Endocr Pract : Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2011;17:456–520.
Persson M, Pasupathy D, Hanson U, Norman M. Birth size distribution in 3,705 infants born to mothers with type 1 diabetes: a population-based study. Diabetes Care. 2011;34:1145–9.
Al-Agha R, Firth RG, Byrne M, et al. Outcome of pregnancy in type 1 diabetes mellitus (T1DMP): results from combined diabetes-obstetrical clinics in Dublin in three university teaching hospitals (1995-2006). Ir J Med Sci. 2011.
Aman J, Hansson U, Ostlund I, Wall K, Persson B. Increased fat mass and cardiac septal hypertrophy in newborn infants of mothers with well-controlled diabetes during pregnancy. Neonatology. 2011;100:147–54.
Mulder EJ, Koopman CM, Vermunt JK, de Valk HW, Visser GH. Fetal growth trajectories in Type-1 diabetic pregnancy. Ultrasound Obstet Gynecol : Off J Int Soc Ultrasound Obstet Gynecol. 2010;36:735–42.
Maresh M, Holmes VA, Patterson CP, Diabetes and Pre-eclampsia Intervention Trial Study Goup, et al. Glycemic targets in the second and third trimester of pregnancy for women with type 1 diabetes. Diabetes Care. 2015;38:34–42. A1C > 6% in the 3rd trimester of pregnancy is associated with increasing prevalence of LGA.
Combs CA, Gunderson E, Kitzmiller JL, Gavin LA, Main EK. Relationship of fetal macrosomia to maternal postprandial glucose control during pregnancy. Diabetes Care. 1992;15:1251–7.
Jovanovic-Peterson L, Peterson CM, Reed GF, et al. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development--Diabetes in Early Pregnancy Study. Am J Obstet Gynecol. 1991;164:103–11.
Mello G, Parretti E, Mecacci F, et al. What degree of maternal metabolic control in women with type 1 diabetes is associated with normal body size and proportions in full-term infants? Diabetes Care. 2000;23:1494–8.
Kyne-Grzebalski D, Wood L, Marshall SM, Taylor R. Episodic hyperglycaemia in pregnant women with well-controlled Type 1 diabetes mellitus: a major potential factor underlying macrosomia. Diabet Med. 1999;16:702–6.
Murphy HR, Steel SA, Roland JM, et al. Obstetric and perinatal outcomes in pregnancies complicated by Type 1 and Type 2 diabetes: influences of glycaemic control, obesity and social disadvantage. Diabet Med. 2011;28:1060–7.
Lepercq J, Coste J, Theau A, Dubois-Laforgue D, Timsit J. Factors associated with preterm delivery in women with type 1 diabetes: a cohort study. Diabetes Care. 2004;27:2824–8.
Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane database of systematic reviews 2006:CD004454.
USPSTF. 2014. http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication#Pod11. The USPSTF issued a statement recommending use of ASA 81 mg during weeks 12-36 of pregnancy to help reduce the risk of preeclampsia in women with type 1 and 2 diabetes.
Riviello C, Mello G, Jovanovic LG. Breastfeeding and the basal insulin requirement in type 1 diabetic women. Endocrine Pract : Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2009;15:187–93.
Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222–31.
Dorheim SK, Bondevik GT, Eberhard-Gran M, Bjorvatn B. Sleep and depression in postpartum women: a population-based study. Sleep. 2009;32:847–55.
Knip M, Åkerblom HK, Becker D, et al. TRIGR Study Group. Hydrolyzed infant formula and early β-cell autoimmunity: a randomized clinical trial. JAMA. 2014;311(22):2279–87. Among infants at risk for type 1 diabetes, the use of a hydrolyzed formula, when compared with a conventional formula, did not reduce the incidence of diabetes-associated autoantibodies after 7 years. These findings do not support a benefit from hydrolyzed formula.
Hummel S, Pfluger M, Kreichauf S, Hummel M, Ziegler AG. Predictors of overweight during childhood in offspring of parents with type 1 diabetes: Response to Rodekamp et al. Diabetes Care. 2009;32:e139.
Schoen S, Sichert-Hellert W, Hummel S, Ziegler AG, Kersting M. Breastfeeding duration in families with type 1 diabetes compared to non-affected families: results from BABYDIAB and DONALD studies in Germany. Breastfeed Med : Off J Acad Breastfeed Med. 2008;3:171–5.
Hawdon JM. Babies born after diabetes in pregnancy: what are the short- and long-term risks and how can we minimise them? Best Pract Res Clin Obstet Gynaecol. 2011;25:91–104.
Hummel S, Winkler C, Schoen S, et al. Breastfeeding habits in families with Type 1 diabetes. Diabet Med. 2007;24:671–6.
Hartmann P, Cregan M. Lactogenesis and the effects of insulin-dependent diabetes mellitus and prematurity. J Nutr. 2001;131:3016S–20S.
Mahmood I, Jamal M, Khan N. Effect of mother-infant early skin-to-skin contact on breastfeeding status: a randomized controlled trial. J Coll Physicians Surg Pak : JCPSP. 2011;21:601–5.
Trevathan WR, Smith EO, McKenna J, editors. Evolutionary medicine and health, new perspectives. New York: Oxford University Press; 2007.
Food and Drug Administration. Safety. Propylthiouracil tablets. U.S. Department of Health & Human Services. (Accessed at http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm209256.htm.
Kjos SL. Contraception for women with diabetes, in diabetes in women. In: Tsatsoulis A, Wyckoff J, Brown FM, editors. Pathophysiology and therapy. New York: Humana press; 2009.
Huang T, Brown FM, Curran A, James-Todd T. Association of pre-pregnancy BMI and postpartum weight retention with postpartum HbA1c among women with Type 1 diabetes. Diabet Med. 2015;32(2):181–8. Pre pregnancy BMI and postpartum weight retention were positively associated with A1C during the first postpartum year in women with T1DM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Anna Z. Feldman and Florence M. Brown declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Treatment of Type 1 Diabetes
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Feldman, A.Z., Brown, F.M. Management of Type 1 Diabetes in Pregnancy. Curr Diab Rep 16, 76 (2016). https://doi.org/10.1007/s11892-016-0765-z
Published:
DOI: https://doi.org/10.1007/s11892-016-0765-z