Abstract
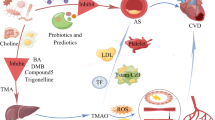
What we understand about diabetes from decades of genetics research is now being supplemented with exciting new evidence based on a better understanding of how one of the biggest “environmental” factors the body is exposed to is influencing the pathogenesis of disease. The recent discovery that certain dietary nutrients possessing a trimethylamine (TMA) moiety (namely choline/phosphatidylcholine and L-carnitine) participate in the development of atherosclerotic heart disease has renewed attention towards the contributions of gut microbiota in the development of cardiovascular diseases. Collectively, animal and human studies reveal that conversion of these nutrient precursors to trimethylamine N-oxide (TMAO) depends on both microbial composition and host factors, and can be induced by dietary exposures. In addition, circulating TMAO levels are strongly linked to cardiovascular disease risks and various adverse cardio-renal consequences. Our group and others have further demonstrated that circulating TMAO levels are elevated in patients with type 2 diabetes mellitus compared to healthy controls and gut microbiota-dependent phosphatidylcholine metabolism has been implicated in metabolic dysregulation and insulin resistance in animal models. Therefore, preventive strategies to minimize adverse consequences associated with TMAO generation in the diabetic population are warranted.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Eckburg PB et al. Diversity of the human intestinal microbial flora. Science. 2005;308(5728):1635–8.
Claesson MJ et al. Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS One. 2009;4(8):e6669.
Turnbaugh PJ et al. The human microbiome project. Nature. 2007;449(7164):804–10.
Backhed F et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–23.
Backhed F et al. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104(3):979–84.
Turnbaugh PJ et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31.
Amar J et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011;54(12):3055–61.
Moreno-Navarrete JM et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond). 2012;36(11):1442–9.
Cani PD et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72.
Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81(3):1031–64.
Cummings JH et al. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr. 1979;32(10):2094–101.
Goncalves P, Martel F. Butyrate and colorectal cancer: the role of butyrate transport. Curr Drug Metab. 2013;14(9):994–1008.
Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12(6):499–507.
Pouteau E et al. Acetate, propionate and butyrate in plasma: determination of the concentration and isotopic enrichment by gas chromatography/mass spectrometry with positive chemical ionization. J Mass Spectrom. 2001;36(7):798–805.
Ray TK et al. Long-term effects of dietary fiber on glucose tolerance and gastric emptying in noninsulin-dependent diabetic patients. Am J Clin Nutr. 1983;37(3):376–81.
Mendeloff AI. Dietary fiber and human health. N Engl J Med. 1977;297(15):811–4.
Robertson MD et al. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia. 2003;46(5):659–65.
Karlsson FH et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. These two studies (18,19) provide the first metagenomic evidence of differing profiles between diabetes and normal individuals and suggest the potential to predict development of disease from metagenomic sequences.
Qin J et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. These two studies (18,19) provide the first metagenomic evidence of differing profiles between diabetes and normal individuals and suggest the potential to predict development of disease from metagenomic sequences.
Le Poul E et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278(28):25481–9.
Brown AJ et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278(13):11312–9.
Kimura I et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829.
Samuel BS et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767–72.
Tolhurst G et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–71.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39.
Freeland KR, Wolever TM. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2010;103(3):460–6.
Vrieze A et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6. e7.
Amar J et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87(5):1219–23.
Shi H et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–25.
Saemann MD et al. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14(15):2380–2.
Roelofsen H, Priebe MG, Vonk RJ. The interaction of short-chain fatty acids with adipose tissue: relevance for prevention of type 2 diabetes. Benefic Microbes. 2010;1(4):433–7.
Peng L et al. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–25.
Koeth RA et al. gamma-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20(5):799–812. This study and reference [47] provide novel insights into the metabolism of TMAO and its associations with various gut microbiome profiles.
Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124(10):4204–11.
Gregory JC et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2014;290:5647–60.
Wang Z et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. In this study, Wang et al. uses a metabolomic screen to identify TMAO as a novel gut microbiota-dependent metabolite that is a potential modifiable risk factor for cardiovascular disease.
Tang WH et al. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol. 2014;64(18):1908–14.
Tang WH et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. In this clinical study with over 4000 participants, the authors reconfirmed the gut microbiota dependence of TMAO in human subjects and found that serum TMAO levels predicted 3-year incident cardiovascular risk in a dose-dependent manner.
Tang WH et al. Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction and adverse clinical outcomes in chronic systolic heart failure. J Card Fail. 2014;21:91–6.
Wang Z et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35(14):904–10.
Wang TJ et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–53.
Lever M et al. Betaine and trimethylamine-N-oxide as predictors of cardiovascular outcomes show different patterns in diabetes mellitus: an observational study. PLoS ONE. 2014;9(12), e114969.
Gao X et al. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118(4):476–81.
Miao J et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. This study shows that knockdown of FMO3 and subsequently TMAO levels can prevent the development of hyperglycemia, hyperlipidemia, and atherosclerosis in a diabetes mouse model.
Shih DM et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56(1):22–37.
Tang WH et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55.
Koeth RA et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. See reference 33.
Zeisel SH, da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. 2009;67(11):615–23.
Chu DM et al. Choline and betaine food sources and intakes in Taiwanese. Asia Pac J Clin Nutr. 2012;21(4):547–57.
Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49(6):1187–94.
Paoletti L et al. Role of phosphatidylcholine during neuronal differentiation. IUBMB Life. 2011;63(9):714–20.
Lever M, Slow S. The clinical significance of betaine, an osmolyte with a key role in methyl group metabolism. Clin Biochem. 2010;43(9):732–44.
Lever M et al. Variability of plasma and urine betaine in diabetes mellitus and its relationship to methionine load test responses: an observational study. Cardiovasc Diabetol. 2012;11:34.
Lever M et al. Betaine and secondary events in an acute coronary syndrome cohort. PLoS One. 2012;7(5), e37883.
Wijekoon EP, Brosnan ME, Brosnan JT. Homocysteine metabolism in diabetes. Biochem Soc Trans. 2007;35(Pt 5):1175–9.
Konstantinova SV et al. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138(5):914–20.
Schartum-Hansen H et al. Assessment of urinary betaine as a marker of diabetes mellitus in cardiovascular patients. PLoS One. 2013;8(8), e69454.
Lever M et al. Plasma lipids and betaine are related in an acute coronary syndrome cohort. PLoS One. 2011;6(7), e21666.
Rajaie S, Esmaillzadeh A. Dietary choline and betaine intakes and risk of cardiovascular diseases: review of epidemiological evidence. ARYA Atheroscler. 2011;7(2):78–86.
Danne O et al. Whole blood choline and plasma choline in acute coronary syndromes: prognostic and pathophysiological implications. Clin Chim Acta. 2007;383(1–2):103–9.
Danne O et al. Prognostic implications of elevated whole blood choline levels in acute coronary syndromes. Am J Cardiol. 2003;91(9):1060–7.
LeLeiko RM et al. Usefulness of elevations in serum choline and free F2)-isoprostane to predict 30-day cardiovascular outcomes in patients with acute coronary syndrome. Am J Cardiol. 2009;104(5):638–43.
Bidulescu A et al. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2007;7:20.
Bidulescu A et al. Repeatability and measurement error in the assessment of choline and betaine dietary intake: the Atherosclerosis Risk in Communities (ARIC) study. Nutr J. 2009;8:14.
Dumas ME et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103(33):12511–6.
Mingrone G. Carnitine in type 2 diabetes. Ann N Y Acad Sci. 2004;1033:99–107.
Capaldo B et al. Carnitine improves peripheral glucose disposal in non-insulin-dependent diabetic patients. Diabetes Res Clin Pract. 1991;14(3):191–5.
Ferrannini E et al. Interaction of carnitine with insulin-stimulated glucose metabolism in humans. Am J Physiol. 1988;255(6 Pt 1):E946–52.
Mynatt RL. Carnitine and type 2 diabetes. Diabetes Metab Res Rev. 2009;25 Suppl 1:S45–9.
Vidal-Casariego A et al. Metabolic effects of L-carnitine on type 2 diabetes mellitus: systematic review and meta-analysis. Exp Clin Endocrinol Diabetes. 2013;121(4):234–8.
DiNicolantonio JJ et al. L-carnitine in the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Mayo Clin Proc. 2013;88(6):544–51.
Shang R, Sun Z, Li H. Effective dosing of L-carnitine in the secondary prevention of cardiovascular disease: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2014;14:88.
Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109(52):21307–12.
Zhu Y et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111(11):4268–73.
Qin J et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
Turnbaugh PJ et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4.
Furet JP et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57.
Zhang X et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8(8), e71108.
Remely M et al. Effects of short chain fatty acid producing bacteria on epigenetic regulation of FFAR3 in type 2 diabetes and obesity. Gene. 2014;537(1):85–92.
Karlsson CL et al. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring). 2012;20(11):2257–61.
Everard A et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. 2011;60(11):2775–86.
Everard A et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–71.
Shin NR et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63(5):727–35.
Walker AW et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5(2):220–30.
Wu GD et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8.
David LA et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63.
Lozupone CA et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220–30.
Acknowledgments
This work is supported in part by grants from the National Institutes of Health and the Office of Dietary Supplements (R01HL103931, P20HL113452).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Daniel Li, Jennifer Kirsop, and W. H. Wilson Tang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pathogenesis of Type 2 Diabetes and Insulin Resistance
Rights and permissions
About this article
Cite this article
Li, D., Kirsop, J. & Tang, W.H.W. Listening to Our Gut: Contribution of Gut Microbiota and Cardiovascular Risk in Diabetes Pathogenesis. Curr Diab Rep 15, 63 (2015). https://doi.org/10.1007/s11892-015-0634-1
Published:
DOI: https://doi.org/10.1007/s11892-015-0634-1