Abstract
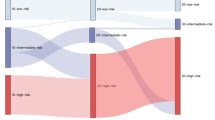
This report details the development, validation, and utility of the Diabetes Prevention Trial-Type 1 (DPT-1) Risk Score (DPTRS) for type 1 diabetes (T1D). Proportional hazards regression was used to develop the DPTRS model which includes the glucose and C-peptide sums from oral glucose tolerance tests at 30, 60, 90, and 120 min, the log fasting C-peptide, age, and the log BMI. The DPTRS was externally validated in the TrialNet Natural History Study cohort (TNNHS). In a study of the application of the DPTRS, the findings showed that it could be used to identify normoglycemic individuals who were at a similar risk for T1D as those with dysglycemia. The DPTRS could also be used to identify lower risk dysglycemic individuals. Risk estimates of individuals deemed to be at higher risk according to DPTRS values did not differ significantly between the DPT-1 and the TNNHS; whereas, the risk estimates for those with dysglycemia were significantly higher in DPT-1. Individuals with very high DPTRS values were found to be at such marked risk for T1D that they could reasonably be considered to be in a pre-diabetic state. The findings indicate that the DPTRS has utility in T1D prevention trials and for identifying pre-diabetic individuals.



Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Gale EA, Bingley PJ, Emmett CL, Collier T, the European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1. Lancet. 2004;363:925–31.
Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of oral insulin in relatives of patients with type 1 diabetes. Diabetes Care. 2005;28:1068–76. This was one of the prevention trials from which the DPTRS was derived.
Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–91. This was one of the prevention trials from which the DPTRS was derived.
Bottazzo JF, Mann JI, Thorogood M, Baum JD, Doniach D. Autoimmunity in juvenile diabetics and their families. Br Med J. 1978;2(6131):165–8.
Cudworth AG, Woodrow JC. Evidence of HL-A linked genes in “juvenile” diabetes mellitus. Br Med J. 1975;3(5976):133–5.
Vardi P, Crisa L, Jackson RA. Predictive value of intravenous glucose tolerance test insulin secretion less than or greater than the first percentile in islet cell antibody positive relatives of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1991;34:93–102.
Rosenbloom AL, Hunt SS, Rosenbloom EK, Maclaren NK. Ten-year prognosis of impaired glucose tolerance in siblings of patients with insulin-dependent diabetes. Diabetes. 1982;31:385–7.
Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473–9.
Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, et al. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1 (DPT-1). Diabetes Care. 2009;32:2269–74.
Sosenko J, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, et al. Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes. Diabetes Care. 2007;30:38–42.
Sosenko JM, Krischer JP, Palmer JP, Mahon J, Cowie C, Greenbaum CJ, et al. A risk score for type 1 diabetes derived from autoantibody positive participants in The Diabetes Prevention Trial-Type 1. Diabetes Care. 2008;31:528–33. This is the first article to describe the development of the DPTRS.
Sosenko JM, Skyler JS, Mahon J, Krischer JP, Beam CA, Boulware DC, et al. Validation of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS) in the TrialNet Natural History Study. Diabetes Care. 2011;34:1785–7.
Sosenko JM, Skyler JS, Mahon J, Krischer JP, Greenbaum CJ, Rafkin LE, et al. The use of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS) for improving the accuracy of the risk classification of type 1 diabetes. Diabetes Care. 2014;37:979–84.
Sosenko JM, Skyler JS, Mahon J, Krischer JP, Beam CA, Boulware DC, et al. The application of the type 1 diabetes risk score for identifying a preclinical state of type 1 diabetes. Diabetes Care. 2012;35:1552–5.
Ilonen J, Hammais A, Laine A, Lempainen J, Vaarala O, Veijola R, et al. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes. 2013;62:3636–40.
Sosenko JM, Skyler JS, Palmer JP, Krischer JP, Yu L, Mahon J, et al. The prediction of type 1 diabetes by multiple autoantibody levels and their incorporation into an autoantibody risk score in relatives of type 1 diabetic patients. Diabetes Care. 2013;36:2615–20.
Acknowledgments
This work was funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute for Child Health and Human Development and National Center for Research Resources; the Juvenile Diabetes Research Foundation International; and the American Diabetes Association. Dr. Jay Sosenko is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
The sponsor of the trial was the Type 1 Diabetes TrialNet Study Group (see Appendix 1). Type 1 Diabetes TrialNet Study Group is a clinical trial network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061016, U01 DK061034, U01 DK061036, U01 DK061040, U01 DK061041, U01 DK061042, U01 DK061055, U01 DK061058, U01 DK084565, U01 DK085453, U01 DK085461, U01 DK085463, U01 DK085466, U01 DK085499, U01 DK085505, U01 DK085509, and a contract HHSN267200800019C; the National Center for Research Resources, through Clinical Translational Science Awards UL1 RR024131, UL1 RR024139, UL1 RR024153, UL1 RR024975, UL1 RR024982, UL1 RR025744, UL1 RR025761, UL1 RR025780, UL1 RR029890, UL1 RR031986, P30 DK017047, and General Clinical Research Center Award M01 RR00400; the Juvenile Diabetes Research Foundation International (JDRF); and the American Diabetes Association (ADA). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, JDRF, or ADA.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Jay M. Sosenko, Jay S. Skyler, and Jerry P. Palmer declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional review boards and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Author Contributions
Jay Sosenko contributed by writing the manuscript, Jay Skyler contributed by reviewing the manuscript, and Jerry Palmer contributed by reviewing the manuscript.
This article is part of the Topical Collection on Pathogenesis of Type 1 Diabetes
The Diabetes Type 1 TrialNet and Diabetes Prevention Trial-Type 1 Study Groups (see Appendix 1)
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 41 kb)
Rights and permissions
About this article
Cite this article
Sosenko, J.M., Skyler, J.S., Palmer, J.P. et al. The Development, Validation, and Utility of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS). Curr Diab Rep 15, 49 (2015). https://doi.org/10.1007/s11892-015-0626-1
Published:
DOI: https://doi.org/10.1007/s11892-015-0626-1