Abstract
Purpose of Review
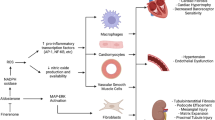
Finerenone is a novel, non-steroidal mineralocorticoid receptor antagonist (MRAs) that has been investigated for the management of cardiorenal conditions. This article provides an overview of recent evidence of benefits on cardiovascular (CV) outcomes.
Recent Findings
The recently published phase III FIDELIO-DKD and FIGARO-DKD, alone and pooled, in patients with CKD and diabetes demonstrate that finerenone reduces the composite of CV death, non-fatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure (HF) with hospitalization for HF being the primary driver of this composite.
Summary
Finerenone is indicated to reduce renal and CV outcomes in patients with CKD and diabetes. Future investigations of this agent include patients with non-diabetic CKD, HF with preserved ejection fraction, and with the use of sodium-glucose transporter type 2 inhibitors.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Bauersachs J, Lother A. Mineralocorticoid receptor activation and antagonism in cardiovascular disease cellular and molecular mechanisms. Kidney Int Suppl. 2011;2022(12):19–26. https://doi.org/10.1016/j.kisu.2021.11.001.
Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9:459–69. https://doi.org/10.1038/nrneph.2013.110.
Terker AS, Yarbrough B, Ferdaus MZ, et al. Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol. 2016;27:2436–45. https://doi.org/10.1681/ASN.2015070815.
Rangaswami J, Bhalla V, Blair JEA, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139:e840–78. https://doi.org/10.1161/CIR.0000000000000664.
Agarwal R, Kolkhof P, Bakris G, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152–61. https://doi.org/10.1093/eurheartj/ehaa736.
Clarisse D, Deng L, de Bosscher K, et al. Approaches towards tissue-selective pharmacology of the mineralocorticoid receptor. Br J Pharmacol. 2022;179:3235–49. https://doi.org/10.1111/bph.15719.
Fuller PJ, Yao Y, Yang J, et al. Mechanisms of ligand specificity of the mineralocorticoid receptor. J Endocrinol. 2012;213:15–24. https://doi.org/10.1530/JOE-11-0372.
Kintscher U, Bakris GL, Kolkhof P. Novel non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Br J Pharmacol. 2022;179:3220–34. https://doi.org/10.1111/bph.15747.
Kolkhof P, Barfacker L. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234:T125–40. https://doi.org/10.1530/JOE-16-0600.
Kolkhof P, Joseph A, Kintscher U. Nonsteroidal mineralocorticoid receptor antagonism for cardiovascular and renal disorders - new perspectives for combination therapy. Pharmacol Res. 2021;172:105859. https://doi.org/10.1016/j.phrs.2021.105859.
Pandey AK, Bhatt DL, Cosentino F, et al. Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Eur Heart J. 2022;43:2931–45. https://doi.org/10.1093/eurheartj/ehac299.
Barrera-Chimal J, Lima-Posada I, Bakris GL, et al. Mineralocorticoid receptor antagonists in diabetic kidney disease - mechanistic and therapeutic effects. Nat Rev Nephrol. 2022;18:56–70. https://doi.org/10.1038/s41581-021-00490-8.
Pitt B, Zannad F. Remme WJ et al The effect of spironolactone on morbidity and mortality in patients with severe heart failure Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17. https://doi.org/10.1056/NEJM199909023411001.
Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. https://doi.org/10.1056/NEJMoa030207.
Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. https://doi.org/10.1056/NEJMoa1009492.
Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. https://doi.org/10.1056/NEJMoa1313731.
Lu R, Zhang Y, Zhu X, et al. Effects of mineralocorticoid receptor antagonists on left ventricular mass in chronic kidney disease patients: a systematic review and meta-analysis. Int Urol Nephrol. 2016;48:1499–509. https://doi.org/10.1007/s11255-016-1319-7.
Greenblatt DJ, Koch-Weser J. Gynecomastia and impotence: complications of spironolactone therapy. JAMA. 1973;223:82.
Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med. 2004;351:543–51. https://doi.org/10.1056/NEJMoa040135.
Struthers A, Krum H, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin Cardiol. 2008;31:153–8. https://doi.org/10.1002/clc.20324.
Bramlage P, Swift SL, Thoenes M, et al. Non-steroidal mineralocorticoid receptor antagonism for the treatment of cardiovascular and renal disease. Eur J Heart Fail. 2017;19:811. https://doi.org/10.1002/ejhf.888.
Amazit L, Le Billan F, Kolkhof P, et al. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem. 2015;290:21876–89. https://doi.org/10.1074/jbc.M115.657957.
Bayer HealthCare Pharmaceuticals I. Kerendia (finerenone). Whippany, NJ. 2022.
Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34:2453–63. https://doi.org/10.1093/eurheartj/eht187.
Filippatos G, Anker SD, Bohm M, et al. A randomized controlled study of finerenone vs eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37:2105–14. https://doi.org/10.1093/eurheartj/ehw132.
Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314:884–94. https://doi.org/10.1001/jama.2015.10081.
•• Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–29. https://doi.org/10.1056/NEJMoa2025845. This clinical trial is one of the landmark trials used to help gain an approval finernone by the FDA and currently provide an overview of the most appropriate patient population for use and the expected benefit/risk for the patient population.
•• Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021;385:2252–63. https://doi.org/10.1056/NEJMoa2110956. This clinical trial is one of the landmark trials used to help gain an approval finernone by the FDA and currently provide an overview of the most appropriate patient population for use and the expected benefit/risk for the patient population.
•• Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022;43:474–84. https://doi.org/10.1093/eurheartj/ehab777. This clinical trial is one of the landmark trials used to help gain an approval finernone by the FDA and currently provide an overview of the most appropriate patient population for use and the expected benefit/risk for the patient population.
Agarwal R, Joseph A, Anker SD, et al. Hyperkalemia risk with finerenone: results from the FIDELIO-DKD Trial. J Am Soc Nephrol. 2022;33:225–37. https://doi.org/10.1681/ASN.2021070942.
ClinicalTrial.gov. A trial to learn how well finereone works and how safe it is in adult participants with non-diabetic chronic kideny disease (FIND-CKD). US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT05047263.
ClinicalTrial.gov. A study to learn how well the treatment combination of finerenone and empagliflozin works and how safe it is compared to each treatment alone in adult participants with long-term kidney disease (chronic kidney disease) and type 2 diabetes (CONFIDENCE). US National Library of Medicine. 2022.
Chiu N, Aggarwal R, Bakris GL, et al. Generalizability of FIGARO-DKD and FIDELIO-DKD Trial criteria to the US population eligible for finerenone. J Am Heart Assoc. 2022;11:e025079. https://doi.org/10.1161/JAHA.121.025079.
Mares D, Rodriguez T, Deoker A, et al. Effect of mineralocorticoid receptor antagonists in heart failure with preserved ejection fraction and with reduced ejection fraction - a narrative review. Curr Vasc Pharmacol. 2022;20(1):46–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Craig J. Beavers declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Ischemic Heart Disease
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beavers, C.J. The Role of the Non-Steroidal Mineralocorticoid Antagonist Finerenone in Cardiorenal Management. Curr Cardiol Rep 24, 1785–1790 (2022). https://doi.org/10.1007/s11886-022-01795-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01795-1