Abstract
Overactivation of the renin–angiotensin–aldosterone system (RAAS) has been shown to be pathologic in heart failure and albuminuric chronic kidney disease (CKD), triggering pro-inflammatory and pro-fibrotic cellular pathways. The standard of care in these disease states includes treatment with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers. Mineralocorticoid receptor antagonists (MRAs) are also a mainstay in the treatment of heart failure with reduced ejection fraction; however, therapy is often limited by treatment-related hyperkalemia. In albuminuric CKD, the risk of hyperkalemia, acute kidney injury (AKI), and hypotension also remains significant. Finerenone is a novel non-steroidal MRA that may obviate some of these concerns and have therapeutic potential in additional patient populations. Finerenone was developed using the chemical structure of a dihydropyridine channel blocker but optimized to create a bulky MRA without any activity at the L-type calcium channel. It has several novel cellular mechanisms that may account for its ability to reduce cardiac hypertrophy and proteinuria more efficiently than an equinatriuretic dose of a steroidal MRA, while retaining anti-inflammatory and anti-fibrotic properties. Finerenone also has a lower rate of treatment-related hyperkalemia and AKI than steroidal MRAs with a smaller effect on systolic blood pressure, greatly expanding its therapeutic utility. The recently published FIGARO-DKD and FIDELIO-DKD trials demonstrate that treatment with finerenone in patients with type II diabetes and albuminuric CKD results in improved cardiovascular outcomes and a lower risk of CKD progression. Patients enrolled in these studies were already on maximally tolerated ACE inhibitor or angiotensin receptor blocker therapy. Trials investigating finerenone’s therapeutic effect in patients with heart failure with preserved ejection fraction (HFpEF) and non-diabetic CKD, as well sodium–glucose cotransporter 2 (SGLT2) and finerenone combination therapy in patients with diabetic nephropathy, are ongoing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Aldosterone is a pathologic agent in heart failure and albuminuric chronic kidney disease (CKD); blockade of the renin–angiotensin–aldosterone system is known to reduce morbidity and mortality in these disease states. |
Finerenone is a novel non-steroidal mineralocorticoid receptor antagonist (MRA) that has a unique chemical structure as compared to steroidal MRAs (i.e., spironolactone and eplerenone) with a lower incidence of treatment-related hyperkalemia and acute kidney injury and a smaller effect on systolic blood pressure. |
The FIGARO-DKD and FIDELIO-DKD trials demonstrated that treatment with finerenone in participants with type II diabetes and albuminuric CKD, already on angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker therapy, resulted in improved cardiovascular outcomes and a lower risk of CKD progression. |
Randomized controlled trials are ongoing to assess use in patients with heart failure with preserved ejection fraction (HFpEF) and non-diabetic CKD, as well as the effects of sodium–glucose cotransporter 2 (SGLT2) and finerenone combination therapy in diabetic nephropathy. |
Introduction
Aldosterone plays critical physiological roles in maintaining normotension, eunatremia, and normokalemia. However, overactivation of aldosterone and the renin–angiotensin–aldosterone system (RAAS) has also been implicated in the pathology of various cardiorenal disease states, including heart failure and hypertension. Mineralocorticoid receptor antagonists (MRAs) have become key components of treatment strategies in these disorders. Currently, the most used MRAs include spironolactone and eplerenone, both steroidal MRAs that bind to the mineralocorticoid receptor (MR) similarly to its natural ligand, aldosterone [1]. Their salutary benefits are multiplex, including management of hypervolemia through diuresis, neurohormonal blockade, and abrogation of cardiac remodeling.
In heart failure, excessive aldosterone secretion drives sodium and water retention, sympathetic nervous system activation, myocardial hypertrophy and fibrosis, and depression of baroreflex sensitivity [2]. The use of an MRA is now routinely recommended in patients with New York Heart Association (NYHA) class II–IV heart failure and who have left ventricular ejection fraction (LVEF) of 35% or less, given that estimated glomerular filtration rate (eGFR) is greater than 30 ml/min/1.73 m2 and serum potassium is less than 5.0 mEq/L [3]. The use of MRAs is also recommended selectively in patients with heart failure with preserved ejection fraction (HFpEF) to reduce the incidence of hospitalizations [4]. In patients with chronic kidney disease (CKD), MRAs reduce albuminuria when combined with angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) therapy; however, the risks include acute kidney injury (AKI), hyperkalemia, and hypotension [5, 6]. KDIGO guidelines recommend a more conservative approach with this combination, noting a marked risk of hyperkalemia and declining kidney function in patients with eGFR less than 45 ml/min/1.73 m2 [7].
Finerenone is a novel, non-steroidal MRA that may have unique utility in both albuminuric kidney disease and heart failure. In preclinical models, finerenone reduced cardiac hypertrophy and proteinuria more efficiently when compared to an equinatriuretic dose of a steroidal MRA [8]. These effects have been shown to be mediated through potent anti-inflammatory and anti-fibrotic pathways. These benefits have been achieved with a lower risk of hyperkalemia when compared to steroidal MRAs like eplerenone and spironolactone [9]. Furthermore, finerenone appears to have a smaller effect on blood pressure, making it a useful agent for patients prone to hypotension or for patients with heart failure who are often already taking multiple heart failure therapies that each independently lower blood pressure [9].
This review will summarize the molecular mechanisms of finerenone activity, its role in cardiorenal disease, review clinical trial data, and discuss future directions for investigation. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Drug Characteristics and Mechanisms
Drug Characteristics
Spironolactone was developed using the chemical structure of progesterone and was the first MRA approved by the US Food and Drug Administration (FDA) in 1960 for edema related to cirrhosis, nephrotic syndrome, heart failure, idiopathic edema, essential hypertension, and cirrhotic ascites [10]. Spironolactone was noted to have high affinity for the MR, binding at the same site as its natural ligand, aldosterone; however, its partial affinity for the progesterone and androgen receptors was evidenced in its adverse effects, including gynecomastia, dysmenorrhea, and erectile dysfunction [10]. Spironolactone is also known to cause hyperkalemia with a renal concentration sixfold higher than its cardiac concentration [8].
A more selective steroidal MRA, eplerenone, was approved by the FDA in 2002. This molecule was developed using the chemical structure of spironolactone with the 17α-thioacetyl group replaced with a carbomethoxy group and a 9,11-epoxide added to the lactone ring [10]. These changes increased eplerenone’s specificity for the MR and had a more favorable side effect profile with regards to gynecomastia, dysmenorrhea, and erectile dysfunction than spironolactone [10]. However, despite eplerenone having a renal concentration only threefold higher than its cardiac concentration, the risk of hyperkalemia remains present [8].
Finerenone is one of several non-steroidal MRAs that was developed using the chemical structure of a dihydropyridine channel blocker but optimized to create a bulky MR antagonist without any activity at the L-type calcium channel [10]. Finerenone is both highly potent and has strong selectivity for the MR, overcoming significant barriers posed by previous generations of MRAs [11]. Preclinical models showed reduction in markers of cardiorenal damage and a lower risk of hyperkalemia [8, 9], postulated to be a result of the roughly 1:1 distribution to the renal and cardiac tissues, as compared to 6:1 for spironolactone and 3:1 for eplerenone [8].
Mechanisms of Action
Despite spironolactone and finerenone binding the same ligand domain on the MR, these molecules have significant differences in their downstream signaling. First, finerenone appears to delay the nuclear accumulation of the MR–aldosterone complex more effectively than spironolactone [11]. Second, finerenone may be more effective than spironolactone at blocking the recruitment of critical transcription cofactors. Normally, after aldosterone binds the MR, the MR–aldosterone complex recruits the transcription cofactors steroidal coactivator 1 (SRC-1) and RNA polymerase II onto a key regulatory region of the gene that encodes the α-subunit of the epithelial sodium channel (ENAC). Spironolactone promotes a lesser but still significant recruitment of these transcription cofactors, while finerenone binding appears to impair recruitment of these cofactors and reduce the binding of existing MR–aldosterone complexes [11]. Finally, finerenone has bulky substituent groups that, when bound to the MR, leads to the formation of an unstable MR–ligand complex that cannot recruit cofactors [11, 12]. In contrast, spironolactone destabilizes critical contacts between aldosterone and the MR but does not physically prevent agonist conformation and subsequent cofactor binding [13].
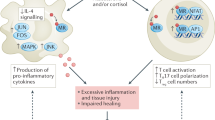
Aldosterone has been implicated in the pathogenesis of cardiovascular disease through inflammatory and fibrotic pathways (Fig. 1). Patients with primary hyperaldosteronism experience more cardiovascular events, and aldosterone is an independent risk factor for cardiovascular disease and all-cause mortality, even at levels below the threshold for hyperaldosteronism [14, 15]. The aldosterone-mediated damage appears dependent on an environment of high salt intake that deranges the homeostasis between aldosterone and serum sodium; populations experiencing chronic sodium deficiency have extremely high levels of plasma aldosterone with minimal cardiorenal damage [16]. In mouse models, aldosterone administration triggers the formation of reactive oxygen species (ROS) in a multitude of tissues, including macrophages, cardiomyocytes, and vascular smooth muscle cells [17, 18]. These ROS trigger the activation of transcription factors that mediate inflammation, including activator protein (AP)-1 and nuclear factor (NF)-κB [19, 20]. Aldosterone also reduces the bioavailability and production of nitric oxide to further promote inflammation [21,22,23]. This inflammation leads to fibrosis in animal models, with improvement in fibrosis and cardiac remodeling after treatment with spironolactone and eplerenone [24,25,26,27,28].
Finerenone has also demonstrated potent anti-fibrotic effect in the heart with improvements in cardiac hypertrophy and fibrosis [29] and was more effective than eplerenone at an equinatriuretic dose [30, 31]. Distinct gene expressions patterns were also observed, with finerenone-treated mice showing reduced expression of BNP (brain natriuretic peptide) as compared to eplerenone-treated mice in a model of pressure overload [30]. Finerenone-treated mice also showed significantly reduced expression of Tnnt2 (cardiac troponin T), but eplerenone-treated mice showed no reduction when compared to vehicle control; however, cardiac troponin T’s role in maladaptive cardiac remodeling still remains unclear [30]. Finally, finerenone but not eplerenone potently blocks the expression of TNX, a known pro-fibrotic gene; this expression appeared to be mediated by differential cofactor modulation by the MR [31].
Aldosterone also triggers a variety of pathogenic changes in the kidney, leading to proteinuria and impairment in kidney function. Patients with primary aldosteronism have higher rates of albuminuria than patients with essential hypertension, implicating aldosterone in proteinuric kidney disease [32, 33]. In the glomerulus, aldosterone appears to have direct deleterious effect on the mesangium and on podocytes. Rats chronically infused with aldosterone demonstrated increased ROS via NADPH oxidase and podocyte foot process effacement. Treatment with eplerenone showed suppression of oxidative stress markers and prevented podocyte effacement [34]. Aldosterone infusion also triggers mesangial injury, cell proliferation, and matrix expansion via ERK/MAPK signaling pathway; these changes were again prevented through treatment with eplerenone [35, 36]. Finally, aldosterone infusion combined with salt intake triggers direct injury to the proximal tubule cells mediated by ROS [37]. This injury leads to increased expression of pro-inflammatory molecules and tubulointerstitial fibrosis, damage that was partially attenuated by eplerenone [38].
Finerenone shows a similar kidney protective effect to steroidal MRAs. In a rat model of aldosterone-induced cardiorenal disease, treatment with finerenone provided protection from glomerular, tubular, and vascular damage, resulting in decreased proteinuria [8]. Finerenone also prevented endothelial cell apoptosis and smooth muscle cell proliferation in a murine model of vascular injury [39]. In a rat model, finerenone increased nitric oxide bioavailability and reduced ROS levels, resulting in reduced endothelial dysfunction, improved systolic blood pressure, and reduced albuminuria [40].
Clinical Trial Data in Humans
Steroidal Antagonist Clinical Trials
A series of clinical trials in both kidney and cardiovascular disease have been conducted with MRAs (Table 1). The landmark RALES trial showed that treatment with spironolactone in participants with NYHA class III or IV heart failure with reduced ejection fraction (HFrEF) resulted in a 35% relative risk reduction in the primary endpoint of all-cause mortality compared with standard of care. A reduction in the frequency of hospitalization for worsening heart failure was also observed [41]. In the subsequent EPHESUS trial, eplerenone compared with standard of care in participants post-myocardial infarction (MI) with reduced LVEF resulted in a significant reduction in the co-primary endpoints of all-cause death as well as cardiovascular death or hospitalization [42]. Finally, the EMPHASIS-HF trial showed a reduced risk of cardiovascular death or hospitalization in patients with NYHA class II HFrEF and LVEF of 35% or less with eplerenone compared with standard of care [43]. These large randomized controlled trials became the foundation behind the recommended use of MRAs in patients with NYHA class II–IV HFrEF [3].
The role for spironolactone in heart failure with preserved ejection fraction (HFpEF) is less clear. In the TOPCAT trial, spironolactone did not reduce cardiovascular mortality and only modestly reduced heart failure hospitalizations in participants with HFpEF; this modest benefit came with an increased the risk of hyperkalemia and renal failure [44]. However, the low 3-year mortality rate of the study population and normal diastolic function on echocardiography found in a subset of participants raised questions about generalizability of the results [45]. A subgroup analysis showed that participants enrolled on the basis of elevated NT-proBNP rather than prior heart failure hospitalization did show improvement in the composite outcome of death from cardiovascular causes, aborted cardiac arrest, or hospitalization for the management of heart failure [44]. This trial led to a class IIb/level B-R recommendation that spironolactone “might be considered” in patients with HFpEF and appropriate kidney function to decrease hospitalizations [4].
With regards to kidney outcomes, studies have shown that inhibition of the renin–angiotensin system, independent of changes in blood pressure, reduces the risk of major kidney events [46, 47]. Further studies demonstrated a continued risk of kidney disease progression, partly explained by the incomplete blockade of the RAAS system [48, 49]. Studies also showed a reduction of proteinuria when steroidal MRAs were added to maximal tolerated doses of ACEi or ARB in participants with diabetic nephropathy [50,51,52,53]. Unfortunately, samples sizes were small, follow-up was short, hard kidney outcomes were not evaluated, and the risk of hyperkalemia remained high.
Phase II Finerenone Clinical Trials
After the safety and tolerability of different oral doses of finerenone were assessed and confirmed in healthy volunteers, a series of randomized clinical trials (RCTs) with finerenone began with the ARTS trial. This phase 2a study enrolled participants with HFrEF and mild or moderate CKD (60 to less than 90 ml/min/1.73 m2 and 30 to 60 ml/min/1.73 m2, respectively) and showed that finerenone at a dose of 5 mg or 10 mg daily had larger reductions in NT-proBNP as compared to spironolactone 25 mg or 50 mg daily [9]. In participants with mild CKD, the incidence of hyperkalemia was negligible in both finerenone treatment arms; however, finerenone did show larger rises in serum potassium than placebo among participants with moderate CKD. Despite this, all finerenone groups showed significantly smaller rises in serum potassium compared to the spironolactone group and also showed a smaller drop in systolic blood pressure and eGFR [9].
With success of the ARTS trial, the ARTS-DN trial was launched—a multicenter phase 2b RCT that evaluated the safety and efficacy of finerenone compared to placebo in reducing albuminuria in participants with diabetic albuminuria (urine albumin–creatinine ratio or UACR of 30 mg/g or higher) and mild-to-moderate CKD (eGFR of 30 ml/min/1.73 m2 or higher) [54]. The study showed a significant reduction in UACR in participants treated with 7.5, 10, 15, and 20 mg/day of finerenone compared to placebo [54]. The incidence of significant eGFR decline (defined as at least 30% at any time post-baseline) was not statistically different between all finerenone treatment groups and placebo. The overall incidence of hyperkalemia leading to discontinuation of finerenone was low at 1.8% (specifically, 0% in the 10 mg group, 3.2% in the 15 mg group, and 1.7% in the 20 mg group) [54]. Blood pressure reduction was modest with statistically significant reduction only seen in the 15 mg and 20 mg groups (decrease of 5.1 mmHg systolic and 4.7 mmHg systolic).
A second phase 2b trial soon followed: the ARTS-HF trial enrolled participants with HFrEF, type 2 diabetes mellitus (T2DM) and/or CKD (eGFR greater than 30 ml/min/1.73 m2 if T2DM or 30–60 ml/min/1.73 m2 without T2DM) to establish the safety and efficacy of five different finerenone dosing regimens compared to eplerenone [55]. All finerenone treatment groups had a similar percentage of participants with reduction in NT-proBNP levels compared to the eplerenone group, as well as a similar risk of hyperkalemia. From 35.6% to 45.2% of participants treated with finerenone showed a greater than 30% reduction in NT-proBNP levels with no statistically significant difference between groups. Rates of hyperkalemia at any point from introduction to any time post-baseline ranged from 3.6% to 6.3%. All but one (2.5 mg daily, uptitrated to 5 mg daily) finerenone treatment group showed a lower incidence of the composite endpoint of death from any cause, cardiovascular hospitalization, or emergency presentation for worsening HF at day 90 [55].
Phase III Finerenone Clinical Trials
Building on the aforementioned safety and efficacy data, the phase 3 FIGARO-DKD trial enrolled participants already on ACEi/ARB therapy with either stage 2–4 CKD (eGFR 25–90 ml/min/1.73 m2) with moderately elevated albuminuria (UACR 30 to at most 300 mg/g) or stage 1–2 CKD (eGFR 60 ml/min/1.73 m2 or higher) with severely increased albuminuria (UACR 300–5000 mg/g) [56]. The presence of symptomatic (NYHA class II–IV) HFrEF was an exclusion criterion. Participants treated with finerenone showed a reduced incidence of the primary composite outcome (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) with a relative risk reduction of 13%. However, this signal was primarily driven by a lower incidence of hospitalization for heart failure in the finerenone group, even as symptomatic HFrEF was used an exclusion criterion [56].
No significant difference in the incidence of the first composite kidney outcome (kidney failure, a sustained decrease from baseline of at least 40% in the eGFR, or death from renal causes) between treatment or placebo was observed [56]. Though an exploratory endpoint in nature, end-stage kidney disease occurred less often in the finerenone group compared to placebo. Additionally, a second kidney composite outcome (kidney failure, sustained decrease eGFR of at least 57% from baseline, or death from renal causes) occurred in 108 participants (2.9%) in the finerenone group compared to 139 participants (3.8%) in the placebo group (HR 0.77; 95% CI 0.60–0.99). Hyperkalemia occurred more often in the finerenone group compared to placebo (10.8% vs. 5.3%), but events leading to discontinuation of the study drug only occurred in 1.2% of participants in the finerenone group compared to 0.4% in the placebo [56].
The phase 3 FIDELIO-DKD trial was also initiated in parallel: this multicenter, double-blind trial assigned 5734 participants already on ACEi/ARB therapy in a 1:1 ratio to receive finerenone or placebo. This study enrolled participants with T2DM and CKD, defined as moderately elevated albuminuria (UACR 30–300 mg/g) and eGFR 25–60 ml/min/1.73 m2 and presence of diabetic retinopathy or severely elevated albuminuria (UACR 300–5000 mg/g) and eGFR 25–75 ml/min/1.73 m2 [57]. At baseline, almost all participants were on maximum tolerated dose of ACEi or ARB, and most participants had moderately increased albuminuria (300 mg/g or higher) with an average eGFR of 44 ml/min/1.73 m2; approximately half of participants had eGFR between 25 and 45 ml/min/1.73 m2. Patients with NYHA class II–IV HFrEF were again excluded from this trial. After a median follow-up of 2.6 years, FIDELIO-DKD showed that finerenone reduced the incidence of the secondary cardiovascular outcome (death from cardiovascular causes, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) with a relative risk reduction of 12.2%. These benefits appeared as early as 1 month into the trial and persisted throughout. The rate of investigator-reported hyperkalemia was greater with finerenone as compared to placebo but rarely (2.3%) resulted in medication discontinuation [57]. Acute kidney injury-related adverse events were similar between the two groups.
The finerenone treatment group showed a decreased incidence of the kidney composite outcome (kidney failure, a sustained decrease of at least 40% in the eGFR from baseline, or death from renal causes) with a relative risk reduction of 15.6% [57]. Finerenone treatment was also associated with a 31% relative reduction in the UACR at month 4 of treatment. After 4 months, the decline in eGFR was also slower in the finerenone group [57]. The benefits appear independent of blood pressure changes as finerenone had only a slight impact on blood pressure at 12 months, with a reduction of 2.1 mmHg in the finerenone group versus 0.9 mmHg in the placebo group. With regard to safety, serious hyperkalemia and hyperkalemia leading to discontinuation were higher in the finerenone group compared to placebo (1.6% vs. 0.4% and 2.3% vs. 0.9%, respectively) [57].
Given that these trials enrolled complementary population groups, the investigators also conducted a pooled analysis of the two studies. The FIDELITY pooled analysis analyzed a population of 13,026 participants with a mean eGFR of 57.6 ml/min/1.73 m2, median UACR 515 mg/g, and 48.3% of whom had very high KDIGO risk scores (eGFR less than 30; eGFR 30–44 and UACR 30 or greater; or eGFR 45–59 and UACR of 300 or greater). At a median follow-up of 3 years, the finerenone treatment group showed a significant reduction in a composite outcome of time to cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure [58]. Again, a reduction in heart failure hospitalizations was driven by the cardiovascular benefit with finerenone with a relative risk reduction of 22%, even as patients with chronic HFrEF were excluded from the study.
The prespecified composite kidney outcome (time to onset of kidney failure, sustained at least 57% decrease in eGFR from baseline over at least 4 weeks, or renal death) occurred in 360 (5.5%) participants receiving finerenone and 465 (7.1%) participants receiving placebo (HR, 0.77; 95% CI 0.67–0.88; P = 0.0002). The component of sustained decrease in eGFR of at least 57% showed a 30% relative risk reduction, and the time to kidney failure component showed a 20% relative risk reduction. In the entire pooled analysis, the incidence of hyperkalemia leading to permanent discontinuation was 1.7% in the finerenone group and 0.6% in placebo [47].
Future Directions and Conclusion
The data from clinical trials with finerenone has expanded the treatment options for cardiorenal disease management for patients with T2DM. The FIDELIO-DKD and FIGARO-DKD trials established therapeutic kidney and cardiac benefit among patients with diabetic nephropathy. The FDA approved finerenone in July 2021 for adult patients with CKD and eGFR greater than 25 ml/min/1.73 m2 secondary to type 2 diabetes to “reduce the risk of kidney function decline, kidney failure, cardiovascular death, nonfatal heart attacks, and hospitalization for heart failure” [59]. The approval was based on results of the phase 3 FIDELIO-DKD and FIGARO-DKD trials, following the previously granted priority review designation. Future directions include the potential therapeutic benefit in patients with non-diabetic proteinuric kidney disease and in the context of other newer treatments (Table 2). The FIND-CKD trial, now recruiting, is a multicenter phase 3 trial to investigate any therapeutic utility in patients with non-diabetic CKD [60]. The planned phase 2 CONFIDENCE trial will investigate the safety and efficacy of empagliflozin plus finerenone in patients with diabetic nephropathy already on maximally tolerated ACEi or ARB.
ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, BNP brain natriuretic peptide, CV cardiovascular, CKD chronic kidney disease, eGFR estimated glomerular filtration rate, ESKD end-stage kidney disease, HbA1c glycated hemoglobin, HF heart failure, HFpEF heart failure with preserved ejection fraction, KCCQ Kansas City Cardiomyopathy Questionnaire, LVEF left ventricular ejection fraction, NT-proBNP N-terminal pro-B-type natriuretic peptide, T2DM type 2 diabetes mellitus, TSS total symptom score, UACR urine albumin–creatinine ratio
With regard to finerenone’s use in patients with HFrEF, the phase 3 FINESSE trial had originally been planned to enroll patients with HFrEF and T2DM, CKD, or both to finerenone vs. eplerenone to assess a composite cardiovascular endpoint [61]. While a trial comparing finerenone head-to-head with a steroidal MRA in the HFrEF population would be useful, the sponsor decided not to move forward with this study [61]. After TOPCAT did not show a reduction in the primary endpoint among patients with HFpEF, angiotensin receptor–neprilysin inhibitors (ARNIs) were also shown to be ineffective in reducing heart hospitalizations or cardiovascular mortality in the HFpEF population [62]. These patients remained with minimal therapeutic options until the breakthrough EMPEROR-Preserved trial, showing a reduced combined risk of hospitalization or cardiovascular death among HFpEF participants treated with empagliflozin [63]. The FINEARTS-HF trial, currently recruiting, is investigating the potential therapeutic benefit of finerenone in the HFpEF population (with and without T2DM) with cardiovascular deaths and heart failure events as the primary outcome [64]. HFpEF remains a formidable clinical entity and a source of significant morbidity and mortality; another tool in the therapeutic arsenal would be extremely welcome.
Ever since the first trials demonstrated that blocking the RAAS slowed progression of kidney disease, many tried to show—without success or leading to increased adverse events—that dual blockade of the RAAS could delay kidney progression even further [12, 14]. The development of finerenone has created a new opportunity to achieve this goal, heralding a potential new era of “triple therapy” to slow the progression of CKD [65]. Finerenone also shows promise in both the treatment of HFpEF and treating patients with HFrEF who would otherwise not be a candidate for MRA therapy. This novel medication has expanded the universe of patients who could benefit from MRA treatment.
References
Kolkhof P, Barfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. 2017;234(1):T125–40.
Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345(23):1689–97.
Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–52.
Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017;23(8):628–51.
Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;4:CD007004.
Currie G, Taylor AH, Fujita T, et al. Effect of mineralocorticoid receptor antagonists on proteinuria and progression of chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol. 2016;17(1):127.
Kidney Disease: Improving Global Outcomes Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–87.
Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69–78.
Pitt B, Kober L, Ponikowski P, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. 2013;34(31):2453–63.
Yang J, Young MJ. Mineralocorticoid receptor antagonists-pharmacodynamics and pharmacokinetic differences. Curr Opin Pharmacol. 2016;27:78–85.
Amazit L, Le Billan F, Kolkhof P, et al. Finerenone impedes aldosterone-dependent nuclear import of the mineralocorticoid receptor and prevents genomic recruitment of steroid receptor coactivator-1. J Biol Chem. 2015;290(36):21876–89.
Fagart J, Hillisch A, Huyet J, et al. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem. 2010;285(39):29932–40.
Fagart J, Wurtz JM, Souque A, Hellal-Levy C, Moras D, Rafestin-Oblin ME. Antagonism in the human mineralocorticoid receptor. EMBO J. 1998;17(12):3317–25.
Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31(10):1237–47.
Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–8.
Funder JW. Primary aldosteronism and salt. Pflugers Arch. 2015;467(3):587–94.
Keidar S, Kaplan M, Pavlotzky E, et al. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation. 2004;109(18):2213–20.
Hirono Y, Yoshimoto T, Suzuki N, et al. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system. Endocrinology. 2007;148(4):1688–96.
Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20(9):1546–8.
Stas S, Whaley-Connell A, Habibi J, et al. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. 2007;148(8):3773–80.
Bauersachs J, Heck M, Fraccarollo D, et al. Addition of spironolactone to angiotensin-converting enzyme inhibition in heart failure improves endothelial vasomotor dysfunction: role of vascular superoxide anion formation and endothelial nitric oxide synthase expression. J Am Coll Cardiol. 2002;39(2):351–8.
Nagata D, Takahashi M, Sawai K, et al. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48(1):165–71.
Leopold JA, Dam A, Maron BA, et al. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13(2):189–97.
Brilla CG, Weber KT. Mineralocorticoid excess, dietary sodium, and myocardial fibrosis. J Lab Clin Med. 1992;120(6):893–901.
Selye H. Protection by a steroid-spirolactone against certain types of cardiac necroses. Proc Soc Exp Biol Med. 1960;104:212–3.
Young M, Funder JW. Eplerenone, but not steroid withdrawal, reverses cardiac fibrosis in deoxycorticosterone/salt-treated rats. Endocrinology. 2004;145(7):3153–7.
Suzuki G, Morita H, Mishima T, et al. Effects of long-term monotherapy with eplerenone, a novel aldosterone blocker, on progression of left ventricular dysfunction and remodeling in dogs with heart failure. Circulation. 2002;106(23):2967–72.
Rocha R, Rudolph AE, Frierdich GE, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283(5):H1802–10.
Lavall D, Jacobs N, Mahfoud F, Kolkhof P, Bohm M, Laufs U. The non-steroidal mineralocorticoid receptor antagonist finerenone prevents cardiac fibrotic remodeling. Biochem Pharmacol. 2019;168:173–83.
Grune J, Benz V, Brix S, et al. Steroidal and nonsteroidal mineralocorticoid receptor antagonists cause differential cardiac gene expression in pressure overload-induced cardiac hypertrophy. J Cardiovasc Pharmacol. 2016;67(5):402–11.
Grune J, Beyhoff N, Smeir E, et al. Selective mineralocorticoid receptor cofactor modulation as molecular basis for finerenone’s antifibrotic activity. Hypertension. 2018;71(4):599–608.
Rossi GP, Bernini G, Desideri G, et al. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48(2):232–8.
Sechi LA, Novello M, Lapenna R, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295(22):2638–45.
Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T. Podocyte as the target for aldosterone: roles of oxidative stress and Sgk1. Hypertension. 2007;49(2):355–64.
Nishiyama A, Yao L, Fan Y, et al. Involvement of aldosterone and mineralocorticoid receptors in rat mesangial cell proliferation and deformability. Hypertension. 2005;45(4):710–6.
Nishiyama A, Yao L, Nagai Y, et al. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension. 2004;43(4):841–8.
Whaley-Connell AT, Habibi J, Nistala R, et al. Mineralocorticoid receptor-dependent proximal tubule injury is mediated by a redox-sensitive mTOR/S6K1 pathway. Am J Nephrol. 2012;35(1):90–100.
Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int. 2003;63(5):1791–800.
Dutzmann J, Musmann RJ, Haertle M, et al. The novel mineralocorticoid receptor antagonist finerenone attenuates neointima formation after vascular injury. PLoS ONE. 2017;12(9): e0184888.
Gonzalez-Blazquez R, Somoza B, Gil-Ortega M, et al. Finerenone attenuates endothelial dysfunction and albuminuria in a chronic kidney disease model by a reduction in oxidative stress. Front Pharmacol. 2018;9:1131.
Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–17.
Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309–21.
Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21.
Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92.
Elguindy AM. TOPCAT misses its primary endpoint: should spironolactone be abandoned in HFpEF? Glob Cardiol Sci Pract. 2013;2013(4):357–60.
Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60.
Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9.
Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41(1):64–8.
Schjoedt KJ, Andersen S, Rossing P, Tarnow L, Parving HH. Aldosterone escape during blockade of the renin-angiotensin-aldosterone system in diabetic nephropathy is associated with enhanced decline in glomerular filtration rate. Diabetologia. 2004;47(11):1936–9.
Schjoedt KJ, Rossing K, Juhl TR, et al. Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int. 2005;68(6):2829–36.
van den Meiracker AH, Baggen RG, Pauli S, et al. Spironolactone in type 2 diabetic nephropathy: effects on proteinuria, blood pressure and renal function. J Hypertens. 2006;24(11):2285–92.
Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2006;1(5):940–51.
Brandt-Jacobsen NH, Johansen ML, Rasmussen J, et al. Effect of high-dose mineralocorticoid receptor antagonist eplerenone on urinary albumin excretion in patients with type 2 diabetes and high cardiovascular risk: data from the MIRAD trial. Diabetes Metab. 2021;47(4): 101190.
Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884–94.
Filippatos G, Anker SD, Bohm M, et al. A randomized controlled study of finerenone vs eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. 2016;37(27):2105–14.
Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385(24):2252–63.
Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219–29.
Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022;43(6):474–84.
Food and Drug Administration. FDA approves drug to reduce risk of serious kidney and heart complications in adults with chronic kidney disease associated with type 2 diabetes [press release]. Food and Drug Adminstration, 2021.
ClinicalTrials.gov. A trial to learn how well finerenone works and how safe it is in adult participants with non-diabetic chronic kidney disease (FIND-CKD). US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT05047263?term=finerenone&draw=2&rank=8. Accessed 28 Feb 2022.
Gay A. Letter to the Editor relative to Clin Chem Lab Med 2018;56(3):360–372. Clin Chem Lab Med. 2018;56(11):251–2.
Solomon SD, McMurray JJV, PARAGON-HF Steering Committee and Investigators. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. Reply. N Engl J Med. 2020;382(12):1182–3.
Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–61.
ClinicalTrials.gov. Study to evaluate the efficacy (effect on disease) and safety of finerenone on morbidity (events indicating disease worsening) & mortality (death rate) in participants with heart failure and left ventricular ejection fraction (proportion of blood expelled per heart stroke) greater or equal to 40% (FINEARTS-HF). US National Library of Medicine. 2020. https://clinicaltrials.gov/ct2/show/NCT04435626?term=finerenone&draw=2&rank=7. Accessed 28 Feb 2022.
ClinicalTrials.gov. A study to learn how well the treatment combination of finerenone and empagliflozin works and how safe it is compared to each treatment alone in adult participants with long-term kidney disease (chronic kidney disease) and type 2 diabetes (CONFIDENCE). 2022. https://clinicaltrials.gov/ct2/show/NCT05254002?term=finerenone&draw=2&rank=1. Accessed 28 Feb 2022.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Srikanth Palanisamy – composition of majority of article’s text. Mario Funes Hernandez – significant composition of article’s text. Tara I. Chang – significant assistance in revision of article. Kenneth W. Mahaffey -- significant assistance in revision of article.
Disclosures
Srikanth Palanisamy, Mario Funes Hernandez, Tara I. Chang, Kenneth W. Mahaffey all have nothing to disclose
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Palanisamy, S., Funes Hernandez, M., Chang, T.I. et al. Cardiovascular and Renal Outcomes with Finerenone, a Selective Mineralocorticoid Receptor Antagonist. Cardiol Ther 11, 337–354 (2022). https://doi.org/10.1007/s40119-022-00269-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-022-00269-3